There are scarce data on the optimal duration and prognostic impact of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) with second-generation drug-eluting stents for left main coronary artery (LMCA) disease. The aim of this study was to investigate the practice pattern and long-term prognostic effect of DAPT duration in patients undergoing PCI with second-generation drug-eluting stents for LMCA disease.
MethodsUsing individual patient-level data from the IRIS-MAIN and KOMATE registries, 1827 patients undergoing PCI with second-generation drug-eluting stents for LMCA disease with valid information on DAPT duration were included. The efficacy outcome was major adverse cardiovascular events (MACE, a composite of cardiac death, myocardial infarction, and stent thrombosis) and the safety outcome was TIMI major bleeding.
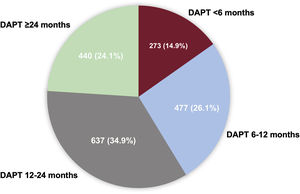
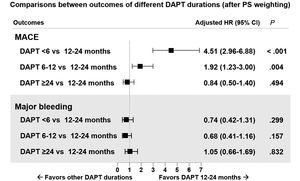
ResultsDAPT duration was <6 months (n=273), 6 to 12 months (n=477), 12 to 24 months (n=637), and ≥ 24 months (n=440). The median follow-up duration was 3.9 [interquartile range, 3.01-5.00] years. Prolonged DAPT duration was associated with lower incidences of MACE. In multigroup propensity score analysis, adjusted HR for MACE were significantly higher for DAPT <6 months and DAPT 6 to 12 months than for DAPT 12 to 24 months (HR, 4.51; 95%CI, 2.96-6.88 and HR 1.92; 95%CI, 1.23-3.00). There was no difference in HR for major bleeding among the assessed groups.
ConclusionsDAPT duration following PCI for LMCA disease is highly variable. Although the duration of DAPT should be considered in the context of the clinical situation of each patient, <12 months of DAPT was associated with higher incidence of MACE. Registration identifiers: NCT01341327; NCT03908463.
Keywords
Recent extended follow-up of landmark randomized clinical trials have shown that percutaneous coronary intervention (PCI) with drug-eluting stents (DES) is associated with similar incidences of hard endpoints and mortality to those of coronary artery bypass grafting in patients with left main coronary artery (LMCA) disease and low-to-intermediate anatomic complexity.1–3 Current practice guidelines usually recommend the duration of dual antiplatelet therapy (DAPT) in patients undergoing PCI with DES on the basis of initial clinical presentation, including DAPT for at least 6 months for chronic stable angina and 12 months for acute coronary syndrome (ACS).4 However, there are limited data on the optimal duration of DAPT in patients receiving PCI for complex lesions, including multivessel, bifurcation, and chronic total occlusions, or LMCA disease.
Although some prior studies suggested that prolonged duration of DAPT might be associated with better clinical outcomes in patients undergoing complex PCI,5,6 the proportion of LMCA-PCI was limited, and most studies used first-generation DES. In this clinical context, we investigated the practice pattern and long-term prognostic effect of DAPT duration in patients undergoing PCI with second-generation DES for LMCA disease using merged individual patient-level data from 2 large, real-world, contemporary PCI registries.
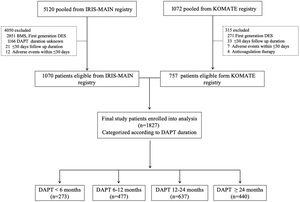
METHODSParticipants and study designThe design and enrollment characteristics of 2 multicenter registries (IRIS-MAIN and KOMATE) have been published previously.7,8 Briefly, the IRIS-MAIN is a nonrandomized, multinational, observational registry gathering data on consecutive patients with unprotected LMCA disease. Another study population was derived from the KOMATE registry, which includes 8 coronary intervention centers in Korea. Both data sources had an “all-comers” design to evaluate the characteristics, treatments, and clinical outcomes of patients with LMCA disease in the real-world setting. Among merged individual-level data, we only included patients with LMCA who were treated with second-generation DES with available accurate information on postprocedural DAPT duration. The exclusion criteria were minimal; patients who underwent bare-metal stent (BMS) or first-generation DES. In addition, we excluded patients in whom we could not accurately judge the effectiveness of DAPT (patients with follow-up loss within 30 days, patients with in-hospital events within 30 days). A flow diagram of the current analysis is shown in figure 1. This study was approved by the institutional review board of each participating center for merged data use and all patients provided written informed consent. The study was performed in accordance with the Declaration of Helsinki and was approved by the research ethics committee of each participating center, and written informed consent was obtained by all participants.
PCI procedures and data collectionAll PCI procedures were conducted in accordance with local guidelines using standard techniques. Intraprocedural anticoagulation was maintained with unfractionated or low molecular weight heparin to achieve an activated clotting time of 250 to 300 s. Other procedural factors, such as access location, stent strategy, stent technique, and use of intravascular ultrasound, were left to the operator's discretion. Although the duration of DAPT (aspirin plus P2Y12 inhibitors [clopidogrel, ticagrelor, or prasugrel]) was usually recommended according to the current practice guidelines,4 the final duration was left to the treating physician's discretion with consideration of the patient's clinical and procedural characteristics and other comorbid medical conditions. The DAPT duration criterion was allowed up to 2 months in addition to the date of use, considering the time of the outpatient department visit. All clinical, angiographic, procedural, and outcome data were collected using a web-based reporting system. To identify the status of antiplatelet therapy, the dates and duration of prescribed antiplatelet agents were obtained from the electronic prescribing system of each hospital. Additional information was obtained by further inquiry into medical records or telephone contact, if necessary.
Clinical outcomes and definitionsThe efficacy outcome of the study was major adverse cardiovascular events (MACE), defined as a composite of cardiac death, fatal or nonfatal acute myocardial infarction, and stent thrombosis events. The secondary outcomes were all-cause mortality and target vessel revascularization. Fatal or nonfatal acute myocardial infarction was defined as an increase in the creatine kinase-myocardial band or troponin level to the 99th percentile of the upper limit of normal with ischemic symptoms or electrocardiographic findings indicative of ischemia not related to the index procedure (ie, procedural myocardial infarction was disregarded). Stent thrombosis was defined as definite stent thrombosis according to the Academic Research Consortium definition.9 The safety outcome was major bleeding. Bleeding events were defined as minor or major bleeding as per the Thrombolysis in Myocardial Infarction (TIMI) bleeding criteria.10 All clinical outcomes were independently adjudicated by an independent group of clinicians who were unaware of DAPT duration and types of DES.
Statistical analysisContinuous variables are reported as mean± standard deviation and were analyzed using 1-way ANOVA. Categorical variables are reported as frequencies (percentages) and were analyzed using chi-square tests. Survival curves were prepared using Kaplan-Meier analysis and analyzed using log-rank tests. Cox proportional hazard regression analysis was used to identify independent predictors of primary endpoints and to estimate hazard ratios (HR) and 95% confidence intervals (95%CI) for clinical outcomes. To minimize confounding and residual selection bias in observational treatment comparisons, a propensity score weighting method was applied to control imbalances in various baseline characteristics across different groups of DAPT duration. For multigroup comparisons, multiple propensity scores were estimated using the Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG) method, and the corresponding inverse probabilities of treatment weight (the reciprocals of the propensity scores) were estimated by using generalized boosted models through an iterative estimation procedure.11 To calculate the propensity score, key clinical, anatomic, and procedural characteristics, such as age, sex, hypertension, diabetes mellitus, dyslipidemia, smoking, ACS, chronic kidney disease, multivessel disease, stent strategy, previous PCI, and intravascular ultrasound, were included. The balance of the pretreatment covariates was assessed, and significant improvement in baseline was achieved after weighting. Furthermore, the performance of this propensity model was confirmed by comparing the distributions of standardized mean differences of covariates and propensity scores between these groups before and after inverse probabilities of treatment weight.
Cox regression models with test for interaction were used to evaluate the consistency of treatment effects in multiple subgroups. The statistical analysis was performed using IBM SPSS version 23.0 (IBM, United States) and R software version 3.5.2 (R Project for Statistical Computing).
RESULTSPattern of DAPT duration and baseline characteristicsFrom July 2006 to August 2017, 1827 patients with LMCA lesions treated by PCI with second-generation DES and with valid data on DAPT duration were included in the final analytic datasets (figure 1). The practice pattern of DAPT duration in these patients is shown in figure 2. The median DAPT duration was 398 (interquartile range [IQR], 360-730) days for the entire population. According to DAPT duration, patients were categorized into 4 groups: DAPT <6 months (n=273), 6 to 12 months (n=477), 1224 months (n=637), and ≥ 24 months (n=440). The median DAPT durations were 99 (IQR, 36.7-180) days in DAPT <6 months, 365 (IQR, 341-365) days in DAPT 6 to 12 months, 523.5 (IQR, 397-730) days in DAPT 12 to 24 months, and 1095 (IQR, 1004-1316.5) days in DAPT ≥ 24 months, respectively. Since we enrolled patients for a long period of time (2006-2017), we investigated the pattern of DAPT duration according to the 2 different periods (2006-2011 and 2012-2017). The median duration of DAPT was longer in the 2006 to 2011 period (IQR, 497days) than in the 2012 to 2017 period (IQR 381 days). The pattern of DAPT between the 2 periods was the same as shown in .
The baseline clinical characteristics of the study population are shown in table 1. The clinical characteristics were similar between the 4 groups, except for dyslipidemia and history of previous PCI. DAPT duration tended to be significantly shorter in patients with stable angina than in those with ACS. The DAPT and procedural characteristics of the study population are shown in table 2. Regarding P2Y12 inhibitors, most patients received clopidogrel (94.6%), and ticagrelor and prasugrel was used in 4.1% and 1.3% of the patients, respectively.
Baseline patient characteristics according to DAPT duration.
| Total population (N=1 827) | DAPT <6 mo (n=273) | DAPT 6-12 mo (n=477) | DAPT 12-24 mo (n=637) | DAPT ≥ 24 mo (n=440) | SMD (unmatched) | P (unmatched) | SMD (after PS weighting) | P (after PS weighting) | |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 64.3±10.6 | 65.0±11.3 | 63.5±10.7 | 64.8±10.7 | 64.1±9.7 | 0.077 | .136 | 0.008 | .997 |
| Male sex | 1,395 (76.4) | 205 (75.1) | 380 (79.7) | 479 (75.2) | 331 (75.2) | 0.057 | .27 | 0.014 | .983 |
| Diabetes mellitus | 609 (33.3) | 87 (31.9) | 158 (33.1) | 204 (32) | 160 (36.4) | 0.049 | .461 | 0.028 | .914 |
| Hypertension | 1,146 (62.7) | 165 (60.4) | 302 (63.3) | 401 (63) | 278 (63.2) | 0.025 | .865 | 0.012 | .993 |
| Dyslipidemia | 1,120 (61.3) | 189 (69.2) | 292 (61.2) | 354 (55.6) | 285 (64.8) | 0.153 | <.0001 | 0.041 | .763 |
| Chronic kidneys disease | 137 (7.5) | 20 (7.3) | 34 (7.1) | 53 (8.3) | 30 (6.8) | 0.031 | .796 | 0.019 | .949 |
| Smoking | 422 (23.1) | 57 (20.9) | 130 (27.3) | 141 (22.1) | 94 (21.4) | 0.076 | .091 | 0.029 | .895 |
| Previous PCI | 326 (17.8) | 45 (16.5) | 72 (15.1) | 109 (17.1) | 100 (22.7) | 0.105 | .017 | 0.017 | .970 |
| Previous CABG | 60 (3.3) | 6 (2.2) | 19 (4) | 20 (3.1) | 15 (3.4) | 0.032 | .612 | 0.039 | .791 |
| Clinical indication for PCI | |||||||||
| Stable angina | 450 (24.6) | 45 (16.5) | 119 (25) | 187 (29.3) | 99 (22.5) | 0.162 | <.0001 | 0.058 | .421 |
| Acute coronary syndrome | 1377 (75.4) | 228 (83.5) | 358 (75) | 450 (70.7) | 341 (77.5) | 0.131 | .001 | 0.036 | .777 |
| Unstable angina | 1060 (77) | 171 (75) | 271 (75.7) | 340 (75.6) | 278 (81.5) | 0.112 | .008 | 0.072 | .370 |
| NSTEMI | 222 (16.1) | 41 (18) | 66 (18.4) | 72 (16) | 43 (12.6) | 0.096 | .103 | 0.074 | .181 |
| STEMI | 95 (6.9) | 16 (7) | 21 (5.9) | 38 (8.4) | 20 (5.9) | 0.050 | .58 | 0.045 | .679 |
| Mean ejection fraction, % | 59.5±12.5 | 59.0±13.1 | 59.8±13.1 | 59.4±13.3 | 59.7±10.2 | 0.059 | .871 | 0.065 | .402 |
CABG, coronary artery bypass grafting; DAPT, dual antiplatelet therapy; NSTEMI, non-ST-elevation acute myocardial infarction; PCI, percutaneous coronary intervention; SMD, standardized mean difference; STEMI, ST-segment elevation myocardial infarction. Values are presented as No. (%) or mean ± standard deviation.
DAPT and procedural characteristics according to DAPT duration.
| Total population (n=1827) | DAPT <6 mo (n=273) | DAPT 6-12 mo (n=477) | DAPT 12-24 mo (n=637) | DAPT ≥ 24 mo (n=440) | SMD (unmatched) | P (unmatched) | SMD (after PS weighting) | P (after PS weighting) | |
|---|---|---|---|---|---|---|---|---|---|
| DAPT duration, d | 589.3±443.9 | 105.1±64.1 | 336.9±52.7 | 546.3±145.4 | 1,225.6±400.2 | 3.181 | <.0001 | 3.224 | <.0001 |
| DAPT score | 0.52±1.30 | 0.45±1.36 | 0.74±1.36 | 0.48±1.27 | 0.39±1.21 | 0.134 | <.0001 | 0.042 | .627 |
| P2Y12inhibitor | 0.074 | .299 | 0.070 | .284 | |||||
| Clopidogrel | 1,729 (94.6) | 255 (93.4) | 458 (96) | 596 (93.6) | 420 (95.5) | ||||
| Ticagrelor | 75 (4.1) | 14 (5.1) | 17 (3.6) | 31 (4.9) | 13 (3) | ||||
| Prasugrel | 23 (1.3) | 4 (1.5) | 2 (0.4) | 10 (1.6) | 7 (1.6) | ||||
| Multivessel disease | 1,281 (70.1) | 177 (64.8) | 339 (71.1) | 447 (70.2) | 318 (72.3) | 0.067 | .186 | 0.056 | .369 |
| Disease extent | |||||||||
| Left main only | 133 (7.3) | 25 (9.3) | 34 (7.2) | 41 (6.4) | 33 (7.5) | 0.056 | .502 | 0.028 | .873 |
| Left main with 1-VD | 459 (25.3) | 73 (27.2) | 114 (24.2) | 173 (27.2) | 99 (22.5) | 0.066 | .281 | 0.047 | .554 |
| Left main with 2-VD | 667 (36.7) | 88 (32.8) | 184 (39.1) | 219 (34.4) | 176 (40) | 0.087 | .094 | 0.054 | .446 |
| Left main with 3-VD | 557 (30.7) | 82 (30.6) | 139 (29.5) | 204 (32) | 132 (30) | 0.028 | .816 | 0.031 | .847 |
| RCA involvement | 805 (44.3) | 120 (44.8) | 202 (42.9) | 286 (44.9) | 197 (44.8) | 0.018 | .911 | 0.040 | .818 |
| Left main lesion location | |||||||||
| Ostium of shaft | 651 (35.8) | 111 (41.4) | 165 (35) | 209 (32.8) | 166 (37.7) | 0.092 | .072 | 0.087 | .143 |
| Distal bifurcation | 1,244 (68.1) | 182 (66.7) | 325 (68.1) | 435 (68.3) | 302 (68.6) | 0.017 | .955 | 0.017 | .975 |
| Stent technique | 0.109 | .003 | 0.039 | .742 | |||||
| 1-stent strategy | 1,512 (83.3) | 216 (80.6) | 381 (80.9) | 558 (87.7) | 357 (81.1) | ||||
| 2-stent strategy | 303 (16.7) | 52 (19.4) | 90 (19.1) | 78 (12.3) | 83 (18.9) | ||||
| Total stent number per patient | 2.21±1.22 | 2.17±1.26 | 2.19±1.16 | 2.16±1.23 | 2.32±1.24 | 0.073 | .156 | 0.068 | .344 |
| Average stent diameter in MV, mm | 3.57±0.43 | 3.51±0.47 | 3.47±0.47 | 3.66±0.38 | 3.63±0.39 | 0.175 | <.0001 | 0.168 | <.001 |
| Average stent length in MV, mm | 21.88±7.64 | 21.85±7.63 | 21.89±7.83 | 20.95±7.38 | 22.92±7.62 | 0.106 | .008 | 0.116 | .028 |
| Postdilatation balloon diameter, mm | 3.71±0.56 | 3.67±0.56 | 3.63±0.58 | 3.71±0.55 | 3.81±0.54 | 0.174 | <.0001 | 0.134 | <.001 |
| Postdilatation balloon pressure, mmHg | 15.61±4.60 | 15.13±4.47 | 15.17±4.12 | 15.83±4.74 | 16.09±4.94 | 0.104 | .011 | 0.115 | .031 |
| FKBI | 848 (46.5) | 164 (60.3) | 213 (44.7) | 250 (39.3) | 221 (50.2) | 0.219 | <.0001 | 0.191 | <.001 |
| IVUS | 1107 (60.7) | 160 (58.8) | 264 (55.3) | 395 (62.1) | 288 (65.5) | 0.103 | .013 | 0.027 | .901 |
DAPT, dual antiplatelet therapy; FKBI, final kissing-balloon inflation; IVUS, intracoronary ultrasound; MV, main vessel; RCA, right coronary artery; VD, vessel disease.
Values are presented as No. (%) or mean±standard deviation.
The percentage of multivessel disease was the lowest in the DAPT <6 months group, and the highest in the DAPT ≥ 24 months group. The extent of CAD was similar between the 4 groups. Distal bifurcation involvement was lowest in the DAPT <6 months group and highest in the DAPT ≥ 24 months group. The average stent diameter and postdilatation balloon diameter were largest in the DAPT ≥ 24 months group. Postdilatation balloon pressure was the highest in the DAPT ≥ 24 months group. Patients who underwent PCI with intravascular ultrasound were significantly more likely to receive DAPT for ≥ 24 months.
Clinical outcomes according to DAPT durationThe median length of follow-up among all patients was 47.9 [36.7-60.8) months. The number of patients lost to follow-up was 73 out of 1827 (3.99%). First, we fit Cox proportional-hazards log-linear models adjusted by baseline and procedural parameters with thin-plate spline curves to DAPT duration (figure 3). When we analyzed the risk of MACE and major bleeding according to DAPT duration as a continuous variable, risk of MACE progressively decreased, but the risk of major bleeding increased with>12 months of DAPT. We investigated Cox proportional-hazards log-linear models with thin-plate spline curves to DAPT duration according to stent strategies and with or without bifurcation lesions. Regardless of these factors, the results remained consistent ().
Spline curves. Duration-response relationships between DAPT duration and MACE (A), and between DAPT duration and major bleeding (B) after PS weighting tested by a log-linear model with thin-plate spline curves. DAPT, dual antiplatelet therapy; HR, hazard ratio; MACE, major adverse cardiovascular event; PS, propensity score.
The cumulative incidences of clinical outcomes according to the categories of DAPT duration are shown in table 3 and in . The cumulative rates of MACE occurred more frequently in the DAPT <6 months group than in other DAPT groups (lowest for the DAPT ≥ 24 months [1.6%] and highest for the DAPT <6 months [5.1%]). There was no difference in the cumulative incidence of major bleeding (lowest for the DAPT <6 months [1.1%] and highest for DAPT ≥ 24 months [3.6%]).
Clinical outcomes according to DAPT duration.
| DAPT <6 mo(n=273) | DAPT 6-12 mo(n=477) | DAPT 12-24 mo(n=637) | DAPT ≥ 24 mo(n=440) | P | |
|---|---|---|---|---|---|
| Major adverse cardiovascular event* | 5.1 (2.0-8.0) | 3.1 (2.0-5.0) | 2.0 (1.0-3.0) | 1.6 (0.0-3.0) | .02 |
| Cardiac death | 3.7 (1.0-6.0) | 2.5 (1.0-4.0) | 1.1 (0.0-2.0) | 1.1 (0.0-2.0) | .03 |
| Myocardial infarction | 2.6 (1.0-4.0) | 1.0 (0.0-2.0) | 1.7 (1.0-3.0) | 1.1 (0.0-2.0) | .35 |
| Stent thrombosis | 1.5 (0.0-3.0) | 0.6 (0.0-1.0) | 0.3 (0.0-1.0) | 0.2 (0.0-1.0) | .12 |
| All-cause death | 12.5 (9.0-16.0) | 6.7 (4.0-9.0) | 5.3 (4.0-7.0) | 3.9 (2.0-6.0) | <.0001 |
| Target vessel revascularization | 11.0 (7.0-15.0) | 5.0 (3.0-7.0) | 4.4 (3.0-6.0) | 8.4 (6.0-11.0) | <.0001 |
| Major bleeding | 1.1 (0.0-2.0) | 1.3 (0.0-2.0) | 2.8 (2.0-4.0) | 3.6 (2.0-5.0) | .07 |
| Minor bleeding | 5.9 (3.0-9.0) | 4.0 (2.0-6.0) | 2.8 (2.0-4.0) | 3.2 (2.0-5.0) | .14 |
DAPT, dual antiplatelet therapy.
Values are presented as cumulative rate (95% confidence interval). Cumulative rates (95% confidence intervals) of events are based on Kaplan-Meier estimates.
The distributional balance of propensity scores according to DAPT duration before and after weighting is shown in . The adjusted risks for adverse clinical events according to the different categories of DAPT duration after application of multiple treatment propensity score weighting are shown in figure 4. With DAPT 12 to 24 months as the reference group, adjusted hazard ratios for MACE were significantly higher for DAPT <6 months and DAPT 6 to 12 months than for DAPT 12 to 24 months (HR, 4.51; 95%CI, 2.96-6.88 and HR 1.92; 95%CI, 1.23-3.00). Major bleeding events tended to be more likely in the ≥ 24 month group than in the other shorter DAPT groups. Multivariate analysis revealed that diabetes mellitus and chronic kidney disease were independent predictors of MACE ().
DISCUSSIONIn this pooled individual patient-level analysis from 2 large-sized, contemporary, real-world registries, DAPT duration was highly variable. Although DAPT duration should be considered in the context of the clinical situation of each patient, <12 months of DAPT was associated with a higher incidence of MACE.
LMCA lesions are one of the most complex anatomic subsets in real-world clinical settings. Recent clinical studies suggested that PCI with second-generation DES for LMCA disease provided favorable procedural and long-term clinical outcomes.2,3 However, there have been few studies on long-term duration and the effect of DAPT in the contemporary PCI practice with second-generation DES for the treatment of LMCA disease. In a recent study, DAPT for> 12 months after an index procedure was associated with a reduced risk of ischemic events among patients with LMCA bifurcation stenting compared with DAPT for ≤ 12 months.12 However, that study might have been hampered by the use of first-generation DES, the exclusion of LMCA ostial and shaft lesions, and nonassessment of bleeding events. Moreover, since events occurring within 12 months were excluded, the exact relationship between shorter or longer DAPT duration and clinical events could not be assessed. In contrast, in the recent EXCEL trial report, continuation of DAPT beyond 12 months was not found to be associated with a reduced risk of ischemic events (death, myocardial infarction, or stroke) after PCI with everolimus-eluting stents in patients with LMCA disease.13 However, that study was a subgroup analysis including a relatively limited number of patients, and, again, there was no assessment of bleeding events. In the current study, we investigated clinical outcomes including ischemic and bleeding events according to different durations of DAPT in patients receiving PCI with contemporary second-generation DES for LMCA disease using 2 large-scaled real-world registries. Following PCI, DAPT was maintained in most patients (84.8%) for at least 6 months according to the current guidelines. After multiple treatment propensity score weighting, with DAPT 12 to 24 months as the reference group, DAPT for <12 months was significantly associated with a higher risk of MACE without a clinical benefit or reducing major bleeding.
Recently, several randomized clinical trials have reported the potential benefit of reduced DAPT duration in patients receiving contemporary second-generation DES.14,15 Most studies have shown that antiplatelet monotherapy was associated with a lower incidence of clinically relevant bleeding compared with DAPT but with a higher risk of ischemic events. However, the observed results of several studies regarding the shortening of DAPT in complex PCI groups were conflicting and the number of patients with LMCA disease was too small to provide clinically meaningful insights. In the RAIN registry subgroup analysis, the incidence of MACE was significantly higher in the ≤ 3 months DAPT group compared with the 3 to 12 and> 12 months DAPT groups, which was mainly driven by the differences in myocardial infarction and stent thrombosis.16 Theoretically, despite the use of second-generation DES, PCI for LMCA disease is more likely to develop stent malapposition due to their large diameter and bifurcation compared with non-LMCA lesions, which might result in insufficient strut coverage and a potential risk of thrombus formation. In previous studies, especially among patients with a 2-stent strategy, implying a high probability of stent malapposition and underexpansion, fatal ischemic events substantially increased when DAPT was discontinued.17 A similar association between shorter DAPT and higher ischemic events was also observed in our study, which might be of paramount clinical significance with regard to the optimal DAPT duration in patients with complex PCI for LMCA disease. In the IDEAL-LM trial, PCI with the biodegradable polymer-coated platinum-chromium DES followed by 4 months of DAPT was noninferior to durable polymer cobalt-chromium DES followed by 12 months of DAPT with respect to MACE at 2 years. However, due to lower than predicted event rates, the trial is underpowered, and the individual components of MACE all trended to be numerically higher in the biodegradable polymer-DES with 4 months of DAPT.18
Several score systems (eg, DAPT score, PRECISE-DAPT score) and validation studies have been designed to determine optimal DAPT duration.19,20 In a previous study, DAPT> 12 months was associated with a lower MACE rate than DAPT ≤ 12 months in a population with DAPT score ≥ 2, but not in a population with DAPT score <2.12 The efficacy and safety of a short DAPT duration of DAPT after LMCA PCI requires further investigation and future studies should focus on individual patient risk of ischemic and bleeding events.
LimitationsThere are several limitations of our study. First, although multiple propensity score treatment analysis was performed, this study was an observational, nonrandomized study; therefore, it might be vulnerable to inherent limitations, including selection bias and unmeasured confounders. Thus, the overall observed findings should be interpretated as provisional and hypothesis-generating only. These findings should be confirmed or refuted through large randomized clinical trials. Second, owing to the limited number of clinical events, our study was underpowered to detect clinically relevant differences regarding hard clinical endpoints including death, stent thrombosis, or major bleeding. Third, we were not able to classify bleeding endpoints to other classifications such as the International Society on Thrombosis and Hemostasis (ISTH) or Bleeding Risk Estimation and the new Bleeding Academic Research Consortium (BARC) classification due to the limited information of our multicenter observational data. Fourth, we could not systematically measure detailed information on atherosclerotic burden and complexity, such as SYNTAX score. Fifth, the study population was enrolled over a wide period (2006-2017). This may have introduced some heterogeneity in the study population due to changes in clinical practice and improvements in PCI technology. Sixth, we did not include data on oral anticoagulation agents. Finally, in the current study, potent P2Y12 inhibitors, such as prasugrel and ticagrelor, were less frequently used. In a recent study, novel P2Y12 inhibitor monotherapy was shown to reduce major bleeding events without increasing ischemic events in patients with complex PCI.21 This concept should be further tested in patients with complex PCI including LMCA disease.
CONCLUSIONSIn this merged individual patient-level analysis of 2 large-scale real-world registries, although the duration of DAPT should be considered in the context of the clinical situation of each patient, DAPT for <12 months was significantly associated with a higher risk of MACE in patients undergoing PCI with second-generation DES for LMCA disease. Future randomized clinical trials are warranted to determine the optimal duration of DAPT for patients receiving complex PCI including LMCA disease.
FUNDINGThis work was supported by the CardioVascular Research Foundation, Seoul, Republic of Korea (2015-09), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (HI20C1566), the Ministry of Science and ICT (2017M3A9E9073585), and the Cardiovascular Research Center (Seoul, Republic of Korea).
AUTHORS’ CONTRIBUTIONSS. Cho, D.Y. Kang, J.S. Kim, and D.W. Park designed the study; T.S. Kang, J.M. Ahn, P.H. Lee, S.J. Kim, S.W. Lee, Y.H. Kim, C.W. Lee, S.W. Park, S.J. Lee, S.J. Hong, C.M. Ahn, B.K. Kim, Y.G. Ko, D. Choi, Y. Jang, M.K. Hong, and S.J. Park assisted with data acquisition and interpretation; S. Cho and I.S. Kim performed statistical analyses; S. Cho, D.Y. Kang, J.S.Kim, D.W. Park, and C.M. Ahn contributed to the discussion; S. Cho, and J.S. Kim drafted the manuscript; S. Cho, J.S. Kim, and D.W. Park revised the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTERESTNone.
- –
Although some prior studies suggested that prolonged duration of DAPT might be associated with better clinical outcomes in patients undergoing complex PCI, the proportion of LMCA-PCI was limited and most studies used first-generation DES.
- –
In this pooled individual patient-level analysis of 2 large-sized, contemporary, real-world registries, we observed that DAPT duration was highly variable. Although DAPT duration should be considered in the context of the clinical situation of each patient, <12 months of DAPT was associated with a higher incidence of MACE.
- –
Further randomized clinical trials are needed to determine the optimal duration of DAPT in patients undergoing PCI for LMCA disease.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.07.007