Keywords
INTRODUCTION
Restenosis continues to be the main problem in percutaneous coronary intervention (PCI), usually defined via angiographic documentation during follow-up as stenosis >50% in the treated segment and/or in the adjacent 5 mm. It occurs in at least 30% of cases after balloon dilatation (G20% with standard stent), and new revascularization procedures are required in over half the cases.
The physiopathology of restenosis after balloon angioplasty includes 3 phenomena: 1) early elastic recoil; 2) negative remodeling, involving a decrease in the total area of the vessel due to shrinking during the weeks following angioplasty; and 3) neointimal hyperplasia, primarily in the 3-5 months after PCI. Compared to balloon angioplasty, stenting decreases the risk of serious complications, and thus the need for emergency surgical revascularization,1 as well as reducing restenosis.2 A reduction in restenosis was initially demonstrated in patients with early elastic recoil3 or suboptimal results4 following balloon dilatation. Subsequently, the BENESTENT, STRESS, and START studies showed that elective stenting also reduces restenosis.5-7 The lesions treated in these studies were favorable and occurred in patients with stable ischemic cardiopathy. However, later studies demonstrated that stenting also reduces restenosis in other contexts (Table 1). Studies on small vessels (<3 mm diameter) have not been conclusive, but a metaanalysis of 11 randomized studies found that there was significantly less restenosis with stenting (25.8% vs 34.2%).8 Once these benefits were demonstrated, the use of high-pressure final balloon dilatations9 and the administration of thienopyridines plus aspirin10 made it possible to decrease the risk of thrombosis due to stenting to G1%, thus leading to their widespread use.
Despite their advantages, the restenosis rate after implantation of standard stents exceeds 20%, and the need for new revascularization procedures, 10%.11 In long and complex lesions, small vessels, and diabetic patients, the restenosis rate can be >50%. This is important given that most lesions currently treated with PCI are of this type.
The lower rate of stent restenosis is basically due to greater acute luminal gain, because late loss (reduction in minimum luminal diameter from implantation until 6 months later) is even higher than that obtained with balloon angioplasty. This is due to the fact that, although the stent virtually eliminates early elastic recoil and negative remodeling, neointimal hyperplasia is even more marked than with balloon angioplasty.12 In addition, there are other factors related to a suboptimal initial procedures ("pseudorestenosis"), such as stent underexpansion, the early protrusion of material through the stent, and the implantation of stents with an incorrect diameter.13
Most drugs with systemic effects (antiplatelet agents, anticoagulants, antiinflammatory agents, hypolipidemic agents, ACE inhibitors, calcium antagonists, antioxidants, etc)14,15 and a variety of mechanical devices have failed to reduce restenosis. Due to parallels between tumor growth and in-stent neointimal growth, it was decided to use antiproliferation agents to reduce in-stent restenosis (ISR). Initially, some drugs failed, probably due to limited effectiveness, insufficient doses, or inappropriate release methods. However, the strong belief that local administration of these drugs was more effective than their systemic use, because greater local concentrations could be obtained with virtually no systemic effects, gave way to the development of antiproliferative drug-eluting stents (DES).
ANTIPROLIFERATIVE DRUG-ELUTING STENTS
Antiproliferative DES consist of 3 components: the stent itself, the drug, and the drug-release mechanism.
1. The stent. This is the scaffold upon which the drug is placed making it possible to reach the vessel wall.
2. Antiproliferative drugs (Table 2). Rapamycin (sirolimus) and paclitaxel are the most-widely used drugs and yield the greatest benefits.
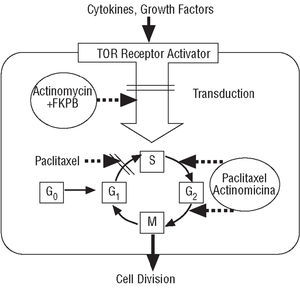
Rapamycin is a macrolide antibiotic, naturally produced via fermentation by Streptomyces hygroscopicus (Figure 1). It was initially used as an antifungal agent, but when its immunosuppressive, antiinflammatory and antiproliferative properties were discovered, its use was suggested in other areas of medicine, such as the prevention of coronary artery bypass graft disease and restenosis in heart transplants as well as for managing rejection after kidney transplantation.16 Rapamycin binds to the intracellular protein FKBP12, inactivates the TOR (Target Of Rapamycin) protein and, finally, inhibits transition from the G1 phase to the S phase (Figure 2). These mechanisms exert an antimigratory and antiproliferative effect on vascular smooth muscle cells.17 When acting in such an early phase of the cell cycle, it blocks proliferation without inducing cell death, thus minimizing possible vascular sequelae.
Figure 1. Chemical structure of rapamycin.
Figure 2. Mechanism of action of rapamycin.
Paclitaxel was initially extracted from the tree Taxus brevifolia (Figure 3). It inhibits proliferation and cell migration by suppressing microtubule dynamics.18 In low doses it acts in the transition between G0 and G1 and between G1 and S, producing cytostasis; however, in high doses it blocks the transition between G2 and M and between M and G1, leading to cell death. Thus, one of the most important aspects regarding paclitaxel has been to find the lowest dose capable of blocking cell response while avoiding vascular damage. Taxol is produced by dissolving 7.0 mmol/L paclitaxel in a lipoid vehicle.
Figure 3. Chemical structure of paclitaxel.
3. Polymer. There are two ways to release the drug: by modifying the stent surface or by using a polymer from which the drug is released. Modifying the stent surface is simpler and cheaper, yet the drug release is less uniform and controlled; in addition, some of the drug can be lost during stent expansion. Using polymers is more expensive and can, in theory, be associated with inflammatory reactions and/or local hypersensitivity, but the dosage is more uniform and the drug is released in a more sustained and controlled manner.
Currently, several commercial antiproliferative DES are available or are about to be launched (Table 3). However, solid evidence regarding effectiveness is only available for the Cypher and Taxus stents; these are the BX Velocity (Cordis Corp.) and Express (Boston Scientific) polymer-based rapamycin- and taxol-eluting stents, respectively.
Antiproliferative Rapamycin-Eluting Stents
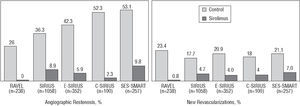
The Cypher stent is a polymer-coated stent that gradually and continuously releases rapamycin (140 μg/mm2) (80% over 28 days) and has drastically reduced restenosis in de novo lesions compared to standard stents as confirmed in several randomized studies19-22 (Figure 4): RAVEL, SIRIUS, E-SIRIUS, and C-SIRIUS.
Figure 4. Benefit of the Cypher stent compared to standard stents in published randomized studies.
In the RAVEL study, 238 patients with lesions =18 mm in vessels measuring 2.5-3.5 mm diameter were randomized to receive a Cypher or a standard bare metal stent,19 obtaining a restenosis rate of 0% and 26.6%, respectively. This resulted in a reduction in the rate of events per year (15.8% vs 28.8%), especially regarding new revascularization procedures. The SIRIUS study included 1,058 patients with a less favorable outcome than those in the RAVEL study (vessels ranging from 2.5 mm to 3.5 mm diameter, lesions from 15 mm to 30 mm, and a higher proportion of diabetic patients).20 The Cypher stent significantly reduced the restenosis rate (8.9% vs 36.3%) and new revascularization procedures (4.1% vs 16.6%). The E-SIRIUS study included 352 patients with lesions of 15-32 mm in small vessels (2.5-3.0 mm diameter). A significant reduction in the restenosis rate (5.9% vs 42.3%) and new procedures (4.0% vs 20.9%) was also found.21 The C-SIRIUS study, with 100 patients similar to those of the E-SIRIUS study, also found a reduction in the restenosis rate (2.3% vs 52.3%) and need for new procedures (4% vs 18%).22
In total, 1748 patients were included in these four studies. The restenosis rate was 6.3% with the Cypher stent and 37.2% with the standard stent, representing an absolute and relative reduction of 30.9% and 83.1%, respectively (3-4 patients would have to be treated with the Cypher stent to avoid one restenosis). The need for new revascularization procedures was reduced from 18.5% to 3.6%, i.e., an absolute and relative reduction of 14.9% and 80.5%, respectively (6-7 patients would have to be treated with the Cypher stent to avoid a new procedure). A key fact is that these benefits have been consistent in all the subgroups of patients included, after stratifying them by vessel diameter, lesion length, presence of diabetes, etc.
Other studies exist, some of which are unpublished or are still under way. The Cypher stent has been evaluated in small vessels in the SVELTE and SES-SMART studies. In the SES-SMART study, 257 patients with vessels ≤2.75 mm diameter were randomized to receive a Cypher or standard stent, with a restenosis rate of 9.8% and 53.1%, respectively.23 The results of the SCANDSTENT study have recently been reported, in which 322 patients with complex lesions were randomized to receive either a Cypher or standard stent. A significant reduction was found in the restenosis rate (2.0% vs 31.1%) and new revascularization procedures (2.4% vs 29.6%). The Cypher stent has been evaluated in patients with ISR in the TROPICAL registry and in the RIBS-II and ISAR-DESIRE randomized studies. This will be addressed later.24,25
The ARTS-II registry consisted of 607 patients with multivessel disease treated with the Cypher stent. Compared to the ARTS-I surgical group, the ARTS-II patients underwent more reinterventions (8.5% vs 4.1%; P=.003), presented less mortality (1.0% vs 2.7%; P=.03) and had a similar incidence of events (10.4% vs 11.6%). In the FREEDOM study, a population of diabetic patients with multivessel disease was randomized to receive the Cypher stent or coronary surgery. In the DIABETES study, which was coordinated in our center, the Cypher stent reduced restenosis and the need for new revascularization.26
Currently, several studies are under way with the Cypher stent--RESEARCH, e-CYPHER, RECIPE, SECURE, and others--where varied clinical situations and angiographic characteristics are being investigated. This means they will reflect the outcomes obtained with the Cypher stent in the "real world."
Antiproliferative Paclitaxel-Eluting Stents
Paclitaxel also reduces in-stent neointimal hyperplasia.27 Antiproliferative paclitaxel DES have been developed with polymer-coating and without. However, only the Taxus antiproliferative paclitaxel-eluting polymer-coated stents have proved to be beneficial when compared to standard stents.
Antiproliferative Paclitaxel-Eluting Non-Polymer-Coated Stents
Antiproliferative paclitaxel-eluting non-polymer-coated stents reduce neointimal hyperplasia, but do not improve clinical evolution. The ASPECT, DELIVER, and ELUTES studies have been the most important in this context. The ASPECT study compared the Supra-G stent (Cook Inc.) without paclitaxel with the same stent but with two different doses of paclitaxel (1.3 μg/mm2 and 3.1 μg/mm2),28 obtaining a restenosis rate of 27%, 12%, and 4%, respectively. However, there were no significant differences in the revascularization rate (8.6%, 6.9%, and 10%, respectively).
In the DELIVER-I study, 1043 patients were randomized to receive the Achieve stent (Cook Inc.) coated with 3 μg/mm2 of paclitaxel or standard stent (Multi-Link Penta). A trend was found toward a smaller rate of restenosis with the Achieve stent (14.9% vs 20.6%; P=.076), but significant clinical benefits were not obtained (new revascularization procedures in 11.9% and 14.5% of patients, respectively; P=.12).29
Finally, in the ELUTES study, 190 patients were randomized to receive one of the five following treatments: standard stent (V-Flex Plus, Cook Inc.) or stent coated with 0.2 μg/mm2, 0.7 μg/mm2, 1.4 μg/mm2, or 2.7 μg/mm2 paclitaxel.30 A dose-response relationship was found with restenosis rates of 21%, 20%, 12%, 14%, and 3%, while new procedure rates were 16%, 5%, 8%, 10%, and 5%, respectively (P=NS).
Antiproliferative Polymer-Coated Paclitaxel-Eluting Stents
The first antiproliferative polymer-coated paclitaxel-eluting stents not only failed to provide clinical benefits but also yielded a higher event rate, basically due to a very high incidence of stent thrombosis. The Quanam QuaDS-QP2 stent was used with very high doses of paclitaxel. It had a very particular design with polymer "sleeves." In the SCORE study, this stent reduced intimal hyperplasia and restenosis, but the thrombosis rate was >10% in the first year.31
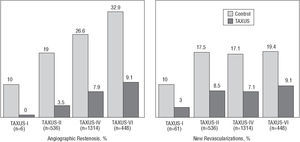
The Taxus polymer-coated paclitaxel-eluting stents have demonstrated a reduced rate of restenosis and new revascularization events (Figure 5), which is not associated with increased risk of stent thrombosis, at least when combined with antiplatelet treatment with aspirin and thienopyridines for 6 months. The benefits of the Taxus stent have been demonstrated in the TAXUS-I, II, IV, and VI studies.32-34
Figure 5. Benefit of the Taxus stent compared with standard stents.
In the TAXUS-I study, 61 patients with lesions ≤12 mm in vessels of 3.0-3.5 mm diameter were randomized to receive a Taxus stent (1.0 μg/mm2, slow release) or standard stent (NIR, Boston Scientific Corp.), obtaining restenosis rates of 0% and 10%, respectively.32
This was a safety study and the primary end-point (death, infarction with Q wave, new revascularization or 30-day stent thrombosis) occurred in 3% and 10%, respectively (P=NS). An important fact is that there was no stent thrombosis in either of the 2 groups over 12 months. The TAXUS II study randomized 536 patients with similar characteristics to those of the TAXUS-I study into 3 groups: standard NIR stent, slow-release Taxus stent, and moderate-release Taxus stent. The restenosis rates were 19%, 2.3%, and 4.7%, and new revascularization procedures 16%, 7.7%, and 6.2%, respectively.33 The TAXUS-IV study randomized 1314 patients with lesions of 10-28 mm in vessels of 2.5-3.75 mm in diameter to receive a standard stent (Express, Boston Scientific Corp.) or a Taxus stent (1 μg/mm2, slow release). The restenosis rate was reduced from 26.6% to 7.9%, and the revascularization rate from 11.3% to 3.0%.34 Taking the TAXUS-I, II, and IV studies into account, the restenosis rate decreased from 23.5% to 6.9% (an absolute and relative reduction of 16.6% and 70.6%, respectively; 6 patients would have to be treated to avoid 1 restenosis).
Other studies using the Taxus stent are under way or still unpublished. In the TAXUS-VI study, 448 patients with long lesions (18-40 mm) were randomized to a moderate-release Taxus stent (an initial release of the drug eight times higher than the slow release) or standard stent. The Taxus obtained a significant reduction in the restenosis rate (35.7% vs 12.4%) and in new procedures (19.4% vs 9.1%). The results of the TAXUS-V study have been reported recently, where 1172 patients with long complex lesions were randomized to receive a Taxus or standard stent. Although in this study the outcomes were better with the Taxus stent than with the standard stent, there was a higher rate of restenosis (18.9% vs 33.9%), and new revascularization procedures (12.1% vs 17.3%). The TAXUS-V US Randomized Pivotal ISR Trial will compare the Taxus stent and intracoronary brachytherapy (ICB) in 488 patients with ISR. The SYRTAX study randomized a group of patients with multivessel disease to receive surgery or the Taxus stent. There also are registries on the use of the Taxus stent in the "real world", such as the WISDOM, T-RESEARCH, MILESTONE, TAXUS-Olympic, and others.
DES Using Other Antiproliferative Drugs
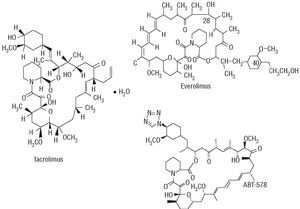
Currently, DES using other antiproliferative drugs, such as sirolimus analogues, are being evaluated (Figure 6). Some have already proven their safety and will be marketed in the coming months in Europe.
Figure 6. Chemical structure of some sirolimus analogues.
Everolimus is an immunosuppressive macrolide developed to prevent rejection in kidney, heart, and lung transplantation and it inhibits proliferation of smooth muscle cells. It is absorbed by local tissue more rapidly than sirolimus and remains in the cells longer. There are several studies where everolimus has been used (FUTURE-I, FUTURE-II, and SPIRIT). Its safety (no stent thrombosis was found) and effectiveness has been demonstrated with significantly reduced late loss, restenosis rates, and need for new revascularization. Nevertheless, these 3 studies included a small number of patients, in favorable angiographic and clinical contexts, and thus these benefits should be confirmed in other studies.
The antiproliferative tacrolimus-(FK-506) eluting stents were initially evaluated in the EVIDENT and PRESENT I and II studies, in saphenous vein bypass grafts and native vessels, respectively. No benefits were observed in the patients treated with tacrolimus. The Janus (Sorin) stent is specially designed with microreservoirs, ensuring a targeted local delivery of tacrolimus directly to the vessel wall. The JUPITER-I study evaluated this stent in a small population of patients. No stent thrombosis was reported, but there was elevated late loss especially in diabetic patients. Thus, the JUPITER-II randomized study (Janus stent vs Tecnic stent) was carried out with a higher dose of tacrolimus (2.3 μg/mm2); its results will be released sometime in 2005.
Although several antiproliferative ABT-578 DES have been developed (Endeavor, TriMaxx, ZoMaxx), the Endeavor stent is the one that has been evaluated in the greatest number of patients up until now. This is a chromium-cobalt ABT-578 (10 μg/mm) polymer-coated stent (Driver, Medtronic Inc.) releasing 70%-80% of the drug in the first 48 h after implantation and the remainder during the following 30 days, approximately. It was evaluated in the non-randomized ENDEAVOR-I study with favorable clinical results (thrombosis 0%, restenosis 3%, clinical events 2%), but with a relatively high late loss (0.61 mm at 12 months). In the ENDEAVOR-II randomized study (n=1197), the patients treated with the Endeavor stent had lower restenosis rates (9.5% vs 32.7%) and fewer new procedures (5.7% vs 12.8%) than those treated with the Driver stent. The randomized ENDEAVOR-III and IV studies will compare the Endeavor stent with the Cypher and Taxus stents, respectively.
The STEALTH-I study, using biolimus A9, randomly compared (2:1) the BioMatrix stent (Biosensors) with a standard stent in 120 patients, obtaining a significant reduction in late loss (from 0.74 mm to 0.25 mm).
In addition to these sirolimus analogues, other antiproliferative drugs have been evaluated: dexamethasone, 17β-estradiol, batimastat, actinomycin-D, methotrexate, angiopeptin, temsirolimus (ICC-779), vincristine, cyclosporin, etc (Table 2). However, the results have been negative or are still in the early stages of research. On the other hand, "coated stents" are normally considered to be those which release antiproliferative agents, given that they have proven to reduce the restenosis rate and need for new revascularization procedures. Nevertheless, the concept of "coated stents" is broader and includes stents coated with other drugs. Heparin-coated stents were developed in attempt to reduce the thrombosis rate whose global incidence was <0.5%. However, the rate obtained was not significantly less than that achieved with standard stents. A reduction in the restenosis rate has not been demonstrated, and therefore the use of this type of stent has been very limited. Phosphorylcholine (Biodivysio stent) and silicone carbide (Tenas stent) are other coatings used in the attempt to reduce stent thrombogenicity. These stents do not reduce the risk of restenosis nor thrombosis.
Current Limitations of Antiproliferative Drug-Eluting Stents
Antiproliferative Drug-Eluting Stents in Non-Favorable Scenarios
Randomized studies have not been done in certain groups of patients, but the data obtained based on registries makes it possible to assume for the time being that antiproliferative DES are probably also more effective than standard stents.
Bifurcated lesions constitute an unfavorable context, not only due to the risk of loss of secondary vessels, but also because of the high rate of restenosis, especially in the secondary vessel. In the SIRIUS-Bifurcations study, 86 patients with bifurcated lesions were randomized to receive the Cypher stent in the main branch and balloon in the side branch versus the Cypher stent in both vessels.35 The results can be summarized as follows: 1) there was a high rate of Cypher-balloon to Cypher-Cypher crossover (51%); 2) there was little restenosis in the main branch (G5% in both groups), a favorable outcome compared to classic series with standard stents; and 3) treatment with the Cypher stent in both vessels did not provide advantages compared to the initial treatment with balloon in the side branch (new revascularization procedures in 11% and 10%, and restenosis in the side branch in 22% and 14%, respectively). Furthermore, all the thromboses occurred in patients treated with Cypher-Cypher stenting. Along with antiproliferative DES, a new technique has been developed for treating bifurcations (crushing technique). This basically consists of first implanting an antiproliferative DES in the side branch, but placed some 4 mm into the main branch. Subsequently, another antiproliferative DES is implanted in the main branch in front of the stent in the side branch. Ideally, this technique should end with simultaneous balloon expansion of both vessels (kissing balloon technique).
Given the concern regarding the possibility of an increase in thrombosis risk after implanting antiproliferative DES, until a short time ago the use of these stents in acute coronary syndromes was relatively limited, especially in ST-segment elevation myocardial infarction. However, in relation to restenosis and stent thrombosis, recent data have shown that the results of treatment with the CYPHER stent in these patients are comparable to those obtained in stable ischemic heart disease.36
Data on the Cypher and Taxus stents in saphenous vein bypass grafts are based on studies with few patients, in which a new revascularization procedure rate of 2.5%-65% was obtained. In a recent study, a lower rate of restenosis (10.0% vs 26.7%; P=.03) and new revascularization procedures (4.9% vs 23.1%; P=.01) was obtained in a group of patients treated with antiproliferative DES (Cypher or Taxus) than in a control group with standard stents.37 Similar results were obtained in another study (new revascularization in 6.4% vs 17.3%, respectively).38 In a recent analysis of the SECURE registry, the need for new revascularization procedures in the patients who received the Cypher stent in saphenous vein bypass grafts was 17%. Although this is a high figure, it is similar to the one found in the patients with lesions in native vessels in the same registry (18%), since it included patients with a particularly unfavorable situation (mainly involving failed ICB).
Traditionally, left main coronary artery disease has been a surgical indication. However, stenting can be an alternative, especially in patients with high surgical risk. Given the great clinical importance of restenosis in the left main coronary artery, antiproliferative DES are especially attractive in this context. A series of patients have been described with left main coronary artery disease treated with Cypher or Taxus stents with a restenosis rate of G5%.39,40 The two most important problems after treating left main coronary artery disease with antiproliferative DES are restenosis of the origin of the circumflex artery when the left main coronary artery lesion is distal, and stent thrombosis; this is the cause of some sudden death events that have taken place after treating the left main coronary artery via antiproliferative DES. Nevertheless, the risk of thrombosis with an antiproliferative DES implanted in the left main coronary artery is probably no higher than that with standard stents.
One problem in the treatment of chronic occlusions is that, although steering the guidewire, dilating the lesion, and implanting a stent may be successful, the restenosis rate is very high. In some series of chronic occlusions treated with antiproliferative DES,41 a restenosis rate of 0%-11% was reported (reocclusion 0%-3%) with a need for new revascularization procedures in 0%-7.5% of cases. These data are favorable when compared to series with standard stents.
Systemic Side Effects of Antiproliferative Drug-Eluting Stents
In experimental studies, systemic high-dose rapamycin can have serious side effects, such as myocardial necrosis, retinal infarction, necrosis of the mucous membrane, and vasculitis. In therapeutic doses, the possible side effects of systemic rapamycin are: headache, polyarthralgia, nosebleed, diarrhea, myelosuppression, and others.42 Furthermore, plasma concentrations of cholesterol and triglycerides can increase in humans.43 Systemic side effects of rapamycin have not been reported when administered via antiproliferative DES, and their risk is virtually nil. In the SIROLIMUS PK study, after implantation of antiproliferative sirolimus-eluting stent, the maximum plasma concentration of rapamycin was 0.80±0.37 ng/mL, with a half-life of 213 h and the presence of detectable concentrations in the plasma for 1 week.
The following side effects have been reported when paclitaxel is administered systemically as an antineoplastic agent: myocardial infarction, heart failure, arrhythmias, hypotension, sudden death, repolarization changes, sinus bradycardia, and atrioventricular blocks.44 However, in these situations, systemic concentrations are 100-1000 times higher than those used in antiproliferative DES. As with rapamycin, the implantation of antiproliferative paclitaxel-eluting stents has not been associated with systemic side effects, although this should be confirmed in a broad series of patients with long-term follow-up.
Side Effects of Antiproliferative Local-Delivery Drug-Eluting Stents
From the inception of DES there has been concern over a possible increase in the risk of stent thrombosis. This was justified by the following facts: 1) parallels with ICB, since antiproliferative drugs can delay stent endothelialization; 2) rapamycin can increase platelet aggregation in vitro45; 3) in some studies, DES have been associated with a greater frequency of late stent malapposition. This fact was reported in 9% and 21% of the patients in the SIRIUS and RAVEL studies, respectively, after implantation of the Cypher stent. On the other hand, in the TAXUS-II study, the risk of late malapposition with the Taxus stent was similar to the standard stent; and 4) in some initial studies with DES, the incidence of stent thrombosis was very high: >10% and 3% per year in the SCORE31 and ASPECT studies, respectively.28
However, in the SCORE study, the high rate of thrombosis was probably due to the stent design and the extremely high doses of paclitaxel.31 In the ASPECT study, all thromboses occurred in patients who received aspirin and cilostazol but not aspirin and thienopyridines.28 No increase in the risk of thrombosis was found in the studies with Cypher or Taxus stents. In a recent metaanalysis of ten randomized studies, the stent thrombosis rate with DES and standard stents (0.58% vs 0.54%) was similar. In these studies, the length of treatment with thienopyridines was 1-6 months.46
Other possible side effects, with clinical implication still not well defined, may also occur, such as hypersensitivity and local inflammation, probably due to the polymer rather than to the antiproliferative drug. In contrast to what occurs in ICB, it has not been demonstrated that antiproliferative DES produce edge effects or more stent-edge restenosis than standard stents. The formation of coronary aneurisms in the long term, after the implantation of DES, has also been reported, but it appears to be infrequent and of little clinical relevance. In the TAXUS II study, for example, coronary aneurisms developed with the Taxus stent at a frequency similar to that of the standard bare metal stent (1.5%).
Finally, since the introduction of DES, the possibility that these would produce a delay rather than a reduction in intimal hyperplasia has been of some concern. However, the benefit of antiproliferative DES has been maintained for at least 2-3 years. In the TAXUS-II study, minimum lumen diameter did not decrease at 6 months and 2 years in patients treated with the Taxus stent, and the number of new revascularization procedures after 1-2 years was even higher with standard bare metal stent stent. Something similar to this occurred in the TAXUS-IV study, where new revascularization procedures were needed 1-2 years in 3.7% and 4.2% of the patients treated with the Taxus and standard stents, respectively. The 3-year results of the RAVEL study were published recently, where the Cypher stent was used.47 Between 1 year and 3 years there was a greater frequency of target-vessel failure in the Cypher group, but the differences were not significant (5.9% vs 4.3%; P=.77) and the difference in the rate of events was preserved at 3 years (16.7% vs 34.5%; P=.002). The 3-year results of the SIRIUS study have also been published recently. In this study the differences in the incidence of new procedures between the Cypher and control groups at 9 months and 3 years were not only maintained, but even increased (18.9% vs 6.4% at 9 months [absolute reduction of 12.5%]; 27.2% vs 11.6% at years [absolute reduction of 15.6%]). The only aspect that remains unsolved is whether the long-term stent thrombosis rate is higher in patients treated with DES than with standard stents, once double antiplatelet aggregation treatment is suspended and more than 1 year has passed since implantation. In a combined analyses of the TAXUS I, II, III, and IV studies, the incidence of stent thrombosis between 6 months and 2 years with the Taxus stent was significantly higher than in the control groups (1.2% vs 0.7%). In contrast, in the SIRIUS study, the thrombosis rate at 3 years was 0.8% in both groups. Thus, the need for administering thienopyridines combined with aspirin over a longer period than in the randomized studies has still not been demonstrated.
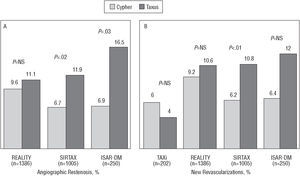
Comparison Between the Cypher and Taxus Stents
We have already mentioned the ISAR-DESIRE study, in which the recurrence of ISR, after it was treated, was 14.3% and 21.7% with the Cypher and Taxus stents, respectively.25 The Cypher and Taxus stents have been compared in several randomized studies in de novo lesions (Figure 7).48-52 In the TAXi study, there were no significant differences between them regarding clinical evolution (there was no angiographic follow-up). In the REALITY, SIRTAX, and ISAR-DIABETES studies, late loss was significantly lower with the Cypher stent and in-lesion restenosis was significantly less frequent with the Cypher stent in the SIRTAX and ISAR-DIABETES studies. Nevertheless, these differences only translated into clinical differences in the SIRTAX study. The preliminary results of the CORPAL study group agree with these data, but it is limited by the fact that angiographic follow-up was done in a small number of patients.
Figure 7. Restenosis rate (A) and new revascularization procedure rates (B) in randomized studies comparing the Cypher and Taxus stents.
Regarding safety, only the REALITY study reported a higher thrombosis rate with the Taxus stent (1.8% vs 0.4% in an analysis by treatment administered), but the differences were not statistically significant in an intention-to-treat analysis (1.6% vs 0.6%; P=.07). In other studies, the incidence of thrombosis was similar in both groups (in the SIRTAX it was 1.6% and 2.0% with the Taxus and Cypher stents, respectively). Although in the studies in which paclitaxel-eluting stents were used there was a trend toward greater duration of treatment with thienopyridines (4.4±2.3 vs 2.0±0.0 months, respectively; P=.08)--and thus we cannot rule out that this masked possible greater thrombogenicity with the paclitaxel-eluting stent--in our metaanalysis the thrombosis rate was not significantly different between rapamycin and paclitaxel DES (0.56% vs 0.66%) and neither were there significant differences in the late stent thrombosis rate (0.11% vs 0.33%).46
Cost of Antiproliferative Drug-Eluting Stents
In our context, the price of antiproliferative DES is 60%-80% higher than standard stents. Furthermore, it is necessary to add the indirect cost of administering thienopyridines over a longer period. The added clinical benefit derived from DES may possibly not be sufficient to justify such a difference in price (we should not forget that DES do not reduce mortality nor the infarction rate). Given current prices, we do not recommend the systematic use of DES, but do recommend them preferably in contexts in which the reduction of restenosis involves greater clinical benefit.
Despite previous considerations, economic analyses of the SIRIUS and TAXUS-IV studies shows that most of the extra cost of DES is compensated for by the savings derived from the reduction in the need for new revascularization procedures. The "cost neutral price" of a device (in this case, the DES) is that in which the initial extra cost is totally compensated for by the reduction in expenditures derived from its clinical benefit (in this case, reduction in expenditure due to fewer new revascularization procedures). An economic study has recently been carried out in Spain which estimated that the cost neutral price of a DES would be somewhat less than 1,500 Euros.53
Treatment of in-Stent Restenosis Via Antiproliferative Drug-Eluting Stents
Conceptually, the use of devices in de novo lesions is a restenosis "primary prevention" strategy, whereas their use in restenotic lesions (especially ISR) would correspond to "secondary prevention" strategies. In-stent restenosis has been classified into four angiographic types with prognostic implications (Table 4).54 The risk of ISR is inversely related to the minimum lumen diameter after stent implantation, and is higher in diabetic patients, long lesions, small vessels, restenotic lesions, saphenous vein bypass grafts, and ostial lesions.55 Some characteristics related to the type of stent could also have a relationship with restenosis.56 Furthermore, some genetic factors can also be related to ISR, such as platelet glycoprotein IIIa PIA polymorphism and a mutant form of methylenetetrahydrofolate reductase, while the allele 2 of interleukin 1 appears to be associated with a lower risk. On the other hand, positive reactions to nickel and molybdenum allergy tests (components of the coronary stents) have also been related to ISR.57
With the majority of devices, percutaneous treatment of ISR is associated with a very high initial success rate (G100% in most series) and a low rate of complications. This is due to the fact that, in ISR, the vessel wall is "protected" by the stent's metallic mesh, thus reducing lesion grade and risk of dissection. However, in contrast, ISR treatment is associated with a restenosis rate higher than that of de novo lesions. Although many devices have been evaluated in the treatment of ISR, only antiproliferative DES and ICB are more effective than balloon angioplasty.
Although the incidence of events was relatively high in some of the first registries of ISR treated with DES, angiographic results were consistently favorable, with low rates of late loss and restenosis.24,25,58-60 In the São Paulo experiment, with 25 ISR patients treated with the Cypher stent, there was no stent thrombosis and there was only one recurrence of ISR.85 In the TAXUS III study, with 28 ISR patients treated with the Taxus stent, the ISR recurrence rate was 16% (4/25). At 12 months, 6 patients (21%) underwent new revascularization (three due to restenosis and three due to intravascular ultrasound findings).59 In another series of 16 patients with complex ISR, a higher restenosis rate has been reported (19% at 4 months).58
In the TROPICAL registry, 162 patients with ISR were treated with the Cypher stent and compared with the control groups from the GAMMA 1 and 2 studies, which had restenosis rates of 9.7% vs 40.3%, respectively. In the TROPICAL registry, the benefit of the Cypher was higher, since ISR length was significantly higher in the control group. In the RIBS-2 study 150 patients with ISR were randomized to receive treatment with the Cypher stent or balloon.24 The provisional results showed an ISR recurrence rate of 11% and of 39% (P<.01), and the need for new revascularization procedures of 9% and 30% (P<.01), respectively. In the recently published ISAR-DESIRE study, 300 patients with ISR were randomized to 3 groups: balloon angioplasty, Cypher stent, and Taxus stent; there was ISR recurrence in 44.6%, 14.3%, and 21.7%, respectively.25
In the context of ISR treatment with DES, the patients in whom ICB has failed beforehand constitute a higher risk subgroup. In a recent update of the SECURE registry, with 193 patients with ISR treated with the Cypher stent (142 of them after failed ICB), the need for new procedures was 17%, but was higher after failed ICB (19%) than in the rest of the patients (12%).61 The same occurred with thrombosis, which only occurred (1.4%) in the patients with previous failed ICB. In a study by Waksman et al,62 treatment with DES in patients with ISR, in whom ICB had already failed, was associated with an even higher risk of events than that of patients treated with new ICB.
Restenosis After the Implantation of Antiproliferative Drug-Releasing Stents
The predictors of DES ISR are similar to those of standard stent ISR. In an evaluation of the RESEARCH study, the predictors of restenosis after implantation of the Cypher stent were treatment for ISR, ostial location, greater total stented length, small vessels, diabetes, and location in the left anterior descending artery.63
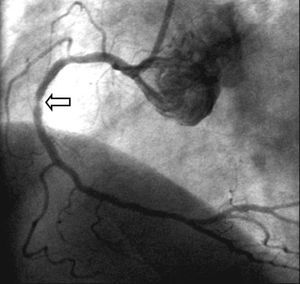
Although antiproliferative DES ISR is usually located within the stent, restenosis located at the edges is frequent (20%-30% of antiproliferative DES ISR), and also where 2 stents overlap (Figure 8). The incidence of restenosis in the stent edges can be reduced by attempting to cover the entire length dilated with the balloon with the stent. ISR of DES usually has a focal pattern and diffuse ISR is infrequent, either with rapamycin64 or paclitaxel.65 Possible explanations to this predominantly focal pattern of ISR after implantation of antiproliferative DES include underexpansion of the stent, non-homogeneous distribution of the drug, or incomplete coverage of the lesion with the stent.
Figure 8. Focal restenosis located in the overlap area of two Cypher stents implanted in the right coronary artery.
There is very little data on the treatment of DES ISR. The best treatment is probably another DES. The RESEARCH study investigated 24 patients (27 lesions) undergoing percutaneous procedures with ISR related to the Cypher stent.64 Approximately 85% were treated with another DES (Cypher or Taxus) and 15% with balloon or a conventional bare metal stent. There was ISR recurrence in 43% of the cases, but only in 18% of the patients treated with another DES. Intracoronary brachytherapy could also be a therapeutic alternative in these patients66 but the devices used have been removed from the market quite recently.
OTHER DEVICES FOR THE PREVENTION AND TREATMENT OF RESTENOSIS
DES have revolutionized interventionist cardiology. Nevertheless, the beginning of the DES era is recent and other devices had already been evaluated in the prevention of restenosis (Table 5).
In the treatment of de novo lesions, no device other than the stent has proven to reduce restenosis. Regarding ISR, DES and ICB have only proven to reduce the recurrence of ISR as compared to conventional treatment. Balloon angioplasty is the conventional treatment for ISR and has been the most-used strategy in the pre-DES era, especially in centers without ICB. This treatment is technically simple and has few complications, but the recurrence of ISR is around 50% and the need for new revascularization procedures 14%-46%. These figures are especially high in type III-IV ISR, diabetics, saphenous vein bypass grafts, and when ISR is early (before 4 months after implantation of the stent).67-69
Initially, the implantation of a new stent for ISR was restricted to the patients with suboptimal outcomes or complications after the failure of other devices, but subsequently the possibility of electively implanting a new stent was evaluated. Most data on the treatment of ISR via stenting intra-stent have been obtained from the RIBS study, where 450 patients with ISR were randomized to receive intra-stent stenting or balloon angioplasty. There were no significant differences in restenosis rate and need for new procedures, but this study has demonstrated that elective implantation of an intra-stent stent is safe and is even associated with a smaller rate of periprocedural events than balloon dilatation.70 This is important at present, when it is beginning to appear that DES can be the best treatment for ISR after the implantation of a standard stent.
Plaque Modification, Reduction, and Elimination Devices (Table 4)
Directional atherectomy (DA) yields some immediate favorable angiographic results, but in randomized studies a reduction in the rate of restenosis and new revascularization procedures has not been demonstrated. Furthermore, DA can increase periprocedural complications.71 It has been demonstrated in the AMIGO and DESIRE studies that DA before the implantation of a stent neither reduces restenosis nor the need for new revascularization procedures. In most studies, DA was used with a relatively conservative strategy, but some later works have shown that a more aggressive strategy not only produces better immediate results, but also a lower rate of restenosis.72 However, the use of DA is currently infrequent. Regarding ISR, DA is able to eliminate intra-stent neointimal tissue and obtain greater immediate luminal gain than balloon angioplasty. However, the relatively high rate of complications in de novo lesions and the possibility of stent strut deterioration have impeded the use of this device in the treatment of ISR.
In the treatment of de novo lesions, rotational atherectomy (RA) achieves better initial outcomes than balloon angioplasty in calcified lesions, and is especially useful in lesions which cannot be dilatated with balloon. In some studies, such as ERBAC and COBRA, RA obtained a higher initial angiographic success rate than balloon angioplasty in complex lesions, but did not reduce restenosis.73,74 It is currently used in selected cases, especially when dilatation with balloon cannot be done and in severely calcified lesions where it can be predicted that a good outcome is unachievable. Currently, in the DES era, this technique still has its role, because it facilitates the implantation and correct expansion of DES in these types of lesions. In the treatment of ISR, RA acts by eliminating neointimal tissue and, if it is followed by expansion with balloon, an additional expansion of the stent is done and neointimal tissue is extruded outside the stent. Although ISR RA is associated with a high initial angiographic success rate and few complications,75 contradictory results have been obtained in 2 randomized studies. In the ROSTER study (n=150), the IRS clinical recurrence rate was significantly lower with RA.76 However, in the ARTIST study (n=298), the angiographic ISR recurrence rate was higher with RA.77 Although it has been argued that the failure of RA in the ARTIST study could be due to an overly conservative strategy (low pressures used to expand the balloon after RA and absence of controls via intravascular ultrasound), the use of ISR RA is currently very limited.
In several randomized studies cutting balloon (CB) has been compared to standard balloon. In some studies, the results were favorable with CB, but in the majority there was no reduction in the rate of new revascularization procedures.78-80 In the REDUCE-3 study, the benefit of CB was evaluated in comparison with standard balloon before the implantation of a stent; although the rate of angiographic restenosis was lower with CB, the incidence of new revascularization procedures was not significantly reduced.81 The theoretical advantages of CB in the treatment of ISR are twofold. In the first place, the small incisions can facilitate the extrusion of neointimal tissue outside the stent lumen. Second, they can help prevent "watermelon seeding" (displacement of the balloon during inflation), a phenomenon that can cause damage to the segments of the vessel adjacent to the stent. This advantage means that CB is especially used when ICB is applied to avoid the phenomenon of geographic miss and thus the appearance of edge restenosis. In several observational studies favorable outcomes82 have been obtained, but randomized studies have not demonstrated a significant reduction in the recurrence of ISR.83-86
In a randomized pilot study, the recurrence of ISR was less frequent with CB (4% vs 28%; P=.047).83 In another randomized pilot study, a lower ISR recurrence rate was obtained in the group treated with CB, although without significant differences (12% vs 20%; P=NS).84 However, in other studies with more patients, CB was not better than standard balloon. In the RESCUT study, there was a trend toward less need for implanting a new stent due to dissection in the group treated with CB, (4% vs 8%; P=.07), but the IRS recurrence rate (29% vs 31%) and the new procedure rate (17% vs 16%) was similar.85 In the REDUCE II study, the recurrence of ISR was also similar with both treatments (24% vs 22%).
Exciser laser has been evaluated in several randomized studies,73,87,88 but in addition to failing to reduce the incidence of new revascularization procedures it was associated with an increase in periprocedural infarction. When treating ISR, the laser produces an additional expansion of the stent, and ablation and extrusion of neointimal tissue. The preliminary studies showed the efficacy and safety of this device but the recurrence of ISR is high, with no difference in benefit when compared to balloon angioplasty.89
The application of ultrasound can reduce cell viability, and the production of cavitations through the use of high energy can inhibit the migration and adhesion of smooth muscle cells in vivo. Ultrasound can also directly inhibit the proliferation of smooth muscle cells in vivo. In some animal studies, the use of intravascular ultrasound reduced neointimal hyperplasia after the implantation of stents.90 However, in humans, ultrasound has not been effective in de novo lesions. In the Euro-SPAH study, 403 stented patients were randomized to receive treatment or not with intravessel ultrasonography, without there being any improvement in clinical outcomes or restenosis.91
Intracoronary Brachytherapy
Ionized radiation inhibits cell proliferation and has been applied to several pathological tumorous and non-tumorous processes. Given the parallels between neointimal hyperplasia in restenosis (especially in ISR) and tumor processes, the use of radioactive isotopes in the prevention of restenosis was relatively prompt. Local application of radiation therapy has an antiproliferative and antimigratory effect on the smooth muscle cells, and in this way reduces neointimal hyperplasia.92 The positive effect of ICB on vessel remodelling also helps to reduce restenosis.93
There are 2 ways to apply ICB: via a catheter and via radioactive stents, with radiation therapy via catheter being the standard technique. Two different types are available for ICB: β and γ (Table 6). Basically, β is high energy/low tissue penetration and thus does not require additional radiation protection measures. In contrast, γ has low energy, but greater tissue penetration and a longer half-life, and exposes the operator to significantly higher radiation than β. Compared to γ, β could, in theory, be less effective due to lower penetration of the vessel wall and less homogeneous exposure, but in clinical studies with ICB β has obtained similar effectiveness to γ. In view of the fact that its application is less problematical, it is the type normally used in centers that offer ICB.
Although ICB has been used particularly in ISR, it was originally applied in de novo lesions.94-100 It inhibits neointimal hyperplasia in such lesions, but is not effective (Table 4). This is basically due to the edge effect and late thrombosis which are more evident when a coronary stent has been implanted. In the BETA-CATH study, 1455 patients with de novo lesions were randomized to receive β ICB or placebo after standard treatment (a stent was implanted in G50% of the patients); the rate of events was not statistically significant (15.6% vs 17.4%, respectively). In the BRIDGE study, 112 patients were treated with stenting and randomized to receive ICB or not. Restenosis and the need for new revascularization procedures in the target segment were more frequent in the patients treated with ICB.96 In a study conducted in our center with 92 diabetic patients, ICB after stent implantation was associated with a significant reduction in ISR, but the rate of new revascularization procedures was similar and the death or infarction rate was higher in the ICB group.100
Intracoronary brachytherapy was the first useful approach to the treatment of ISR, and until the development of DES it was the most effective98,101-108 (Table 7).
γ radiation was used in the GAMMA-I and WRIST studies which randomized 252 and 130 patients with ISR, respectively, to receive treatment with 192Ir or placebo.101,103 In the SCRIPPS study, 55 patients were randomized to receive 192Ir or placebo after the implantation of a stent for the treatment of restenotic lesions, 62% of which were ISR.102 A significantly lower ISR recurrence rate was found in these three studies than that found with ICB. Subsequently, studies in subgroups of patients have been carried out (long lesions, saphenous vein bypass grafts, etc.) with similar results.105
Most of the studies with β radiation came after those with γ radiation. In the Beta-WRIST study, with 90-yttrium, a control group was used with the same characteristics as the original WRIST study,92 with similar results.106 In the START study,107 the ISR recurrence rate was significantly lower in the patients treated with 90Sr. In the INHIBIT study, with 332 patients, ICB also reduced the recurrence of ISR.104 Finally, in the PREVENT study, although most of the patients had de novo lesions, 24% had ISR. There was a reduction in the recurrence of ISR with ICB.98
One of the limitations of ICB in the treatment of ISR is the partial loss of benefit over time. In the GAMMA-1 study, for example, the reduction in the rate of revascularization of the target lesion in the group of patients treated with balloon angioplasty only was 34%, 23%, 14%, and 11% at 1, 2, 3, and 4 years, respectively. At 5-year follow-up in the SCRIPPS study, the number of new procedures between 1 and 5 years was also greater in the ICB group.106
Some studies are under way which randomly compare DES and ICB for ISR. In 1 study, 97 diffuse ISR were randomized to receive treatment with the Cypher stent or ICB (188Re). Both the recurrence of ISR (2% vs 27%; P=.003) and the rate of events (4% vs 13%; P=.145) favored the Cypher stent (Park SJ. Scientific sessions of the American Heart Association, 2004). The SISR study compared the Cypher stent and ICB (β or γ) in 400 patients with ISR. Its results will be published in 2005. In the TAXUS-V-ISR study, 488 patients with ISR were randomized to receive the Taxus stent or β ICB. Despite these studies, the emergence of DES as an effective strategy in the treatment of ISR, as well as the expected reduction in the incidence of ISR with the use of DES in de novo lesions, has meant that devices for the application of ICB which were available in this context have recently been withdrawn from the market.
The problems associated with ICB in the treatment of de novo lesions and ISR are now described in greater detail:
Local side effects: 1) ICB delays stent endothelialization, which can increase the risk of late thrombosis. In the first series, the risk was >5%, but was especially associated with early withdrawal (30 days) of thienopyridines and implantation of a new stent.100 The establishment of prolonged treatment with aspirin and thienopyridines has succeeded in strongly reducing this complication; 2) second, ICB can be associated with positive remodeling and late stent malapposition which can also favor late thrombosis109; 3) third, ICB can be associated with restenosis in the edges or extremes of the irradiated area (edge or candy wrapper effect). Regarding the physiopathology of this phenomenon, there is the possibility of vascular damage caused at the ends of the irradiated segment and heterogeneity regarding the dose received, with a smaller dose being applied at the ends (geographic miss)110; and 4) finally, due to multiple factors (tortuosity and modifications in vessel caliber, source movements during the cardiac cycle, etc), the radiation dose administered is not homogeneous over the entire segment treated, which can contribute to partially limiting the antirrestenotic effect of ICB.
Logistic limitations: the use of ICB requires the collaboration and coordination of personnel not associated with catheterization laboratories (radiology service, etc). On the other hand, when γ radiation is applied the catheterization laboratory must be properly equipped. As a result of these obstacles, very few centers have carried out this technique.
Elimination versus delay in restenosis. In view of the fact that radiation therapy depopulates smooth muscle cells, theoretically a greater number of cell divisions is all that is required (and, thus, more time) to finally produce the same degree of intimal proliferation. In fact, this is not the case, since the restenosis process may be finished before this occurs. What in fact occurs over the years is a partial loss of the benefit obtained with ICB. Some studies have found a significant reduction in minimum lumen diameter and an increase in the number of new revascularization procedures between 6 months and 2-3 years.102,103
Costs. Intracoronary brachytherapy involves a significant increase in PCI costs, not only due to the price of the material used, but also because of the costs involved in organizing the infrastructure needed to carry out this technique. Although part of this cost is compensated by the reduction in the need for new revascularization procedures in the target vessel, the final cost continues to be higher than that of standard techniques.
All these limitations have led to the use of ICB being minimal, even in the treatment of ISR. This meant that, together with the advent of antiproliferative DES, the ICB devices available have been withdrawn from the market by the manufacturers and, thus, from a practical standpoint, ICB has disappeared from the therapeutic armamentarium of the interventional cardiologist, at least in our setting.
The use of radioactive stents reduces instent neointimal hyperplasia compared to standard stents. However, the edge restenosis rate is very high (G40%) since, by definition, the irradiated area (the stent) does not succeed in covering the entire area damaged by the balloon.111 This has impeded them from being used in clinical practice.
CONCLUSIONS AND FUTURE PROSPECTS
The Cypher and Taxus stents have demonstrated significant reductions in restenosis and the need for new revascularization procedures. This benefit is maintained for at least 3 years. Nevertheless, their costs need to reduce in order to allow a more diffuse use in all types of lesions. On the other hand, if the results from the studies comparing DES and surgery in patients with multivessel disease show that DES are better, this fact will probably reduce the number of patients undergoing surgical revascularization procedures.
Other DES have been recently marketed or will be shortly, but their effectiveness has to be demonstrated in randomized equivalence studies in comparison with the Cypher or Taxus stents. In the near future, DES with polymers or even with biodegradable scaffolds will be evaluated regarding their capacity to minimize the risk of late stent thrombosis and eliminate the probability of allergic reactions or late hypersensitivity occurring. Some studies are under way investigating stents coated with monoclonal antibodies that attract endothelial precursor cells to accelerate stent endothelialization. Other approaches include stents with a combination of drugs (antiproliferative agents and drugs that improve endothelial function, etc). On the other hand, gene therapy is also under evaluation for preventing restenosis, although the evidence regarding its effectiveness and potential clinical application is probably still distant.
Although ICB has proven to be more effective than balloon angioplasty in ISR, its use is minimal. The recent launch of DES has led to fewer patients being treated with ICB in the last 2 years. Thus, the manufacturers have decided to withdraw ICB devices and, from a practical standpoint, ICB has disappeared from our setting.
Concerning the remaining devices, CB and RA can still have a role in the DES era since they facilitate the implantation of these stents in especially complex and/or calcified lesions.
ACKNOWLEDGEMENTS
I would like to thank Drs. Fernando Alfonso, Manuel Sabaté, Rosana Hernández, and Carlos Macaya for their critical review of this article.
Section Sponsored by the Dr Esteve Laboratory
Correspondence: Dr. R. Moreno.
Unidad de Cardiología Intervencionista. Hospital Clínico San Carlos.
Martín Lagos, s/n. 28040 Madrid. España.
E-mail: raulmorenog@terra.es