Direct oral anticoagulant (DOAC) therapy has been shown to be safe and effective in patients with atrial fibrillation (AF). However, outcomes in AF patients with bioprosthetic valves are unclear, as this population has been underrepresented in clinical trials. The aim of this study was to assess the safety and efficacy of DOACs in this population based on the existing published literature.
MethodsA systematic search and review were conducted to identify randomized clinical trials and comparative observational studies published from 2017 to January 2022 that compared DOACs and vitamin K antagonists (VKAs) in AF patients with bioprosthetic valves. Hazard ratios (HR) were collected to compare the 2 treatments in terms of cardiovascular and all-cause mortality, stroke/systemic embolism, and major bleeding. A meta-analysis combining the results was performed.
ResultsWe included 12 studies (30 283 patients). DOACs and VKAs were compared based on HRs at the 95% confidence interval. DOAC therapy was associated with a significant 9% reduction in all-cause mortality (HR, 0.91; 95%CI, 0.85-0.97; P=.0068; I2=8%), with no significant differences in the risk of stroke/systemic embolism (HR, 0.87; 95%CI, 0.67-1.14; P=.29; I2=45%) or major bleeding (HR, 0.82; 95%CI, 0.67-1.00; P=.054; I2=48.7%).
ConclusionsDOAC therapy in AF patients with bioprosthetic valves may be associated with a significant reduction in all-cause mortality, with no reduction in the efficacy of stroke/systemic embolism prevention or increase in major bleeding risk.
Keywords
Direct oral anticoagulants (DOACs) have been increasingly used for cardioembolic prophylaxis in patients with nonvalvular AF. The large randomized clinical trials published to date have shown that these agents are as effective or more so than vitamin K antagonists (VKAs) in the prevention of thromboembolic events and generally have a better bleeding risk profile. However, patients with bioprosthetic valves have been underrepresented in these studies. Furthermore, the studies used to validate the CHA2DS2-VASc score have not included bioprosthesis patients.1 The European Clinical Guidelines2 make a IIA recommendation with level of evidence C for the use of DOACs in this group (IIB in the first 3 months following valve replacement) based on the results of substudies conducted as part of these clinical trials.3 In contrast, the North American guidelines do not support this recommendation4 because 2 of these substudies were analyzed post hoc in a small group of patients,5,6 and another study comparing warfarin vs dabigatran was discontinued prematurely because of low recruitment.7
Even though the evidence on DOAC safety and efficacy in this patient population has not yet been well established, these drugs are increasingly being used in the clinical practice.8
The objective of this study was to assess the safety and efficacy of DOAC in patients with AF and bioprosthetic valves through a systematic review and meta-analysis including recent evidence from comparative cohort studies and real-world results.
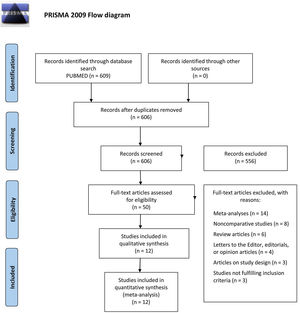
METHODSA systematic review of the scientific literature and a subsequent meta-analysis were carried out following the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement guidelines9 for systematic reviews (figure 1).
Systematic searchThe first nonformal searches were performed in January 2022 combining the terms ‘bioprosthetic valves’ and ‘direct anticoagulants’ in the PubMed and Scopus databases. A systematic search was subsequently conducted in the PubMed database using Boolean operators, restricting the search to articles published from 2017 (inclusive) to January 2022. The combination of terms providing the best results is presented in the . Specifically, 609 results were obtained. Secondary sources were subsequently searched by searching references from initially identified articles and reviews. Inclusion and exclusion criteria were established prior to study selection (table 1).
Inclusion and exclusion criteria for the selection of studies for the meta-analysis
| Inclusion criteria | Exclusion criteria |
|---|---|
| - RCTs or comparative observational studies.- Comparing DOACs vs vitamin K antagonists.- In patients with AF or atrial flutter and bioprosthetic valves.- Including all-cause mortality, major bleeding, and systemic embolism results.a- Published from January 2017 to January 2022. | - Noncomparative studies.- Patients without AF or atrial flutter.- Meta-analysis. |
AF, atrial fibrillation; DOAC, direct oral anticoagulant; RCT, randomized clinical trial.
Screening for study selection was conducted separately by 2 independent and blinded authors through title and abstract reading, to select the studies based on our inclusion and exclusion criteria. Disagreements were resolved by consensus.
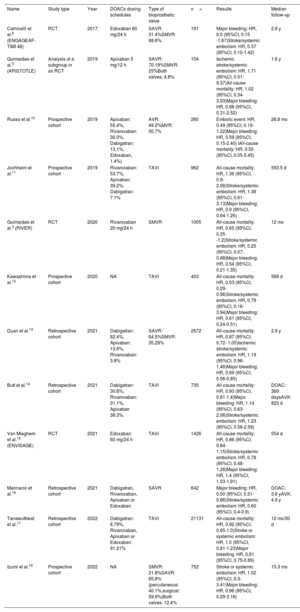
Of all the screened studies, 50 were eligible for a comprehensive evaluation and 12 were finally selected for their inclusion in the quantitative analysis. This process and the reasons for exclusion are summarized in the PRISMA flow diagram (figure 1). The characteristics of the studies included in the meta-analysis are described in table 2.
Key features of the studies included in the meta-analysis
| Name | Study type | Year | DOACs dosing schedules | Type of bioprosthetic valve | n= | Results | Median follow-up |
|---|---|---|---|---|---|---|---|
| Carnicelli et al.6 (ENGAGEAF-TIMI 48) | RCT | 2017 | Edoxaban 60 mg/24 h | SAVR: 31.4%SMVR: 68.6% | 191 | Major bleeding: HR, 0.5 (95%CI, 0.15 -1.67)Stroke/systemic embolism: HR, 0.37 (95%CI, 0.10-1.42) | 2.8 y |
| Guimarães et al.5 (ARISTOTLE) | Analysis of a subgroup in an RCT | 2019 | Apixaban 5 mg/12 h | SAVR: 70.19%SMVR: 25%Both valves: 4.8% | 104 | Ischemic stroke/systemic embolism: HR, 1.71 (95%CI, 0.31-9.37)All-cause mortality: HR, 1.02 (95%CI, 0.34-3.03)Major bleeding: HR, 0.88 (95%CI, 0.31-2.52) | 1.6 y |
| Russo et al.10 | Prospective cohort | 2019 | Apixaban: 55.4%, Rivaroxaban: 30.0%, Dabigatran: 13.1%, Edoxaban, 1.4%) | AVR: 49.2%MVR: 50.7% | 260 | Embolic event: HR, 0.49 (95%CI, 0.19-1.22)Major bleeding: HR, 0.59 (95%CI, 0.15-2.40) IAll-cause mortality: HR, 0.50 (95%CI, 0.05-5.45) | 26.8 mo |
| Jochheim et al.11 | Prospective cohort | 2019 | Rivaroxaban: 53.7%, Apixaban: 39.2%, Dabigatran: 7.1% | TAVI | 962 | All-cause mortality: HR, 1.36 (95%CI, 0.9-2.06)Stroke/systemic embolism: HR, 1.38 (95%CI, 0.61-3.13)Major bleeding: HR, 0.9 (95%CI, 0.64-1.26) | 593.5 d |
| Guimarães et al.3 (RIVER) | RCT | 2020 | Rivaroxaban 20 mg/24 h | SMVR | 1005 | All-cause mortality: HR, 0.65 (95%CI, 0.35--1.2)Stroke/systemic embolism: HR, 0.25 (95%CI, 0.07-0.88)Major bleeding: HR, 0.54 (95%CI, 0.21-1.35) | 12 mo |
| Kawashima et al.12 | Prospective cohort | 2020 | NA | TAVI | 403 | All-cause mortality: HR, 0.53 (95%CI, 0.29-0.96)Stroke/systemic embolism: HR, 0.79 (95%CI, 0.16-3.94)Major bleeding: HR, 0.61 (95%CI, 0.24-0.51) | 568 d |
| Duan et al.13 | Retrospective cohort | 2021 | Dabigatran: 82.4%, Apixaban: 13.6%, Rivaroxaban: 3.8% | SAVR: 64.5%SMVR: 35.29% | 2672 | All-cause mortality: HR, 0.87 (95%CI, 0.72- 1.05)Ischemic stroke/systemic embolism: HR, 1.19 (95%CI, 0.96-1.46)Major bleeding: HR, 0.69 (95%CI, 0.56-0.85) | 2.9 y |
| Butt et al.14 | Retrospective cohort | 2021 | Dabigatran: 30.6%, Rivaroxaban: 31.1%, Apixaban 38.3%. | TAVI | 735 | All-cause mortality: HR, 0.93 (95%CI, 0.61-1.4)Major bleeding: HR, 1.14 (95%CI, 0.63-2.06)Stroke/systemic embolism: HR, 1.23 (95%CI, 0.58-2.59) | DOAC: 369 daysAVK: 823 d |
| Van Mieghem et al.15 (ENVISAGE) | RCT | 2021 | Edoxaban: 60 mg/24 h | TAVI | 1426 | All-cause mortality: HR, 0.86 (95%CI, 0.64-1.15)Stroke/systemic embolism: HR, 0.78 (95%CI, 0.48-1.28)Major bleeding: HR, 1.4 (95%CI, 1.03-1,91) | 554 d |
| Mannacio et al.16 | Retrospective cohort | 2021 | Dabigatran, Rivaroxaban, Apixaban or Edoxaban | SAVR | 642 | Major bleeding: HR, 0.50 (95%CI, 0.31-0.86)Stroke/systemic embolism: HR, 0.60 (95%CI, 0.4-0.9) | DOAC: 3.8 yAVK: 4.9 y |
| Tanawuttiwat et al.17 | Retrospective cohort | 2022 | Dabigatran: 8.79%, Rivaroxaban, Apixaban or Edoxaban: 91.21% | TAVI | 21131 | All-cause mortality: HR, 0.92 (95%CI, 0.85-1.0)Stroke or systemic embolism: HR, 1.0 (95%CI, 0.81-1.23)Major bleeding: HR, 0.81 (95%CI, 0.75-0.89) | 12 mo/30 d |
| Izumi et al.18 | Prospective cohort | 2022 | NA | SMVR: 21.8%SAVR: 65.8% (percutaneous: 40.1%,surgical: 59.9%)Both valves: 12.4% | 752 | Stroke or systemic embolism: HR, 1.02 (95%CI, 0.3-3.41)Major bleeding: HR, 0.96 (95%CI, 0.29-3.16) | 15.3 mo |
95%CI, 95% confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; RCT, randomized clinical trial; NA, not available; SAVR, surgical aortic valve replacement; SMVR, surgical mitral valve replacement; TAVI, transcatheter aortic valve replacement.
The primary outcome of interest was all-cause mortality. Secondary outcomes were systemic embolism or stroke and cardiovascular mortality. The primary safety outcome was major bleeding. We accepted the criterion of major bleeding from each study. Systemic embolism or stroke was defined as ischemic stroke, systemic embolism, and/or transient ischemic attack.
Statistical analysisMeasure of interest and softwareThe data extracted from all the studies consisted of the HR for each outcome of interest. The measure of uncertainty was calculated using a 95% confidence interval (95%CI). Although the meta-analysis was initially based on 12 studies, those with missing data, either for the effect size (hazard ratio [HR]) or the 95%CI, were excluded from each analysis.
The data extracted to evaluate the effect of the intervention were those derived from intention-to-treat analysis (in randomized clinical trials [RCTs]) and fully-adjusted models (for observational studies). The selected intervention effects were those derived from the longest follow-up period for each study.
The metagen function in the meta library of the statistical R package19 was used to work with HRs on a logarithmic scale. Both the random effects model and the fixed effects model were simultaneously fitted to the data and then updated based on heterogeneity tests.
The inverse variance method was used to obtain the estimator of the overall effect size and the Paule-Mandel estimator for the random error. The Q-profile method was used for the CI of this error, and the Hartung-Knapp correction was applied when a random effects model was used.
Moderator of subgroup analysisGiven the initial differentiation among studies (RCT and observational studies), we tested a priori differences through the Borenstein and Higgins fixed-effects plural model.20
Meta-analysis and heterogeneityThe conclusions regarding heterogeneity between studies were based on the CI for the random error tau (μ), the Cochran Q test, the Higgins and Thomson (I2) statistic (describing the percentage of variability of the effect sizes due to the heterogeneity), and the H statistic based on Cochran's Q comparing the observed with the expected variability. When any of the tests provided evidence of heterogeneity, the random effects model was chosen. The overall effect size was estimated with the chosen model (fixed or random effects) and conclusions were based on its significance and CI.
Results were also displayed in forest plots where weights given to the combined studies—based on the precision of the estimates—were also shown.
Influence analysisAn influence analysis to identify outlier studies using the find.outliers function of the meta library in R was carried out. An influence analysis was also done for those studies with high weights in the meta-analysis in order to study its sensitivity (or robustness) to dispensing with them.
Publication biasThe publication bias analysis was performed using Duval and Tweedie's “trim and fill” method,21 only when the random effects model had been fitted to the available studies. This procedure replicates studies with an effect size that differs significantly from those of the other studies using mirror values (from the mean) and readjusts the random effects meta-analysis.
Risk of bias in individual studiesStudy quality was assessed using the Cochrane Collaboration ROB-2.0 tool22 () for RCTs and the Newcastle-Ottawa Scale23 () for observational studies.
RESULTSOur analysis included 12 studies, 4 RCTs and 8 observational studies that enrolled a total of 30 283 patients.
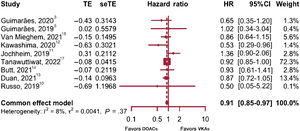
All-cause mortality and cardiovascular mortalityThe subgroup analysis did not provide evidence of differences between observational studies and RCT (P=.37), or heterogeneity within subgroups (P=.32), therefore 6 observational and 3 RCT providing data on all-cause mortality were combined (figure 2).3,5,10–15,17.
The study by Tanawuttiwat et al.17 was the most precise of all to estimate the HR and the study with the greatest impact on the meta-analysis (72% weight). There was no evidence of heterogeneity and a fixed effects model was therefore used. A 9% reduction was found for all-cause mortality in patients treated with DOACs (HR, 0.91; P=.0068; 95%CI, 0.85-0.97; I2=8%). The influence analysis did not identify any outlier studies.
Excluding the study by Tanawuttiwat et al.17 from the sensitivity analysis did not result in significant differences between the 2 treatments (HR, 0.88; P=.06; 95%CI, 0.77-1.01; I2=16.3%). However, this study was not identified as an outlier, and after its inclusion there was no evidence of heterogeneity, and therefore no concerns were found for its inclusion in the analysis.
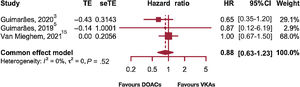
Cardiovascular mortality was also assessed, with just 3 studies in the meta-analysis providing results, which inevitably yielded little evidence and represented a limitation for the interpretation of the findings in conjunction with the other conclusions drawn from this study (figure 3).3,5,15.
No significant differences were found between treatments (HR, 0.88; P=.446; 95%CI, 0.63-1.23; I2=0%) and no evidence of heterogeneity was revealed. There was no evidence of outliers. The study by Van Mieghem et al.15 was found to be the most precise of all to estimate the HR and received a 68% weight. Excluding this study for the sensitivity analysis did not result in significant differences between the 2 treatments.
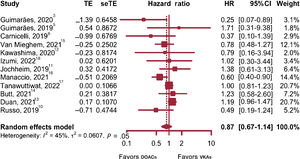
Stroke and systemic embolismThe subgroup analysis showed no differences between observational studies and RCT (P=.22), or heterogeneity within subgroups (P=.08); therefore 4 RCT and 8 observational studies were combined (figure 4).3,5,6,10–18
Weights varied widely, and both studies by Tanawuttiwat et al.17 and Duan et al.13 were assigned the maximum weight of approximately 20.7%. As there were signs of significant heterogeneity, a random effects model was used. No evidence showing a significant effect of DOAC therapy on risk reduction for stroke and embolism was obtained (HR, 0.87; P=.29; 95%CI, 0.67-1.14; I2=45%).
The influence analysis did not identify any outlier studies and we found no evidence of publication bias.
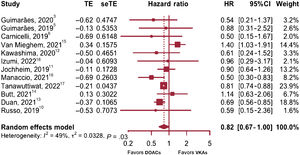
Major bleedingThe subgroup analysis showed no differences between observational studies and RCT (P=.1471) or heterogeneity within subgroups (P=.1827). Therefore, the results of the 12 studies were combined to obtain the relative risk of major bleeding associated with DOAC therapy (figure 5).3,5,6,10–18
In this case, weights varied widely and the study by Tanawuttiwat et al.17 was found to have the maximum weight (23.9%) with an estimation error clearly smaller than the rest (0.04 for the standard error). Although the random error (τ) was not significantly >0 and the H statistic's CI contained the value of 1, thus providing no evidence of heterogeneity, up to 48.7% of the variation between the effect sizes was seen to result from between-study heterogeneity, as inferred from the I2 value and Cochran's heterogeneity test (P=.029). The random effects model was therefore considered superior and provided an overall effect size estimate of 0.82 based on HR (P=.054; 95%CI, 0.67-1.00). This was not significant at the 95% level although the results were borderline (the P value was greater than the usual 5% and the value 1 is the limit of the CI for HR estimation); however, a significant DOAC therapy effect on bleeding reduction could not be said to occur.
For the assessment of potential publication bias, a “trim and fill” adjustment21 was performed by adding 3 studies to complement the meta-analysis (Guimarães et al., 3 Carnicelli et al.,6 and Mannacio et al.16). As no evidence of between-study heterogeneity was found, a fixed effects model was used, and the conclusions reached, based on the findings obtained, were similar to those already mentioned. Even when we used a random effects model was used, we found no significant overall effect in favor of a bleeding risk reduction with DOAC therapy.
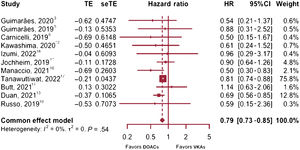
A robustness analysis was performed subsequently, and the study by Van Mieghem et al.15 was identified as atypical compared with the other studies included. We therefore proceeded to re-run the meta-analysis without the results of that study and, because we found no evidence of heterogeneity, a fixed effects model was used. In this case, the overall effect size was HR, 0.79 (P=.0001; 95%CI, 0.73-0.85; I2=0%), with a 21.1% reduction in the risk of major bleeding with DOAC therapy compared with VKA (figure 6).3,5,6,10–14,16–18
Major bleeding forest plot excluding ENVISAGE.15 DOACs, direct oral anticoagulants; VKAs, vitamin K antagonists.
The study by Tanawuttiwat et al.17 exhibited a high weight (75.8%), but by excluding this study from the sensitivity analysis, similar results were shown with a fixed effects model (HR, 0.73; P=.0001; 95%CI, 0.63-0.84).
DISCUSSIONDOACs have been shown to have an enhanced safety profile and have made anticoagulant therapy monitoring and follow-up easier in AF patients. However, these benefits are less clear among patients with bioprosthetic valves.
Conclusions were first derived from substudies that included few patients and were part of pivotal trials conducted with different DOACs.
The findings of the post hoc analysis of the RIVER3 trial demonstrated the noninferiority of rivaroxaban vs warfarin in patients with AF and mitral bioprosthetic valves for a combined outcome of death and cardiovascular or major bleeding events at 12 months.
A substudy of the ARISTOTLE5 trial compared a subgroup of 108 bioprosthetic valve patients with AF receiving apixaban (n=56) vs warfarin (n=52) and found no differences in stroke or systemic embolism, major bleeding, or all-cause mortality.
In the ENGAGE AF-TIMI 48 trial, a prespecified analysis6 comparing warfarin and edoxaban in patients with bioprosthetic valves and AF showed similar rates of stroke and systemic embolism, as well as decreased bleeding with edoxaban.
Finally, in the recent ENVISAGE TAVI- AF15 RCT, in which AF patients who had undergone successful TAVI were randomly assigned to receive either VKAs or edoxaban, noninferiority for edoxaban was shown for the composite outcome of adverse clinical events (all-cause mortality, myocardial infarction, ischemic stroke or systemic embolism, valvular thrombosis, or major bleeding). However, edoxaban did not meet the noninferiority criteria for safety as major bleeding events due to gastrointestinal bleeding increased in the group treated with this drug. These findings are consistent with the increase in gastrointestinal bleeding found in the ENGAGE AF-TIMI 48 trial.24
Various cohort studies providing additional evidence of DOACs in patients with bioprosthetic valves have also reached contrasting conclusions. In the OCEAN prospective cohort,12 which included TAVI patients, all-cause mortality decreased in the DOACs group. A study based on the France-TAVI and FRANCE-2 registries also found a decrease in all-cause mortality and major bleeding, and comparable rates of ischemic stroke in patients receiving DOACs vs VKAs.25 Contrasting results have also been obtained for the risk of stroke and systemic embolism. In a multicenter study with 962 TAVI patients,11 a higher rate of ischemic events and a similar rate of bleeding events were found with DOACs. However, in a Japanese cohort of 894 patients with bioprosthetic valves (BPV-AF Registry),18 of whom approximately 30% were receiving DOAC therapy, the risk of major bleeding, stroke, and systemic embolism was similar to that of patients receiving warfarin. Similar findings were obtained in a Danish cohort of TAVI patients.14
Additionally, Tanawuttiwat et al.17 analyzed data from 21 131 patients receiving VKAs or DOACs identified from one of the largest cohorts of patients with TAVI (STS/ACC Registry) and found that DOACs were associated with a similar risk of stroke but also with a lower risk of bleeding, intracranial hemorrhage, and 1-year mortality.
Last, the various published meta-analyses have generally yielded either similar results for both therapies26,27 or findings in favor28–30 of DOACs in terms of safety and efficacy.
Our study showed a 9% reduction in all-cause mortality among DOAC-treated patients. This reduction could be attributed to a decrease in major bleeding reported in the included studies (except for ENVISAGE). Although this result suggests a benefit in favor of the superiority of NOACs in those patients, it should be noted that, in the registry of Tanawuttiwat et al., the lower risk of bleeding among NOAC-treated patients could be attributed to this group having fewer risk factors for bleeding. Therefore, this finding, reported mainly in observational studies, could be due to significant baseline differences between the 2 groups because of the lack of randomization.
In the study by Yokoyama et al.,30 which combined data from 4 RCTs and 6 comparative cohort studies, DOAC use was associated with a significant reduction in the relative risk of bleeding and no increase in the risk of embolic events or all-cause mortality. The differences in major bleeding reduction between the study by Yokoyama et al. and our own could be due to the inclusion of the results of the ENVISAGE trial.15
In our meta-analysis, the sensitivity analysis indicated that the ENVISAGE study15 was an outlier, in statistical terms, for major bleeding risk. Of note, exclusion of this study from the meta-analysis revealed significant differences in favor of DOACs. However, the results on major bleeding in that study were finally included in the meta-analysis as it was one of the most recent RCTs, had high-quality data and was the only RTC assessing the use of VKAs vs edoxaban in patients with AF after TAVI. In addition, the study provided information on its influence on the global analysis, and we therefore deemed its findings to deserve mention. The higher risk of gastrointestinal bleeding in the edoxaban group may have been due to the inclusion of older patients with greater comorbidity, who may also have had a higher likelihood of having an acquired von Willebrand factor deficiency. Additionally, the indication of concomitant antiplatelet therapy was based on the clinician's judgment, and dual antiplatelet therapy could be used during the first 3 months. The study also reported higher treatment discontinuation rates in patients treated with VKAs. A further factor that may have contributed to these findings is that, although the target international normalized ratio (INR) was 2.0 to 3.0 in general, it was 1.6 to 2.6 in the Japanese patients> 70 years. However, this does not appear to be that relevant as this age group did not account for the largest group of patients in the total study population. In addition, in the cohort of Izumi et al.,18 in which up to 52% of patients aged <70 years had an INR below the target INR <2.0, and 91.1% of patients> 70 years had an INR <2.6, no significant differences were detected in the risk of major bleeding between the 2 groups. However, that study included patients with other types of bioprosthetic valves (only 26.4% of patients had undergone TAVI).
In contrast to the findings of the ENVISAGE trial, Tanawuttiwat et al.17 reported a significant decrease in bleeding and all-cause mortality, although edoxaban was the least represented DOAC in that study. Another reason that could therefore explain this discrepancy may be the existence of different bleeding risk profiles between the various DOACs. Indeed, the ATLANTIS RCT31 attempted to demonstrate the superiority of apixaban over VKAs (if anticoagulation was indicated) or antiplatelet therapy (if there was no such indication) in TAVI patients, but found no differences in major bleeding.
The present study has several limitations. First, it has the inherent limitations of the studies included and the combination of findings from comparative observational cohort studies and ad hoc studies from RCTs, entailing a potential source of bias.
Second, sample sizes varied widely among the studies. The study by Tanawuttiwat et al.17 had by far the largest sample size among the included studies, accounting for up to 70% of patients included in our meta-analysis. Furthermore, since that study had a retrospective cohort design, it may have introduced a potential source of bias.
Third, follow-up times were in general short, being less than 18 months in most studies and longer than 24 months in only 4,6,10,13,16 of which only 1 was an RCT.6
Fourth, individual risks for each type of bioprosthetic valve were not studied and they were instead included in a single group. The proportion of different types of bioprosthetic valves assessed in each study varied and some included only TAVI patients.11,12,14,15,17 In our meta-analysis, only 18% of patients approximately underwent surgical aortic valve replacement and/or surgical mitral valve replacement, with TAVI patients representing the majority. Outcomes in surgical aortic valve replacement and/or surgical mitral valve replacement patients seem to favor of DOAC therapy vs VKAs in terms of efficacy and safety, whereas the results in TAVI patients seem to be more heterogeneous, particularly for major bleeding. Furthermore, antiplatelet regimens also differed and may have contributed to greater differences in bleeding events.
This variability reflects the lack of an optimal standard treatment for all the bioprosthetic valves, as is evident from current clinical practice guidelines.2,4 Further studies are needed to provide solid evidence on the optimal anticoagulant therapy for each type of bioprosthetic valve according to its location (aortic or mitral) and type of approach (surgical or percutaneous).
Fifth, the 4 currently marketed DOACs were included in our meta-analysis, but their safety and efficacy were not evaluated separately. Their use varied between studies, most of which did not clearly define DOAC treatment initiation following bioprosthetic valve implantation. Therefore, the findings of our study cannot be extrapolated to the different DOACs individually.
Finally, except for the RIVER trial,3 there are no data on relevant events during the first 3 months following valve replacement. Therefore, our study cannot not provide new information on the safety and efficacy of DOACs during that time period.
CONCLUSIONSThis meta-analysis combines the results of 30 283 patients with bioprosthetic valves and AF and is therefore one of the meta-analyses with the largest patient population to date, combining findings from RCTs and from comparative cohorts providing real-world data.
Our study found that DOAC therapy is associated with a significant reduction of all-cause mortality with no significant increase in the risk of systemic embolism or stroke. Our results therefore suggest that DOACs could be safer than VKAs in this group of patients (figure 7). Although our findings do not point to significant differences in bleeding risk between the 2 groups, they should be considered with caution. Studies based primarily on TAVI patient cohorts may not provide evidence of improved safety or reduced major bleeding with DOAC therapy because of these patients’ higher bleeding risk profile or the concomitant use of antiplatelet drugs; in addition, the benefits may differ between different types of DOACs and bioprosthetic valves. There is a need for future RCTs to compare the different DOACs currently available on the market with VKAs in patients with bioprosthetic valves and AF, and to assess the different types of DOACs and bioprostheses separately.
- •
The benefit of DOACs in patients with AF and bioprosthetic valves is not established given that this population is underrepresented in the literature.
- •
Our meta-analysis, combining the results of a large number of patients from RCTs and the most recent population-based registries, reveals a significant relative risk reduction in all-cause mortality in patients with AF and bioprosthetic valves treated with DOACs compared with those treated with VKAs, with no significant increase in major bleeding or in the risk of systemic embolism or stroke.
- •
Further research is needed to evaluate the long-term safety and efficacy of different DOACs and types of bioprosthetic valves, including in the first 3 months after the intervention.
Central illustration. A meta-analysis of 4 randomized clinical trials and 8 observational studies comparing DOACs vs vitamin K antagonists (VKAs) for patients with atrial fibrillation (AF) and bioprosthetic valves showing lower all-cause mortality with DOACs compared with vitamin K antagonists. DOACs, direct oral anticoagulants.
None.
AUTHORS’ CONTRIBUTIONSP. Guardia Martínez: study design, data acquisition, systematic search and study selection, reviewing, editing. A. Luis Avilés Toscano: data acquisition, systematic search and study selection, reviewing, editing. M.A. Martínez Mayoral: study design, methodology, statistical analysis, and reviewing. J. Moltó Miralles: study design, methodology, statistical analysis and reviewing. All authors are responsible for reviewing the manuscript and approving the final version.
CONFLICTS OF INTERESTNone declared.
We appreciate the support of the Research Unit of the Agencia Sanitaria Costa del Sol in the translation of the manuscript.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.02.002