Patients who receive drug-eluting stents (DES) require a regimen of dual antiplatelet therapy (DAPT) consisting of aspirin and an adenosine diphosphate (ADP) receptor antagonist.1 This therapy has been proven superior to anticoagulation therapy in terms of periprocedural ischemic and bleeding complications.2 While aspirin is usually prescribed for life, the optimal duration of ADP-receptor blocker therapy as part of DAPT has not been defined. This lack of evidence is also reflected in diverging recommendations in the guidelines of the European and American Societies of Cardiology. While the European guidelines do not recommend a therapy duration beyond 6 months in patients with stable angina and DES,1 the American guidelines stress that patients with DES that are not at high bleeding risk should receive DAPT for at least 12 months.3
THE PASTNotably, the optimal duration of DAPT was not defined in the bare metal stent era, due to the lack of dedicated randomized controlled clinical trials (RCT) on this topic.4 From 2006, several observational studies showed a higher risk of stent thrombosis with first-generation DES than with bare metal stents.5 Although subsequent, more comprehensive analyses confirmed the overall safety of DES, they also found a time shift with an excess of late stent thrombosis with first-generation DES compared with bare metal stents.6 Stent thrombosis is almost inevitably associated with an acute myocardial infarction and a high mortality rate.7 These observational findings triggered a number of RCTs aiming to assess the optimal duration of DAPT. A recent meta-analysis that included 4 open-label RCTs8–11 found no benefit in terms of ischemic protection but reported an increase in major bleeding with prolonged therapy.12 Other, more recently published open-label RCTs including mainly new-generation DES confirmed that ischemic events were not reduced by prolonged DAPT.13–16
THE PRESENTTwo long-awaited double-blind randomized clinical trials on the duration of DAPT after DES implantation were presented at the Annual Scientific Meeting of the American Heart Association in Chicago in November 2014.
The investigator-initiated ISAR-SAFE trial is the largest and the only double-blind trial evaluating the value of shortening DAPT duration from 12 to 6 months after DES implantation. Due to much lower than expected event rates and slow recruitment, the study was stopped prematurely after inclusion of 4005 (of 6000 planned) patients. In this study, no difference was detected with 6 vs 12 months of DAPT regarding a net clinical endpoint, the composite of death, myocardial infarction, stent thrombosis, stroke, or Thrombolysis in Myocardial Infarction (TIMI) major bleeding. The rates of TIMI major bleeding were similar in both groups. However, after application of newer and more sensitive definitions of bleeding, such as the Bleeding Academic Research criteria (BARC) there was indeed a significant reduction in the bleeding rate with the shorter, 6-month regimen (1.4% vs 2.8%; P=.002). However, the results of the trial should be interpreted carefully in light of the premature termination and much lower than expected event rates.17
The double-blind DAPT-trial included 9961 patients with DES implantation and is by far the largest trial aiming to assess optimal DAPT duration after DES implantation and the only trial that is powered for hard clinical endpoints.18 This trial was designed in response to a request by the US Food and Drug Administration (FDA) and was funded by 8 stent and pharmaceutical manufacturers. In this trial, DAPT with either clopidogrel or prasugrel over 30 months was superior to 12 months regarding the 2 coprimary efficacy endpoints: stent thrombosis (definite and probable according to the Academic Research Consortium [ARC] criteria) and the composite of death, myocardial infarction, or stroke (major adverse cardiac and cerebrovascular events, MACCE). Indeed, the rate of definite stent thrombosis was significantly reduced from 1.2% to 0.3% with prolonged DAPT. The advantage regarding the reduction in MACCE was driven by a reduction in myocardial infarction. Interestingly, in 55% of the patients, the myocardial infarction was not associated with stent thrombosis but occurred outside the stented vascular segment. This suggests protection from plaque rupture in coronary vessels outside the stented area with DAPT (secondary prevention). In the DAPT trial, stent thrombosis and myocardial infarction increased in both study groups within the 3 months after thienopyridine discontinuation. A similar clustering of ischemic events after clopidogrel discontinuation had been suggested earlier in epidemiological studies.19 Whether this merely reflects the continued need for DAPT or a transient platelet hyper-reactivity after thienopyridine discontinuation (an effect known as rebound phenomenon) is not known. Preclinical20 and clinical21 studies examining the rebound phenomenon could not confirm its existence. Based on the reduction in stent thrombosis and myocardial infarction observed in the DAPT trial, should patients receive DAPT for 30 months or even for life? To answer this question, it should be noted that the increase in efficacy occurred at the expense of an increased bleeding risk.18 The rate of Global Use of Strategies to Open Occluded Arteries (GUSTO) moderate or severe bleeding (defined as either intracranial hemorrhage or bleeding that causes hemodynamic compromise and requires intervention or bleeding that requires blood transfusion) was significantly increased from 1.6% to 2.5% with prolonged therapy. Moreover, mortality was increased in patients with prolonged DAPT (2% vs 1.5%; P=.05 at 30 months und 2.3% vs 1.8%; P=.04 at 33 months). The opposing effects on ischemic and bleeding endpoints and the increase in overall mortality with prolonged DAPT hamper any definite recommendation for clinical practice. Both bleeding and ischemic complications are associated with long-term mortality.22 The separation of clinical outcomes in ischemic and bleeding endpoints may be useful for the detection of mechanisms of action. However, to evaluate the overall effects of a therapy for patients, it is important to assess mortality and overall morbidity. This is best reflected in “net” clinical outcome endpoints including both bleeding and ischemic outcomes. The FDA, who initiated the DAPT trial, currently advises that, based on the trial results “health care professionals should not change the way they prescribe these drugs at this time”.23
In this article of Revista Española de Cardiología, de la Torre Hernández et al report on the results of a matched analysis of the ESTROFA-DAPT and ESTROFA-2 registries.24 The authors performed propensity score matching of patients receiving new-generation DES that were assigned to either a 6-month period of DAPT in the ESTROFA-DAPT registry or a 12-month period of DAPT in the ESTROFA-2 registry. A total of 1286 patients in each group were included for analysis. The authors found no significant differences in the primary net clinical endpoint, the composite of death, myocardial infarction, stent thrombosis, revascularization, or major bleeding in patients assigned to either 6- or 12-month DAPT at 12 months after DES implantation. The incidence of stent thrombosis was similar in both groups. There was a trend for lower bleeding events in the 6-month group.24 By showing no reduction in ischemic events but signs of an increased bleeding risk with prolonged therapy, the results are in line with a number of previous RCTs that assessed the value of shortening DAPT duration to 6 and even 3 months.9–11,13,15–17 Despite the inherent limitations of such analyses (mainly residual bias introduced by the nonrandomized design, despite sophisticated statistical methods such as propensity score matching and limited sample size), registry results may better reflect an all-comer population than RCTs using rigorous inclusion and exclusion criteria. The authors should also be congratulated for including patients after implantation of new-generation DES. To define optimal DAPT duration, it is important to reconsider the type of DES that has been implanted. The superiority of new-generation DES to first-generation DES regarding both restenosis and stent thrombosis is well documented.25 In the ISAR-SAFE trial, most patients received a new-generation DES and only 11% received either the first-generation sirolimus-eluting Cypher or paclitaxel-eluting Taxus stent.17 In the DAPT trial, only FDA-approved DES were allowed (either the sirolimus-eluting Cypher stent, the paclitaxel-eluting Taxus stent, the zotarolimus-eluting Endeavor stent or the everolimus eluting Xience/Promus stent). Although there was no significant interaction for DES type and the occurrence of stent thrombosis in the DAPT trial, the Taxus stent contributed 27% of the patients but 57% of stent thrombosis. These and other data suggest that newer and safer DES may support the use of a shorter DAPT duration.
Importantly, in the DAPT and ISAR-SAFE trials, the patients were randomized at the time the treatment arms actually started to differ, ie, at 12 and 6 months after DES implantation, respectively. Therefore, patients with recurrent ischemic or bleeding complications after DES implantation were excluded, resulting in a selection bias toward a low-risk cohort. However, randomization at the time of percutaneous coronary intervention (PCI) would have diluted the treatment effect: most events occurred early after PCI, when treatment was actually the same in both arms.
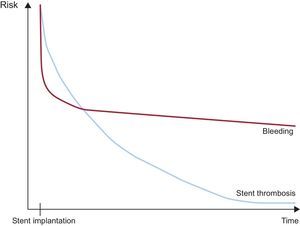
THE FUTURENo recommendation of a single DAPT duration for all patients undergoing DES implantation can be derived on the basis of current evidence. The DAPT trial showed that prolonging DAPT for up to 30 months and probably even longer may prevent myocardial infarction and stent thrombosis. However, that trial also showed that this strategy may cause harm by increasing bleeding and mortality.18 In contrast, a number of RCTs have shown that shortening DAPT duration to 6 months is safe, especially after new-generation DES implantation, and that this strategy does not expose the patient to an increased bleeding risk.9–11,13,15–17 Further analyses will be required to identify those subgroups that either benefit from a shorter or longer duration. The individualization of DAPT duration according to the patient's ischemic and bleeding risk profile will require further analyses to identify the optimal time point of stopping DAPT for individual patients (Figure). At the last Annual Scientific Session of the American College of Cardiology, the results of the PEGASUS-TIMI 54 trial were presented.26 In this trial, 21 162 patients who had had a myocardial infarction 1 to 3 years earlier were randomly assigned to 1 of 3 groups: to ticagrelor at a dose of 90mg twice daily, ticagrelor at a dose of 60mg twice daily, or placebo. At a median of 33 months, the primary efficacy end point-the composite of cardiovascular death, myocardial infarction, or stroke-was significantly reduced by 15% and 16% in the 90mg and 60mg ticagrelor groups, respectively. However, the risk of major bleeding was more than doubled with both dose regimens and the overall mortality was not significantly impacted. In other words, the trade-off for the absolute 1.2% to 1.3% reduction in ischemic complications (mostly myocardial infarctions) was an absolute 1.2% to 1.5% increase in the risk of major bleeding depending on the ticagrelor dose. This is the most recent example of the difficulty of interpreting data from trials that show symmetrical findings for ischemic complications and bleeding that move in opposite directions while mortality is not affected. Nevertheless, the findings of the PEGASUS-TIMI 54 trial may serve to add ticagrelor to the arsenal of antiplatelet drugs that might be used for longer than 1 year in specific subgroups of patients characterized by an excessive risk of ischemic complications and lower risk of bleeding.
Graphic presentation of the risk of stent thrombosis and bleeding over time in patients with drug-eluting stent implantation and dual antiplatelet therapy. The relationship between the risk of stent thrombosis and bleeding over time should be taken into account when defining the optimal duration of dual antiplatelet therapy.
None declared.