Keywords
INTRODUCTION
In recent years, heart failure has become one of most important public health problems of developed nations, both in terms of the increase in its incidence and the enormous personal, social and economic repercussions it is likely to have in the near future.1,2 Currently, heart failure affects more than 22 million people worldwide,3 is the third most common cardiovascular cause of death, and is an important cause of morbidity and hospitalizations.4
Biventricular pacing is an option for patients with heart failure; its benefits have been shown in a number of prospective, randomized trials.5-11 Improvements have been described in functional class, quality of life, and the distance covered in the 6 min walk test.5-7 More recently, reductions in overall mortality,11 as well as in morbidity and mortality due to heart failure, have also been reported.12
The benefits of cardiac resynchronization, however, do not depend solely upon the careful selection of patients, but also on the final position of the implanted leads, especially of that which will stimulate the left ventricle. A number of animal13 and human studies14-16 have shown there to be a close relationship between the site of stimulation and the magnitude of the ventricular functional response to cardiac resynchronization. The available data show the ideal locations to be the lateral or posterolateral regions since it is here where the greatest contractile delay is seen under conditions of left bundle block.17
The implantation of a cardiac resynchronization device can be performed via the venous route without difficulty in about 80%-90% of patientsa consequence of the technological advances made in recent years and the greater experience of medical personnel in implanting left ventricular leads.3,18-22 However, problems can appear in some 10%-20% of cases, the consequences of which can be serious since the implantation of the left ventricular lead at an inadequate site can result in the failure of resynchronization therapy. The only alternative to intravenous implantation is that involving thoracotomy, an option currently regarded as being undesirable.
A number of obstacles can hinder the implantation of the venous coronary lead. The main causes of failure to implant this electrode include the impossibility of advancing the guidewire to the coronary sinus, a complicated venous anatomy, the presence of phrenic stimulation and/or the instability of the lead in the selected position, and (exceptionally) an inability to cannulate the coronary sinus ostium.23-25
The present work examines our experience of the double-wire technique for the implantation of over-the-wire electrodes in patients with complicated anatomy in whom the conventional implantation technique was unviable.
METHODS
The study subjects were 170 patients (age 66.7 ± 9.1 years, 72% men, New York Heart Association [NYHA] functional class 2.98 ± 0.56, left ventricular ejection fraction 29.2 ± 11.3, QRS complex 156.3 ± 26.8 ms); between May 2002 and October 2006 all were fitted with cardiac resynchronization devices at our center. In 20 (11.8%) of these patients the use of a second, parallel, hydrophilic guidewire was required in order to implant the lead in the target vein; implantation of the lead was impossible with the conventional technique. Table 1 shows the clinical characteristics of the patients.
Implantation Technique
In all cases the coronary sinus was accessed with a wide angle guiding catheter (CS-Wide, Guidant Corp., St. Paul, MN, USA), a telescopic catheter, and a hydrophilic guidewire. The use of a hemostatic valve allowed the manipulation of the guidewire and the injection of the contrast medium. Firstly, the guidewire was advanced towards the coronary sinus, and after removing the telescopic catheter a balloon catheter was put in place following the guidewire. Occlusive sinus venography was then performed using 4 mL of contrast medium at a rate of 3 mL/s; images were obtained from the anteroposterior and anterior left oblique 20º views. After retracting the balloon catheter, the target vein was accessed by the guidewire and an attempt made to advance an over-the-wire electrode (Easy Track 2, Guidant Corp., St. Paul, MN, USA) following the guidewire. The conventional technique was considered to have failed if, once the guidewire was in the selected vein, it was impossible to advance the lead into the vein, the guidewire repeatedly retreating. Selective angiography was then used to determine the nature of the obstacle. Once this was complete, a second, polymer-coated, hydrophilic guidewire (Whisper or PT-Graphic) was inserted into the target vein and efforts to advance the lead reinitiated (Figure 1). During this maneuver a second operator controlled the second guidewire in order to prevent any undesirable progression; meanwhile, the first operator performed the implantation as normal. After implanting the lead, the second guidewire was retracted without difficulty and the guiding catheter removed after substituting the guidewire occupying the lumen of the lead for a finishing wire.
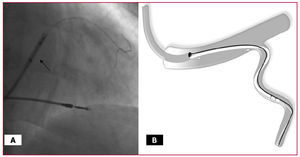
Figure 1. A: This figure shows the guiding catheter close to the mouth of the vein and two hydrophilic guidewires, one inside the lumen of the lead (arrow) and the other parallel to it. B: Diagram showing the guiding catheter with two guidewires (outside the catheter) and the lead in the target vein.
RESULTS
The lead was implanted in a lateral vein of the left ventricle in 18 (90%) of the 20 patients that underwent the double-wire procedure, in a secondary ascending branch (with a trajectory in the free wall of the left ventricle) of a vein with its origin close to the coronary sinus ostium in one patient (5%), and in an anterolateral vein (a tributary of the anterior interventricular vein) in one other (5%). The mean target vein diameter was 4.4 ± 1.3 mm.
In 2 patients the presence of a restrictive, fenestrated Thebesian valve caused the guiding catheter to provide insufficient support during implantation. In one patient it was impossible to advance the guiding catheter beyond the coronary sinus ostium because the Thebesian valve was very large and restrictive, covering 95% of the orifice. In the other, the guidewire passed through a fenestration of the Thebesian valve which, because of its small size, did not permit access to the guiding catheter. In both cases the lead was finally implanted without adequate support from the guiding catheterusing a second, parallel, hydrophilic guidewire.
A prominent Vieussens valve was another anatomical factor that limited the advance of the guidewire inside the coronary venous system (2 patients). Although the guidewire managed to get past the Vieussens valve with some difficulty, the progress of the guiding catheter and/or the lead to be implanted was prevented. The introduction of a second, parallel guidewire allowed the opening of this valve and gave sufficient support for the lead to be implanted in the target vein. Figure 2 shows two examples in which the guiding catheter provides insufficient support in the presence of a restrictive Vieussens valve and a fenestrated Thebesian valve, and in which the use of a second guidewire was decisive in the successful implantation of the lead.
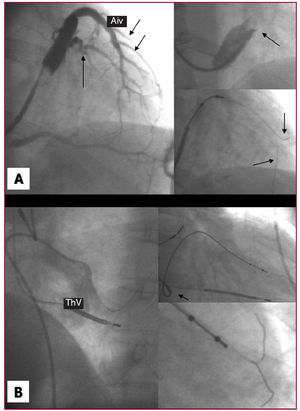
Figure 2. A. Occlusive coronary sinus venography showing a lateral target vein (arrow) and the anterior interventricular vein with multiple anterolateral veins (arrows). The upper right inset shows an amplified image of a very restrictive Vieussens valve impeding the progress of the guiding catheter. The lower inset shows two guidewires (arrows) aiding the advance of the lead. B. Non-selective angiography showing a very prominent Thebesian valve that impeded the advance of the guiding catheter tip. With the help of the second guidewire, it was finally possible to implant the lead in the target vein (previously identified by return venous angiography). Aiv indicates anterior interventricular vein; ThV, Thebesian valve
In the 20 patients in whom the conventional technique was unviable, the target vein showed characteristics that impeded the normal implantation of the lead. In five patients a tortuous origin of the vein was overcome with the use of the double-wire technique. In 7 cases the angle at the origin of the vein was greater than 90º, and the second hydrophilic guidewire was necessary to overcome this. Finally, when it was impossible to advance the lead through an apparently ideal target vein, the existence of a valve structure at the origin of the vein was inferred (eight patients). This was confirmed by the absence of a notch when a balloon was inflated in this area. The advance of the second guidewire, with the other still in place, allowed the valve to be opened, and the lead to be implanted. Figure 3 shows examples of obstacles associated with the target vein in which the double-wire technique was used (extreme tortuosity in the area of the origin of the vein, a pronounced angle at the origin of the target vein, and the presence of a restrictive venous valve at the origin of a large target vein). Table 2 shows a summary of the causes that made the use of the double-wire technique necessary.
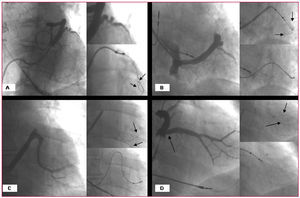
Figure 3. A. Occlusive coronary sinus venography showing a very tortuous target vein in which the double-wire technique (guidewires marked by arrows) permitted the advance of the lead. B. Pronounced tortuosity of a target vein in which implantation was possible thanks to the double-wire technique (see arrows). C. A very pronounced angle at the origin of the vein; this problem was also overcome with the use of the double-wire technique. D. The existence of a valve (arrow) at the origin of this large diameter vein was inferred; after being opened by the second guidewire (see arrows), the lead was able to advance.
The implantation of the lead in the target vein was possible after inserting a second hydrophilic guidewire in all 20 patients that required this technique. The amplitude of the local electrogram was 15.4 ± 7.9 mV, the stimulation threshould was 1.8 ± 1.2 V, and the impedance 1089 ± 215.9 Ohms. Three patients showed phrenic stimulation with energies of above 8 V, but there was no need to relocate the lead since its stimulation threshold was <2 V. In 2 patients a small tattoo appeared after injecting the contrast medium, although this problem disappeared spontaneously within a few minutes and did not impede the implantation of the lead. No other complications were recorded during the procedure. At 21 months of follow-up (range, 6-37 months) all leads implanted were still in place.
DISCUSSION
Current evidence suggests that stimulation in the lateral region of the left ventricle is that which provides the greatest benefit in cardiac resynchronization therapy.13-16 However, different obstacles can appear in this region that impede the progress of the lead towards a target vein of adequate diameter and trajectory. These obstacles, which are often multiple, are identified by angiography and, in some cases (especially when they involve the Vieussens valve), can be inferred from the impossibility of progressing the system. In these situations, a second guidewire can be used after the impossibility of advancing the lead with the conventional technique is confirmed.26
The size of the Thebesian valve in relation to the diameter of the coronary sinus ostium, as well as the presence of fenestrations, are the major difficulties in accessing the coronary venous system.25 The presence of right atrial dilation along with dilation of the left ventricle adds a further degree of difficulty since the ostium is displaced away from the coronary sinus. The Vieussens valve, which is commonly associated with the presence of the oblique vein of Marshall, is another obstacle to progress the system since it must be crossed against the current.24 Extreme tortuosity, a marked angle close to the origin of the vein, and/or a valve close to the origin of the target vein (all of which can be difficult to visualize by angiography) are other anatomical obstacles that can impede the progress of the guiding catheter and therefore prevent sufficient support being provided to the lead.26
The double-wire technique is a maneuver that has been used in the coronary arterial tree for other arteries for other purposes, such as the stabilization of a balloon in the treatment of intra-stent restenosis,27 for advancing a coronary premounted stent in the case of complicated anatomy, and the protection of secondary branches in the treatment of bifurcations. It has also been used to facilitate navegation in other vascular areas, for example in the central nervous system.28 However, in the coronary venous system its use has been described in only a single patient with a marked angle at the mouth of the target vein.29
The advances made in the technology for implanting venous leads (better designed leads, guidewires and guiding catheters), and the greater experience of operators, now allow success rates of 90% to be achieved,3,18-22 although in at least 20% of cases implantation is laborious and requires the use of additional techniques.
In 169 of the 170 (99.4%) patients of the present study, the lead was successfully implanted in the left ventricle, although special techniques (the double-wire technique, the push-and-pull technique, use of a balloon catheter, etc)30 were required in some cases. In 1 patient (0.6%), extreme hypoplasia of the coronary sinus ostium prevented access to the coronary venous system.
This article describes a simple, safe and reproducible technique that allows different types of anatomical complication to be overcome when attempting lead implantation in the left ventricle. We exclusively use polymer-coated hydrophilic guidewires since these provide an appropriate platform for sliding the lead forward. We have never observed any deterioration of the lead due to friction caused by the second guidewire. Size 8 Fr catheters adequately allow the passage of the lead and guidewire while leaving sufficient residual volume for monitoring the procedure by selective injections of contrast medium (if required). The second guidewire should be controlled by another operator to prevent its excessive migration when advancing the lead.
In the future, the appearance of new guidewires may allow the progression of the lead towards the target position with a reduced need for special maneuvers. However, for now, the double-wire technique is a useful tool that can aid in the implantation of a cardiac resynchronization device in patients with complicated venous anatomy.
CONCLUSIONS
The use of a second, parallel, hydrophilic guidewire (the double-wire technique) is a safe, efficient and reproducible procedure for implanting a left ventricular venous lead in patients with a target vein and/or coronary sinus showing complicated anatomy.
Correspondence: Dr. E. Arbelo Lainez.
Crta. General del Centro, 239.
35017 Las Palmas de Gran Canaria. España.
E-mail: elenaarbelo@secardiologia.es
Received August 28, 2006.
Accepted for publication November 2, 2006.