This report presents the clinical characteristics, outcomes and complications of all consecutive patients implanted with a long-term mechanical circulatory support device in Spain between 2007 and 2020.
MethodsAnalysis of the Spanish Registry of durable ventricular assist devices (REGALAD) including data form Spanish centers with a mechanical circulatory support program.
ResultsDuring the study period, 263 ventricular assist devices were implanted in 22 hospitals. The implanted device was an isolated continuous-flow left ventricular assist device in 182 patients (69%), a pulsatile-flow device (58 isolated left ventricular and 21 biventricular) in 79 (30%), and a total artificial heart in 2 patients (1%). The strategy of the implant was as bridge to heart transplant in 78 patients (30%), bridge to candidacy in 110 (42%), bridge to recovery in 3 (1%) and destination therapy in 72 patients (27%). Overall survival at 6, 12 and 24 months was 79%, 74% and 69%, respectively, and was better in continuous-flow left ventricular assist devices (84%, 80%, and 75%). The main adverse events related to this therapy were infections (37% of patients), bleeding (35%), neurological (29%), and device malfunction (17%).
ConclusionsDurable ventricular assist devices have emerged in Spain in the last few years as a useful therapy for patients with advanced heart failure. As in other international registries, the current trend is to use continuous-flow intracorporeal left ventricular devices, which are associated with better results. Adverse events continue to be frequent and severe.
Keywords
Despite recent advances in the management of heart failure (HF), a nonnegligible number of HF patients progresses to advanced HF; at this stage, medical therapy is insufficient to avoid functional deterioration, the involvement of other organs, frequent readmissions, and even death.1
In such patients, heart transplant (HTx) continues to be the treatment of choice. However, because of the shortage of donors and eventual contraindications or comorbidities, many patients cannot access this treatment.2,3
Durable ventricular assist devices (dVADs) have undergone rapid development in recent years.4 Their function is to provide circulatory support to patients with advanced HF on the transplant waiting list (bridge to transplant [BTT]) until a potentially reversible contraindication to transplant resolves (bridge to candidacy), the heart recovers (bridge to recovery), or as final therapy in patients who are not HTx candidates (destination therapy).
We present the first analysis of the Spanish Registry of durable ventricular assist devices (REGALAD). This registry is constituted as a delegated activity by the Heart Failure Association of the Spanish Society of Cardiology with the collaboration of the Spanish Society of Cardiovascular and Endovascular Surgery and aims to collect and analyze the characteristics and outcomes of dVAD procedures performed in Spain.
METHODSRegistryREGALAD is an observational and multicenter study that retrospectively includes all dVAD procedures performed in Spain from 2007 to the date of registry activity initiation (November 30, 2018) and prospectively thereafter.
For the technical management of the registry, an external company was contracted by the registry management. The procedures used in the data collection, handling, and reporting are in line with the regulations established in the Spanish Data Protection Law, including informed consent. The protocol was approved by the ethics committees of the participating centers.
Participating hospitalsAll Spanish hospitals performing dVAD implantation participated in this registry. In each center, a local physician and a surgeon undertook to introduce all dVAD procedures into the online database at least annually. They included both new cases and an update of the status and main complications of previous cases. All registry participants are listed in .
Ventricular assist devicesREGALAD is restricted to the study of what are considered to be long-term devices. These devices are understood to be those that eventually allow patient autonomy and discharge.
Depending on the type of flow provided by the pump, the devices used comprised a paracorporeal pulsatile-flow dVAD model with the possibility of isolated left ventricular or biventricular support—EXCOR (Berlin Heart GmbH, Germany)—and various intracorporeal continuous-flow dVAD models for isolated left ventricular support: INCOR (Berlin Heart GmbH), HeartMate II (Abbott, United States), and Jarvik 2000 (Jarvik Heart Inc, United States), which provide axial flow, and HeartWare HVAD (Medtronic, United States) and HeartMate 3 (Abbott), the latest-generation pumps that provide centrifugal continuous flow. There was also a total artificial heart device that generates pulsatile flow in both circuits (systemic and pulmonary): SynCardia TAH-t (SynCardia Systems LLC, United States).
PatientsThe patients analyzed in this work were all adults with advanced HF who underwent dVAD implantation in Spain between 2007 and 2020.
REGALAD includes most variables from the IMACS (International Society for Heart and Lung Transplantation Registry for Mechanically Assisted Circulatory Support) and EUROMACS (European Registry for Patients with Mechanical Circulatory Support) registries, as well as additional variables considered pertinent by the registry founders. These variables include patients’ demographic, clinical, laboratory, echocardiographic, and hemodynamic characteristics, implantation data, and 3-month, 1-year, and annual follow-up data. Adverse events associated with the device were specifically recorded. A definition of all adverse events can be found in .
HF severity at implantation was graded using the scale proposed by INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support)5 ().
Statistical analysisNormally and nonnormally distributed numerical variables are expressed as mean ± standard deviation or median [interquartile range], respectively. Categorical variables are expressed as absolute and relative frequencies. Overall survival was estimated using the Kaplan-Meier method. Patients were followed up until death or study closure on December 31, 2020. For this survival analysis, patients were censored at transplantion or dVAD explantation. Patients’ clinical course was also examined using Fine-Gray competing risk analysis, which tracks multiple mutually exclusive outcomes (alive with device, death, transplantation, or explantation). At any time, the sum of the proportion (percentage) of patients in each outcome category was equal to 100%. To compare the clinical course in terms of implant characteristics and outcomes, the study time was divided into 3 periods (2007-2010, 2011-2015, and 2016-2020). All tests were 2-sided, and a P value less than .05 was considered statistically significant. The analyses were performed using IBM SPSS Statistics version 24.0 and R version 4.1.2 software. Survival and competing risk analyses were performed using the “survival” and “cmprsk” R libraries.
RESULTSParticipating hospitalsThe 22 Spanish hospitals performing dVAD implantation between 2007 and 2020 participated in the registry; 15 had a HTx program. The number of devices per hospital was highly variable: in particular, 4 hospitals performed more than 20 implants per center, comprising more than half of all activity. The numbers of participating hospitals and of dVADs implanted in each center are shown in table 1.
Ventricular assist devices implanted in Spain, by autonomous community, province, and hospital (2007-2020).
| Community | Hospital | Devices (n=263) |
|---|---|---|
| Andalusia | ||
| Córdoba | Hospital Universitario Reina Sofía de Córdoba | 1 |
| Granada | Hospital Universitario Virgen de las Nieves | 2 |
| Seville | Hospital Universitario Virgen del Rocío | 14 |
| Principality of Asturias | Hospital Universitario Central de Asturias | 14 |
| Balearic Islands | Hospital Universitari Son Espases | 1 |
| Cantabria | Hospital Universitario Marqués de Valdecilla | 3 |
| Castile and León | ||
| Salamanca | Hospital Clínico Universitario de Salamanca | 8 |
| Valladolid | Hospital Clínico Universitario de Valladolid | 11 |
| Catalonia | ||
| Barcelona | Hospital Clínic i Provincial de Barcelona | 7 |
| Hospital Universitari de Bellvitge | 46 | |
| Hospital de la Santa Creu i Sant Pau | 6 | |
| Hospital Germans Trias i Pujol | 2 | |
| Valencian Community | ||
| Valencia | Hospital Universitario La Fe | 12 |
| Galicia | ||
| A Coruña | Complejo Hospitalario Universitario A Coruña | 15 |
| Pontevedra | Hospital Álvaro Cunqueiro | 4 |
| Community of Madrid | Hospital Universitario 12 de Octubre | 24 |
| Hospital General Universitario Gregorio Marañón | 25 | |
| Hospital Universitario La Paz | 2 | |
| Hospital Universitario Puerta de Hierro | 48 | |
| Hospital Universitario Ramón y Cajal | 3 | |
| Region of Murcia | Hospital Clínico Universitario Virgen de la Arrixaca | 3 |
| Chartered Community of Navarre | Clínica Universidad de Navarra | 12 |
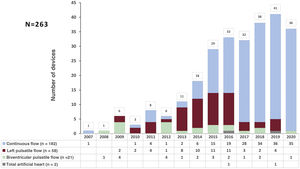
Between 2007 and 2020, 263 dVADs were implanted in Spain. The device was a continuous-flow left ventricular device in 182 patients (69%), a pulsatile-flow device in 79 (30%) (58 left, 21 biventricular), and a total artificial heart in 2 (1%) (figure 1). The different dVAD models used are shown in table 2 by period.
Ventricular assist device model by implantation period.
| 2007-2010 (n = 11) | 2011-2015 (n = 72) | 2016-2020 (n = 180) | Total (n = 263) | |
|---|---|---|---|---|
| Pulsatile flow | ||||
| Left EXCOR | 4 | 34 | 20 | 58 |
| Biventricular EXCOR | 5 | 10 | 6 | 21 |
| Continuous flow | ||||
| INCOR | 2 | 3 | 5 | |
| HeartMate II | 5 | 1 | 6 | |
| HeartWare HVAD | 20 | 55 | 75 | |
| HeartMate 3 | 95 | 95 | ||
| Jarvik 2000 | 1 | 1 | ||
| Total artificial heart | ||||
| SynCardia | 2 | 2 | ||
Patients’ baseline characteristics are summarized in table 3.
Baseline clinical, echocardiographic, and hemodynamic characteristics of the patients (n = 263).
| Age, y | 58 ± 12 |
| Male sex | 220 (84) |
| Body surface area, m2 | 1.9±0.2 |
| Hypertension | 141 (54) |
| Diabetes mellitus | 88 (34) |
| Previous stroke | 27 (10) |
| Previous cardiac surgery | 29 (11) |
| Type of heart disease | |
| Ischemic heart disease | 134 (51) |
| Nonischemic dilated cardiomyopathy | 91 (35) |
| Hypertrophic cardiomyopathy | 9 (3) |
| Myocarditis | 8 (3) |
| Toxic cardiomyopathy | 7 (3) |
| Valvular heart disease | 5 (2) |
| Other | 9 (3) |
| Time from HF diagnosis | |
| <1 mo | 15 (6) |
| 1 mo-1 y | 26 (10) |
| >1 y | 220 (84) |
| Need for outpatient inotropic agents | 69 (26) |
| ICD recipient | 207 (79) |
| CRT recipient | 78 (31) |
| Initial objective of device | |
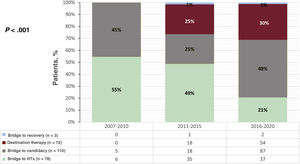
| Bridge to transplant | 78 (30) |
| Bridge to candidacy | 110 (42) |
| Bridge to recovery | 3 (1) |
| Destination therapy | 72 (27) |
| Treatment in the 48 h before implantation | |
| Inotropic agent infusion | 176 (68) |
| Intraaortic balloon pump | 38 (14) |
| Other circulatory support | 22 (8) |
| Respirator | 15 (6) |
| Dialysis/ultrafiltration | 3 (1) |
| Blood parameters in the 24 h before implantation | |
| Creatinine, mg/dL | 1.3±0.5 |
| GOT/AST, U/L | 35±48 |
| Bilirubin, mg/dL | 1.2±0.9 |
| Albumin, mg/dL | 3.8±0.6 |
| NT-proBNP, pg/mL | 4567 (2587-8286) |
| Platelets, ×10/L | 201±74 |
| INR | 1.3±0.4 |
| Echocardiographic parameters | |
| LVEDD, cm | 6.8±1.0 |
| LVEF, % | 23±7 |
| TAPSE, mm | 15±4 |
| Moderate or severe RV dysfunction | 88 (34) |
| Moderate or severe mitral regurgitation | 172 (65) |
| Moderate or severe tricuspid regurgitation | 106 (40) |
| Moderate or severe aortic regurgitation | 14 (5) |
| Hemodynamic parameters | |
| CVP, mmHg | 11±6 |
| sPAP, mmHg | 55±18 |
| mPAP, mmHg | 36±12 |
| PCP, mmHg | 23±8 |
| TPG, mmHg | 13±8 |
| CO, L/min/m2 | 4.1±1.1 |
| CI, L/min/m2 | 2.2±0.6 |
| PVR, UW | 3.3±2.3 |
| RVSWI, mmHg/mL/m2 | 774±456 |
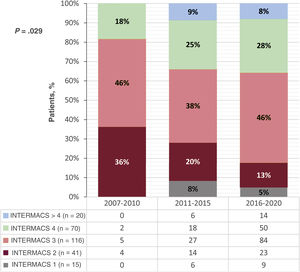
| INTERMACS at implantation | |
| 1 | 15 (6) |
| 2 | 41 (15) |
| 3 | 116 (44) |
| 4 | 70 (27) |
| > 4 | 20 (8) |
AST, aspartate aminotransferase; CI, cardiac index; CO, cardiac output; CRT, cardiac resynchronization therapy; CVP, central venous pressure; GOT, glutamic oxaloacetic transaminase; HF, heart failure; ICD, implantable cardioverter-defibrillator; INR, international normalized ratio; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary arterial pressure; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCP, pulmonary capillary pressure; PVR, pulmonary vascular resistance; RV, right ventricle; RVSWI, right ventricular stroke work index; sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion; TPG, transpulmonary pressure gradient.
Continuous data are expressed as No. (%) while categorical data are expressed as mean ± standard deviation or median [interquartile range].
The initial objective of the dVAD was BTT in 78 patients (30%), with a median time on the waiting list of 61 [21-228] days before device implantation. In 110 patients (42%), the dVAD was implanted as a bridge to candidacy; in these patients, the main contraindications to HTx were pulmonary hypertension (61 patients, 55%) and recent history of cancer (11 patients, 10%). In the 72 patients (27%) whose dVAD was implanted as destination therapy, the most commonly reported contraindications were advanced age (47 patients, 65%) and renal failure (22 patients, 31%). The device was implanted as bridge to recovery in only 3 patients (1%). The changes over time in the initial objective by implantation period are shown in figure 2.
All patients met the criteria for advanced HF and most had an INTERMACS risk profile of 3 (44%) or 4 (27%) at implantation. The changes over time in the risk profile by implantation period are shown in figure 3.
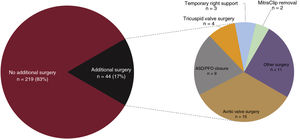
Characteristics of the device implantation surgeryIn 250 patients (95%), the surgery was performed using cardiopulmonary bypass. The surgical approach was median sternotomy in 93% of the patients, minimally invasive surgery in 5%, and left thoracotomy in 2%. The surgery was associated with another cardiac surgery in 44 patients (17%); the most frequent was aortic valve surgery (figure 4).
Clinical course of patients hospitalized for device implantationOf the 263 patients treated, 190 (72%) were discharged after a median of 36 [20-64] days, 44 (17%) died, and 27 (10%) underwent a HTx during admission after 77 [33-121] days. In 23 of the 27 transplanted patients (85%), the devices were pulsatile pumps with events or other conditions that required that the patient be hospitalized until the HTx. Two patients (1%) developed dVAD-related complications that necessitated pump explantation.
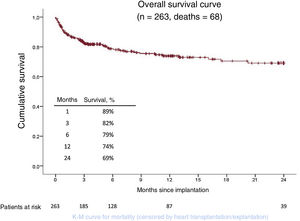
Patient survivalOverall patient survival after a mean follow-up of 358 days (median, 173 [83-430] days) was 89%, 82%, 79%, 74%, and 69% at 1, 3, 6, 12, and 24 months (figure 5).
Figure 6 shows survival by type of device and by the initial implant objective. The best survival was achieved with continuous-flow left ventricular devices (92%, 87%, 84%, 80%, and 75% at 1, 3, 6, 12, and 24 months) vs pulsatile-flow left ventricular devices (82%, 74%, 62%, and 31% at 1, 3, 6, and 12 months) and biventricular/total artificial heart devices (91%, 71%, and 56% at 1, 3, and 6 months).
Patient complicationsDuring follow-up, 611 major ventricular assist device-related complications were recorded (2.3 events/patient) (table 4).
Major complications related to ventricular assistance.
| Events | Patients, n | Patients, % | Incidence (every 100 patients-mo) | |
|---|---|---|---|---|
| Major bleeding | 151 | 91 | 35 | 6.05 |
| Mediastinal/thoracic | 60 | |||
| Gastrointestinal | 57 | |||
| Pleural/pulmonary | 14 | |||
| ORL/dental | 6 | |||
| Intraabdominal | 4 | |||
| Urinary | 3 | |||
| Other | 7 | |||
| Major infection | 139 | 98 | 37 | 5.74 |
| Specific to the device | 60 | |||
| Related to the device | 14 | |||
| Not related to the device | 65 | |||
| Neurological dysfunction | 113 | 76 | 29 | 4.30 |
| TIA | 37 | |||
| Ischemic stroke | 47 | |||
| Hemorrhagic stroke | 21 | |||
| Other | 8 | |||
| Device dysfunction | 56 | 45 | 17 | 2.0 |
| Pump dysfunction | 45 | |||
| Pump thrombosis is a subtype of pump dysfunction | 26 | |||
| Dysfunction of the percutaneous drive cable | 3 | |||
| Dysfunction of another component | 8 | |||
| Right heart failure | 52 | 49 | 19 | 1.88 |
| Mild HF | 23 | |||
| Moderate HF | 23 | |||
| Severe HF | 6 | |||
| Respiratory failure | 15 | 14 | 5 | 0.50 |
| Development of anti-HLA antibodies | 6 | 6 | 2 | 0.20 |
| Arterial thromboembolism without CNS involvement | 2 | 2 | 1 | 0.07 |
| Another type of event | 77 | 55 | 21 | 2.89 |
CNS, central nervous system; HF, heart failure; HLA, human leukocyte antigen; ORL, otorhinolaryngology; TIA, transient ischaemic attack.
The most commonly reported complication was major bleeding. In the first postimplantation week, the most frequent bleeding events were related to the surgery itself; subsequently, they comprised gastrointestinal bleeding.
The second most common complication was major infection. Of the infections, 47% had no direct relationship with the device and most were respiratory or urinary. In addition, 43% were specific device infections, particularly infections of the percutaneous drive cable connecting the intracorporeal pump with the external controller or of the percutaneous cannulae in the case of paracorporeal pumps. The remaining 10% of infections were related to the device but were not specific to it (mediastinitis, bacteremias, and bacterial endocarditis).
Other less frequent, but more severe, complications were neurological, which were fatal in 26 patients (34% of cases): 12 patients died of ischemic stroke after a median of 15 [5-66] days and 12 patients died of hemorrhagic stroke after 162 [33-236] days. In another 10 patients (13%), the neurological complications had severe sequelae.
The most commonly detected dVAD dysfunction was pump thrombosis, which was seen on 26 occasions and affected 5% of patients with continuous-flow devices and 11% of those with pulsatile-flow devices. In most cases, the thrombosis was resolved by intensification of the antithrombotic therapy or by changing the paracorporeal ventricles. However, in 3 patients with severe thromboses and/or repeated thromboses, the pump was eventually explanted and the patients were connected to temporary circulatory support as a bridge to urgent HTx. Moreover, 21% of all device dysfunctions led to the patients being put on the urgent HTx waiting list.
After device implantation, 49 patients (19%) had some degree of right HF. In most patients, the HF was controlled with medical therapy, but 6 required temporary right circulatory support and 2 of these patients died of this condition.
Comparison of complication rates by type of dVAD (continuous left vs pulsatile left vs biventricular/artificial heart) revealed that, except for right HF, which was obviously prevented with biventricular devices, the other events were less frequent in the first group of patients (table 5).
Major complications by type of ventricular assist device (incidence per 100 patient-months).
| Continuous left (n = 182) | Pulsatile left (n = 58) | Biventricular/total artificial heart (n =23) | P | |
|---|---|---|---|---|
| Major bleeding | 5.15 | 13.89 | 26.72 | <.001 |
| Major infection | 4.82 | 15.58 | 16.70 | <.001 |
| Neurological dysfunction | 2.54 | 30.55 | 7.92 | <.001 |
| Device dysfunction | 1.13 | 13.06 | 9.53 | <.001 |
| Pump thrombosis | 0.51 | 6.53 | 4.08 | <.001 |
| Right heart failure | 1.74 | 5.24 | 0 | .01 |
| Respiratory failure | 0.40 | 0.60 | 3.73 | .01 |
| Development of anti-HLA antibodies | 0.07 | 1.18 | 2.52 | .01 |
| Other type of event | 2.72 | 4.52 | 5.11 | — |
HLA, human leukocyte antigen.
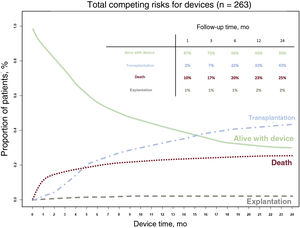
On December 31, 2020, of the 263 patients included in the registry, 101 (38%) had received a HTx after a median of 159 [105-321] days, 90 (34%) were still alive with support after 173 [83-430] days, and 68 (26%) had died after 51 [18-178] days; in addition, the device had been explanted in 4 patients (2%) (3 due to pump dysfunction and 1 due to patient recovery from the heart disease).
Notably, of the subgroup of 110 patients initially implanted with a device as bridge to candidacy, 58 (53%) ultimately underwent transplant, 23 (21%) died, and 29 (26%) were still alive with a device at the end of follow-up; 15 (14%) of these were on the HTx waiting list.
Figure 7 shows the clinical course of patients during the first 2 postimplantation years. shows the clinical course of patients by type of device implanted.
The leading cause of death was a neurological complication (38% of deaths). The remaining causes of death are summarized in table 6.
Primary cause of death of implanted patients.
| Primary cause of death | n (%) |
|---|---|
| Neurological dysfunction | 26 (38) |
| Multiorgan failure | 9 (13) |
| Bleeding | 7 (10) |
| Refractory heart failure | 6 (9) |
| Major infection | 4 (6) |
| Respiratory failure | 4 (6) |
| Device dysfunction | 3 (4) |
| Cancer | 2 (3) |
| Other | 7 (10) |
| Total | 68 (100) |
This work is the first report from REGALAD and reports complete data on all dVAD implants performed in Spain between 2007 and 2020.
As in other international registries, the number of devices implanted progressively increased from their introduction to 2020, which showed a small fall that was probably related to the COVID-19 pandemic. However, our annual numbers are significantly lower than those of other countries. For example, based on INTERMACS registry data,6 9.7 dVADs were implanted per million population in the United States in 2019 vs 0.8 in Spain. This huge difference might be due to the relative ease and speed of HTx receipt in Spain. This, together with the high percentage of patients who underwent urgent transplant with a short-term circulatory assist device,7 would explain the low number of dVADs as BTT in our registry. However, this strategy is much discussed due to its dubious results.8,9 The recent change to the regulations for the prioritization of donor recipients in the United States has had the same effect, facilitating urgent HTx with temporary circulatory support and reducing the number of dVAD implants as BTT but increasing mortality after HTx.10
Another reason for the negligible growth in dVADs in Spain is the poor prevailing knowledge in Spain concerning this treatment and its outcomes, which means that many patients who could benefit are not referred to specialized centers. This, together with the lack of favorable cost-effectiveness results, may strongly underlie the slow incorporation of this treatment into the Spanish public health system, particularly as destination therapy.
In terms of the time of dVAD implantation, there was a markedly low number of severe (1 or 2) INTERMACS profiles (just 21% of patients in our series) vs the American registry (51%).6 This may be related to the logistical difficulty and the concerns that most Spanish centers still have at the time of urgent dVAD implantation, which has classically been associated with worse outcomes.11 For these critically ill patients, many physicians opt for initial implantation of a short-term circulatory assist device as bridge to a dVAD (bridge to bridge strategy); indeed, 22 patients (8%) of our series had a temporary mechanical device (8 ECMOs, 6 Impellas, and 8 Centrimags) at the time of dVAD implantation. These patients did not have more events or worse survival than the other patients in the registry.
The REGALAD results also confirm that male sex (84%) predominates among the HF population treated with advanced therapies, as seen in other dVAD registries such as the INTERMACS (79%)6 and EUROMACS (82%)12 and in HTx registries such as the Spanish Heart Transplantation Registry itself (75%).7
The previously used paracorporeal pulsatile devices have been superseded by intracorporeal continuous-flow devices; due to their smaller size, lower energy use, and longer lifespan, they can be used as definitive treatment. These devices are associated with a lower rate of complications but are more expensive and are less suitable for right ventricle management. The predominant device in the last 3 years has been the HeartMate 3. This device will probably remain the most popular in the coming years because the sale and distribution of another similar model (HeartWare HVAD) was suspended in June 2021 due to an elevated rate of adverse events.13 Our experience with total artificial hearts is negligible due to their extremely complicated implantation and their short durability and numerous complications.14
The outcomes for the patients included in the REGALAD are weighed down by the high percentage of pulsatile-flow and biventricular assist devices, as well as by the participation of centers with highly irregular and sporadic implantation activity, with little experience and a learning curve that remains to be overcome. Nonetheless, the overall survival (74% and 69% at 1 and 2 years) is no worse than that seen in other similar international registries, such as the EUROMACS registry, which included 2268 patients and showed survival rates of 69% at 1 year and 55% at 2 years.12 If we focus on the subgroup of patients with an isolated left continuous-flow dVAD, our outcomes (80% survival at 1 year and 85% at 2 years) are also on a par to those of other international registries such as the INTERMACS, which enrolled 25 551 left continuous-flow dVADs, implanted between 2010 and 2019, and showed survival rates of 82% at 1 year and 73% at 2 years.6
Notably, in our series, no significant differences were seen in survival when patients were compared by the reason for the implantation, in contrast to other registries.6,12 It is no surprise that patients implanted with a dVAD as BTT or as bridge to candidacy, who are younger and have fewer comorbidities, would have better survival rates than those receiving a device as destination therapy. The explanation probably lies precisely in the fact that the vast majority of pulsatile-flow and biventricular devices, which are those with the worst outcomes, were implanted for this objective to treat patients until HTx.15
The complications occurring in these patients continue to be frequent and severe, with some causing death or permanent disability and many requiring hospitalization and worsening of perceived quality of life. These complications are now the main obstacle to the expansion of this therapy.
Stroke, both ischemic and hemorrhagic, is the most dreaded complication due to its sequelae and because it is the leading cause of death in these patients (up to 38% of deaths in our series). Fortunately, with the identification of its risk factors and preventive measures, such as strict control of blood pressure and, of course, with the development of new more hemocompatible dVADs, this complication is significantly less common.
Our registry also shows a progressive decline in device dysfunction. Of these dysfunctions, the most characteristic and severe is pump thrombosis, which occurred in 7% of our patients, particularly with paracorporeal pulsatile pumps, and necessitated device replacement in more than half of cases.
To prevent thromboembolic complications, most of these patients receive dual antiplatelet therapy, which unfortunately favors the development of bleeding events. Particularly typical in the long term is gastrointestinal bleeding, which affected up to 19% of patients in our series. The results of the ARIES HM3 study will show if it is possible to omit antiplatelet agents in selected patients with low thrombotic risk.16
The other most frequent complication are infections; many are similar to those of any postoperative cardiac surgery period, but specific to these patients are infections of the cannulae that exit the skin for paracorporeal pumps and of the drive cable in intracorporeal devices. Once they develop, these infections are difficult to suppress, necessitate the prolonged use of antibiotics, and worsen quality of life.17 As in the other complications, they tend to be less common with the latest-generation devices.
The collaboration between technology and medicine in recent years has permitted the development of increasingly effective and safe mechanical devices that provide long-lasting circulatory support to patients with advanced HF. We need to continue advancing toward simpler devices that can be used in the early phases of HF to facilitate recovery and devices that can be made with hemocompatible materials to decrease the need for anticoagulation, as well as those that can obviate the need for percutaneous cables and adapt to patients’ physiological needs.
The present registry shows that dVADs are already a reality and, although much uncertainty remains concerning their usefulness and indications, their use is already spreading. These devices still require considerable refinement to become a definitive solution for patients with advanced HF.
LimitationsThe main limitations of this registry, in comparison with other international registries, are the small number of patients included and the relatively short follow-up. However, the registry is exhaustive, with the inclusion of all consecutive procedures performed since implantation of these devices began in Spain.
CONCLUSIONSdVADs have become established in Spain in recent years as a useful treatment for selected patients with advanced HF. As in other international registries, the tendency is for the use of intracorporeal continuous-flow left ventricular devices, which are the devices achieving the best outcomes. Complications continue to be frequent and severe and many are associated with the hemocompatibility of the devices.
- -
Durable ventricular assist device are part of the current management of advanced heart failure and are used to provide circulatory support before heart transplant or as definitive treatment in patients with contraindications to the procedure.
- -
We present the characteristics and outcomes of ventricular assist procedures performed in Spain from the first implantation of this type of device.
- -
As in other international registries, the data confirm the trend in recent years toward intracorporeal continuous-flow devices, which are more durable, have lower complication rates, and achieve better survival rates.
None.
AUTHORS’ CONTRIBUTIONSAll authors have contributed equally to the data collection for this work and to the drafting of the final manuscript.
CONFLICTS OF INTERESTM. Gómez-Bueno has received fees for presentations and consultancy work from Abbott; M.D. García-Cosío has received grants for registration fees and travel to conferences from Epycardio; E. Barge-Caballero has received fees for presentations and consultancy work from Abbott; D. Rangel has received grants for registration fees and travel to conferences from Abbott and Medtronic; A. Uribarri has received fees for consultancy work from Abbott; M.A. Castel has received grants for registration fees and travel to conferences from Epycardio and Abbott; P. Codina has received payments for presentations from Abbott; J. González-Costello has received fees for presentations and consultancy work from Abbott. The remaining authors have no conflicts of interest in relation to this work.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2022.07.011