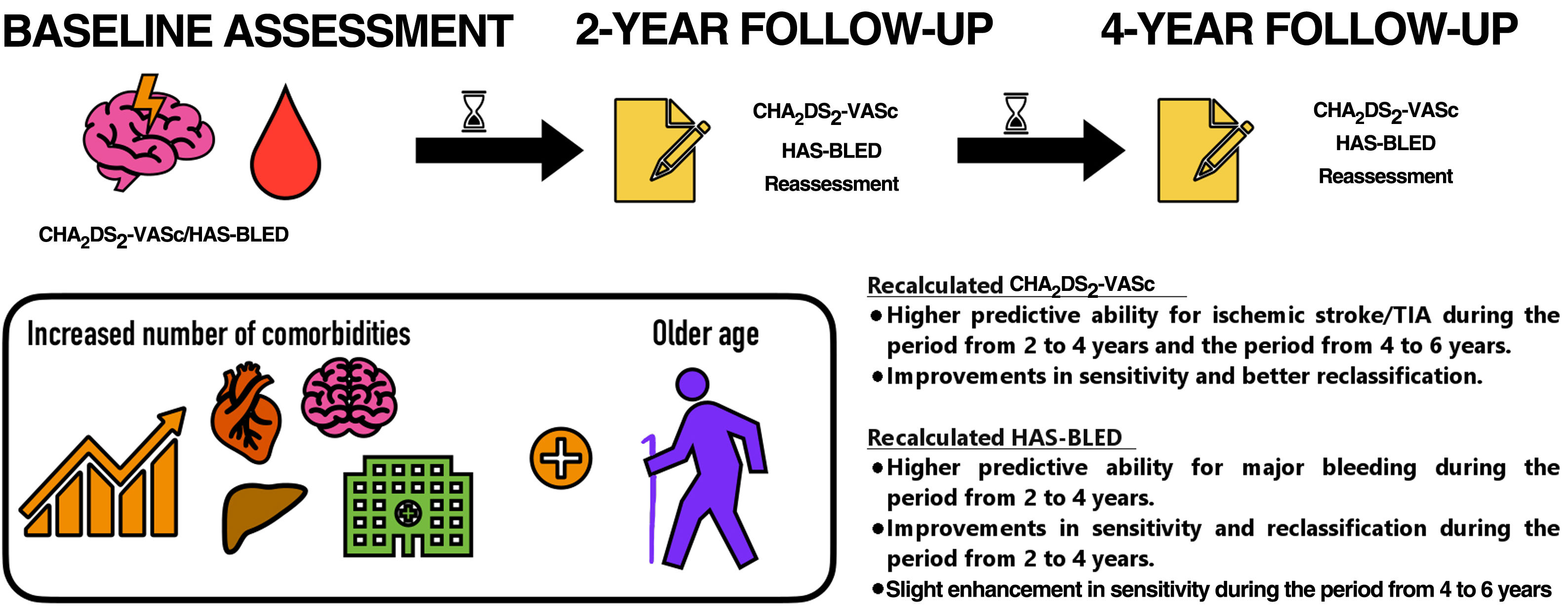
Stroke and bleeding risks in atrial fibrillation (AF) are often assessed at baseline to predict outcomes years later. We investigated whether dynamic changes in CHA2DS2-VASc and HAS-BLED scores over time modify risk prediction.
MethodsWe included patients with AF who were stable while taking vitamin K antagonists. During a 6-year follow-up, all ischemic strokes/transient ischemic attacks (TIAs) and major bleeding events were recorded. CHA2DS2-VASc and HAS-BLED were recalculated every 2-years and tested for clinical outcomes at 2-year periods.
ResultsWe included 1361 patients (mean CHA2DS2-VASc and HAS-BLED 4.0±1.7 and 2.9±1.2). During the follow-up, 156 (11.5%) patients had an ischemic stroke/TIA and 269 (19.8%) had a major bleeding event. Compared with the baseline CHA2DS2-VASc, the CHA2DS2-VASc recalculated at 2 years had higher predictive ability for ischemic stroke/TIA during the period from 2 to 4 years. Integrated discrimination improvement (IDI) and net reclassification improvement (NRI) showed improvements in sensitivity and better reclassification. The CHA2DS2-VASc recalculated at 4 years had better predictive performance than the baseline CHA2DS2-VASc during the period from 4 to 6 years, with an improvement in IDI and an enhancement of the reclassification. The recalculated HAS-BLED at 2-years had higher predictive ability than the baseline score for major bleeding during the period from 2 to 4 years, with significant improvements in sensitivity and reclassification. A slight enhancement in sensitivity was observed with the HAS-BLED score recalculated at 4 years compared with the baseline score.
ConclusionsIn AF patients, stroke and bleeding risks are dynamic and change over time. The CHA2DS2-VASc and HAS-BLED scores should be regularly reassessed, particularly for accurate stroke risk prediction.
Keywords
Atrial fibrillation (AF) is the most common arrhythmia and carries a significant risk of stroke and thromboembolism; hence, oral anticoagulation (OAC) is recommended in guidelines as a fundamental pillar for its appropriate management.1,2
Nonetheless, OAC use requires stroke risk stratification, which should also be balanced with bleeding risk assessment, using clinical risk scores such as the CHA2DS2-VASc and HAS-BLED scores,3,4 as recommended by most clinical practice guidelines. Unfortunately, such risk assessment is usually performed at baseline, as a one-off evaluation that views risk as static process, and these values are usually applied to predict clinical outcomes that occur many years later.
However, risk factors for stroke and bleeding are not static over time, but are rather dynamic in nature, and most AF patients will develop at least 1 new risk factor before presenting with a thromboembolic event.5 These dynamic changes may increase the scores of the CHA2DS2-VASc and HAS-BLED assessed initially, thus modifying the absolute risk (and rate) of stroke and bleeding. Hence, the baseline estimated risk of these outcomes may worsen, due to aging and other incident comorbidities.6,7
The aim of the present study was to investigate whether dynamic evaluation of CHA2DS2-VASc and HAS-BLED scores over time would improve risk prediction in a cohort of AF patients taking OAC therapy and who were prospectively enrolled in the Murcia AF Project.
METHODSFrom May 1, 2007, to December 1, 2007, we consecutively included adult outpatients with permanent or paroxysmal AF who were stable on OAC therapy with vitamin K antagonists in the preceding 6 months (ie, INR from 2-3, so the time in therapeutic range at enrolment was 100%) attending our anticoagulation clinic. Patients with prosthetic valves or rheumatic AF were excluded, as well as those who had had an acute coronary syndrome, stroke, surgical interventions, hospitalizations, or any hemodynamic instability in the previous 6 months.
The study protocol was approved by the Ethics Committee of Hospital General Universitario Morales Meseguer and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All patients gave informed consent for participation.
CHA2DS2-VASc and HAS-BLED scoresAt inclusion, a complete clinical history was recorded. The baseline CHA2DS2-VASc and HAS-BLED scores were calculated for each patient at study entry according to their original definitions (appendix A of the supplementary data).3,4 Based on the characteristics of the cohort at entry (see inclusion criteria), the baseline labile INR criterion was quantified as 0 in all patients.
During the follow-up, all risk variables of the scores were reassessed again and the CHA2DS2-VASc and HAS-BLED were recalculated at the end of each 2-year period. Their predictive abilities for clinical outcomes were tested against the baseline scores in 2-year intervals (from baseline to year 2, from year 2 to 4, and from year 4 to 6). Patients were censored if they died between periods of comparisons. For example, if a patient died at 3 years, data were used to compare the baseline vs recalculated scores at 2 years, but not for the following comparisons since their comorbidities could not be obtained again at 4 years. There were no missing data on clinical variables used to calculate scores.
Follow-up and clinical outcomesFollow-up was performed according to the standard of care at each routine visit to the outpatient anticoagulation clinic or visits for anticoagulation control. If the patient never attended these visits, medical records and telephone calls were used to obtain the information needed and vital status, with no specific interventions and no specific visits for study purposes. Follow-up was extended for 6 years. During this period, all adverse events were recorded. No patient was lost to follow-up.
For the present study, the primary endpoints were ischemic strokes/transient ischemic attacks (TIAs) and major bleeding. Definitions of the primary endpoints are detailed in appendix B of the supplementary data. The investigators identified, confirmed, and recorded all clinical outcomes.
Statistical analysisQuantitative variables are presented as mean±standard deviation or median and interquartile range [IQR], as appropriate after testing for normality by the Kolmogorov-Smirnov test. Categorical variables are expressed as frequencies and percentages. The Pearson chi-square test was used to compare proportions. The Student t or Mann-Whitney U tests were used to compare continuous and categorical variables, as appropriate.
The proportion of patients moving among different risk categories was visualized in an alluvial plot. The predictive ability (expressed as c-indexes) of baseline and dynamic CHA2DS2-VASc and HAS-BLED scores was assessed by receiver operating characteristics (ROC) curves, using the quantitative version of CHA2DS2-VASc and HAS-BLED in all cases. To contrast prognostic accuracy, we compared ROC curves using the method of DeLong et al.8 The net reclassification improvement (NRI) and integrated discriminatory improvement (IDI) for the baseline against the dynamic scores were also calculated as described by Pencina et al.9
We estimated the clinical usefulness and the net benefit of the original (baseline) scores in comparison with the dynamic scores by using decision curve analysis (DCA), as proposed by Vickers et al.10 The DCA shows the clinical usefulness of each new model based on a continuum of potential thresholds for adverse events (x-axis) and the net benefit of using the model to stratify patients at risk (y-axis) relative to assuming that no patient will have an adverse event. In this study, the prediction models are represented by color lines. The farther the prediction models from the dashed black line (ie, assume all adverse events) and the horizontal black line (ie, assume no adverse events), the higher the net clinical benefit.
A P value <.05 was accepted as statistically significant. Statistical analyses were performed using SPSS v. 25.0 (SPSS, United States), Origin v. 2022, (OriginLab Corporation, United States), MedCalc v. 16.4.3 (MedCalc Software bvba, Belgium), STATA v. 16.0 (Stata Corp, College Station, United States), and PredictABEL package for R v. 4.1.2 for Windows.
RESULTSThis study included 1361 patients, of which 693 (50.9%) were women, with a median age of 76 [IQR 71-81] years. A summary of other baseline characteristics is shown in table 1.
Baseline clinical characteristics
| N=1361 | |
|---|---|
| Demographic data | |
| Male sex | 663 (48.7) |
| Age, y | 76 [71-81] |
| Comorbidities | |
| Hypertension | 1116 (82.0) |
| Diabetes mellitus | 363 (26.7) |
| Heart failure | 429 (31.5) |
| History of stroke/TIA/thromboembolism | 267 (19.6) |
| Renal impairment | 144 (10.6) |
| Coronary artery disease | 255 (18.7) |
| Hypercholesterolemia | 443 (32.5) |
| Current smoking habit | 210 (15.4) |
| Current alcohol consumption | 50 (3.7) |
| History of previous bleeding | 113 (8.3) |
| Concomitant malignant disease | 105 (7.7) |
| Concomitant treatment | |
| Amiodarone | 77 (5.7) |
| Digoxin | 272 (20.0) |
| Calcium antagonist | 339 (25.0) |
| Beta-blockers | 470 (34.5) |
| Statins | 331 (24.3) |
| Diuretics | 614 (45.1) |
| Antiplatelet therapy | 243 (18.0) |
| ACE inhibitors/ARBs | 717 (52.7) |
ACE inhibitors, angiotensin-converting-enzyme inhibitors; ARBs, angiotensin II receptor blockers; TIA, transient ischemic attack.
Data are expressed as No. (%), or median [interquartile range].
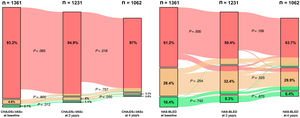
The mean baseline CHA2DS2-VASc was 4.0±1.7, whereas the mean CHA2DS2-VASc scores at 2 years and at 4 years were 4.3±1.6 and 4.5±1.6, respectively. Compared with baseline CHA2DS2-VASc, CHA2DS2-VASc at 2 years and at 4 years were significantly higher (both P <.001). CHA2DS2-VASc at 4 years was also significantly higher than CHA2DS2-VASc at 2 years (P <.001). Accordingly, the proportions of participants categorized as low, intermediate or high risk by CHA2DS2-VASc score at baseline were 2.1% (28), 4.8% (65) and 93.2% (1268), respectively. Of the 1062 patients who were alive at 4 years, 8 (0.8%) were categorized as low risk, 24 (2.3%) as intermediate risk and 1030 (97.0%) as high risk by recalculated CHA2DS2-VASc score (figure 1).
Alluvial plots showing baseline stroke and bleeding risk stratification (baseline CHA2DS2-VASc and HAS-BLED), and reclassification into different risk categories during follow-up. Green, low risk of stroke or bleeding; orange, moderate risk of stroke or bleeding; red, high risk of stroke or bleeding.
The mean HAS-BLED score was 2.7±1.2 at baseline, 2.9±1.3 at 2 years and 3.1±1.2 at 4 years. Compared with baseline HAS-BLED, HAS-BLED at 2 years and at 4 years were significantly higher (both P <.001). HAS-BLED at 4 years was also significantly higher than HAS-BLED at 2 years (P <.001). At baseline, the proportions of participants categorized as low, moderate or high risk by HAS-BLED score were 10.4% (n=141), 28.4% (n=387) and 61.2% (n=833), respectively. Of the 1062 patients who were alive at 4 years, 68 (6.4%) were categorized as low risk, 318 (29.9%) as moderate risk, and 676 (63.7%) as high risk by the recalculated HAS-BLED score (figure 1).
Primary outcomes and predictive abilitiesDuring 6 years of follow-up, 156 (11.5%) patients had an ischemic stroke/TIA and 269 (19.8%) had a major bleeding event. In addition, 472 (34.68%) patients died. The period with the highest incidence rates for ischemic stroke/TIA and major bleeding was from 2 to 4 years, which showed a significantly higher incidence rate ratio compared with the others for major bleeding outcomes. Complementary information on clinical outcomes and incidences among the different periods is shown in appendix C and table 1 of the supplementary data.
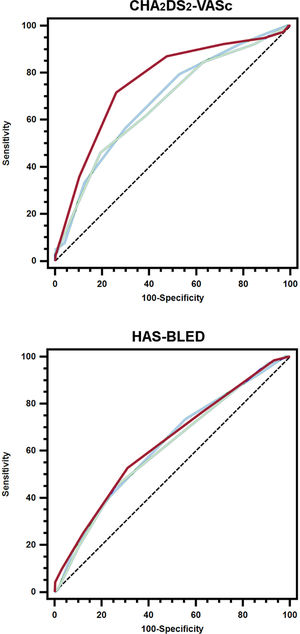
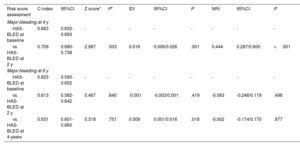
The probability of experiencing an ischemic stroke/TIA increased with the use of the recalculated CHA2DS2-VASc, with the 4-year assessment showing the highest higher hazard ratio even after adjustment (table 2 of the supplementary data). Regarding the predictive performance for ischemic stroke/TIA, the CHA2DS2-VASc recalculated at 2 years had significantly higher predictive ability during the period from 2 to 4 years compared with the baseline CHA2DS2-VASc, whereas IDI and NRI showed improvements in sensitivity and reclassification (table 2). Similarly, the CHA2DS2-VASc recalculated at 4 years yielded significantly better predictive performance for ischemic stroke/TIA during the period from 4 to 6 years compared with the baseline CHA2DS2-VASc (figure 2). Again, IDI reported improvement in sensitivity and there was an enhancement of the reclassification ability based on NRI (table 2).
C-indexes, c-indexes comparison, IDI and NRI of the dynamic CHA2DS2-VASc compared with the original score
| Risk score assessment | C-index | 95%CI | Z score* | P* | IDI | 95%CI | P | NRI | 95%CI | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Ischemic stroke/TIA at 4 y | ||||||||||
| CHA2DS2-VASc at baseline | 0.604 | 0.576-0.631 | - | - | - | - | - | - | - | - |
| CHA2DS2-VASc at 2 y | 0.701 | 0.675-0.727 | 3.628 | <.001 | 0.014 | 0.007/0.020 | <.001 | 0.677 | 0.427/0.926 | <.001 |
| Ischemic stroke/TIA at 6 y | ||||||||||
| CHA2DS2-VASc at baseline | 0.682 | 0.653-0.710 | - | - | - | - | - | - | - | - |
| CHA2DS2-VASc at 2 y | 0.670 | 0.640-0.697 | 0.889 | .374 | 0.002 | -0.001/0.004 | .211 | 0.209 | -0.092/0.511 | .173 |
| CHA2DS2-VASc at 4 y | 0.761 | 0.734-0.786 | 2.234 | .026 | 0.030 | 0.016/0.044 | <.001 | 0.757 | 0.496/1.018 | <.001 |
95% CI, 95% confidence interval; IDI, integrated discrimination improvement; NRI, net reclassification improvement.
Receiver operating characteristic curves of the baseline and dynamic (at 2 and 4 years) CHA2DS2-VASc/HAS-BLED scores for the prediction of ischemic stroke/TIA or major bleeding. Blue line, baseline scores; green line, scores recalculated at 2-years; red line, scores recalculated at 4-years.
As for stroke, the probability of major bleeding was higher with the use of the recalculated HAS-BLED score (table 3 of the supplementary data). At 2 years, the recalculated HAS-BLED score also showed a significantly higher predictive ability than the baseline HAS-BLED score for major bleeding events during the period from 2 to 4 years. IDI and NRI demonstrated significant improvements compared with the baseline HAS-BLED score (table 3). For major bleeds from 4 to 6 years, the c-index of the HAS-BLED score recalculated at 4 years was numerically higher but not statistically significant compared with baseline. There was a slight enhancement in sensitivity as assessed by the IDI but was not significantly better when reclassified by NRI (table 3).
C-indexes, c-indexes comparison, IDI and NRI of the dynamic HAS-BLED compared with the original score
| Risk score assessment | C-index | 95%CI | Z score* | P* | IDI | 95%CI | P | NRI | 95%CI | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Major bleeding at 4 y | ||||||||||
| HAS-BLED at baseline | 0.663 | 0.632-0.693 | - | - | - | - | - | - | - | - |
| vs HAS-BLED at 2 y | 0.709 | 0.680-0.738 | 2.987 | .003 | 0.016 | 0.006/0.026 | .001 | 0.444 | 0.287/0.600 | <.001 |
| Major bleeding at 6 y | ||||||||||
| HAS-BLED at baseline | 0.623 | 0.593-0.652 | - | - | - | - | - | - | - | - |
| vs HAS-BLED at 2 y | 0.613 | 0.582-0.642 | 0.467 | .640 | -0.001 | -0.003/0.001 | .419 | -0.063 | -0.246/0.119 | .498 |
| vs HAS-BLED at 4-years | 0.631 | 0.601-0.660 | 0.318 | .751 | 0.009 | 0.001/0.016 | .018 | -0.002 | -0.174/0.170 | .977 |
95% CI, 95% confidence interval; IDI, integrated discrimination improvement; NRI, net reclassification improvement.
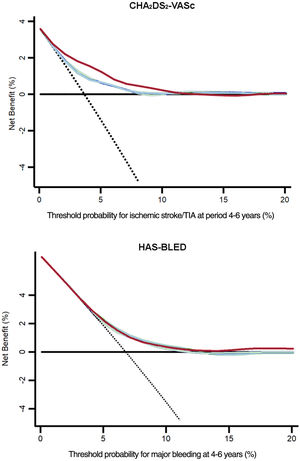
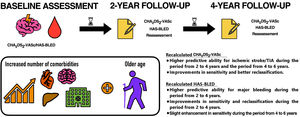
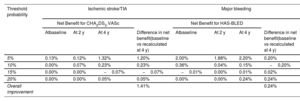
DCAs would help in the estimation of patients who will experience any of the primary endpoints, based on the predictions of the baseline risk scores in comparison with those recalculated at 2 and 4 years of follow-up. In figure 3, the prediction models that are the farthest away from the dashed black line (ie, assume all events) and the horizontal black line (ie, assume none event) had the highest net benefit. DCAs demonstrated that using the dynamic CHA2DS2-VASc and HAS-BLED scores was clinically useful and provided an overall improvement in the net benefit for the prediction of ischemic stroke/TIA and major bleeding, respectively. A detailed estimate of the net benefit at each threshold of probabilities from 0% to 20% is shown in table 4. A summary of the study findings is shown in figure 4.
Decision curve analysis of the baseline and dynamic (at 2 and 4 years) CHA2DS2-VASc/HAS-BLED scores for ischemic stroke/TIA or major bleeding. Solid black line, assumes all patients will suffer an adverse event; dashed black line, assumes no patient will suffer an adverse event; blue line, baseline scores; green line, scores recalculated at 2-years; red line, scores recalculated at 4-years.
Net benefits for baseline, 2-year, and 4-year CHA2DS2-VASc/HAS-BLED at different threshold probabilities
| Threshold probability | Ischemic stroke/TIA | Major bleeding | ||||||
|---|---|---|---|---|---|---|---|---|
| Net Benefit for CHA2DS2-VASc | Net Benefit for HAS-BLED | |||||||
| Atbaseline | At 2 y | At 4 y | Difference in net benefit(baseline vs recalculated at 4 y) | Atbaseline | At 2 y | At 4 y | Difference in net benefit(baseline vs recalculated at 4 y) | |
| 5% | 0.13% | 0.12% | 1.32% | 1.20% | 2.00% | 1.88% | 2.20% | 0.20% |
| 10% | 0.00% | 0.07% | 0.23% | 0.23% | 0.36% | 0.04% | 0.15% | −0.20% |
| 15% | 0.00% | 0.00% | −0.07% | −0.07% | −0.01% | 0.00% | 0.01% | 0.02% |
| 20% | 0.00% | 0.00% | 0.05% | 0.05% | 0.00% | 0.00% | 0.24% | 0.24% |
| Overall improvement | 1.41% | 0.24% | ||||||
In this real-world cohort study, our principal findings are as follows: a) consecutive reassessment of stroke and bleeding risks through CHA2DS2-VASc and HAS-BLED scores demonstrated a significantly higher predictive ability and net benefit compared with the baseline scores; and b) the risks of stroke and bleeding in AF patients are dynamic and change during follow-up.
The incidence of new risk factors and the temporal trends in the CHA2DS2-VASc score have been previously investigated. Chao et al.5 included 14 606 AF patients who did not receive antiplatelet agents or OACs, with a baseline CHA2DS2-VASc score of 0 (men) or 1 (women) and with incident risk factors, and observed a dynamic increase in the CHA2DS2-VASc score.5 Indeed, this often translated into a dynamic change in the risk category.6,11,12 A recent study demonstrated that changes in CHA2DS2-VASc score over time were associated with the incidence of stroke, thus indicating that stroke risk is not static.13 In fact, the CHA2DS2-VASc score of AF patients would increase as patients become older and they accumulate more comorbidities, and we observed a significant progressive increase in the CHA2DS2-VASc score in accordance with previously published literature,5,6,11 again emphasizing the dynamic nature of thromboembolic risk.
Although the concept or risk reassessment is well accepted.5,14,15 prior clinical guidelines did not provide clear and concise recommendations on this issue. The landscape has changed with the publication of the latest international guidelines, which emphasize the need for dynamic assessment, at least annually, of thromboembolic risk.1,2,16–19 However, the time interval in which thromboembolic risk should be reassessed is controversial. In a study published by Chao et al.,5 the CHA2DS2-VASc score in patients initially classified as low risk increased by approximately 12.1%/y. The authors therefore suggest that, among low-risk patients, stroke risk should be reassessed every 4 months, with the aim of prescribing OAC therapy in those with an increased CHA2DS2-VASc score.
Regarding bleeding risk, guidelines recommend the use of HAS-BLED for the evaluation of bleeding risk, suggesting its frequent reassessment with particular attention to modifiable bleeding risk factors in patients with a high bleeding risk (HAS-BLED ≥3).1,2,16,18–21 Although the dynamic nature of the variables associated with bleeding is well accepted, few studies have specifically investigated this. As with stroke risk, bleeding risk assessment is often done at baseline only, at the beginning of OAC therapy, while bleeding events can be observed many years later. This may reflect that bleeding risk assessment has been subject to misuse and misinterpretation, and modifiable bleeding risk factors should be addressed as part of a holistic approach to AF patient assessment and management.22 Bleeding risk stratification should be used to flag patients with a high risk of severe bleeding for a more careful and closer follow-up to manage modifiable factors and reduce the potential risk of a major bleeding event. This approach has been prospectively tested in the mAFA-II trial, where the intervention arm using HAS-BLED had a lower risk of major bleeding at 1 year and an increase in OAC use compared with usual care, which showed higher bleeding and a decline in OAC use.23
One study that focused on the dynamic nature of bleeding risk found that dynamic assessments of HAS-BLED had a better risk predictive ability than baseline assessment alone for the prediction of major bleeding.24 These results are similar to that observed in our study, where the predictive ability of the HAS-BLED score calculated at 2 years was significantly better than the baseline assessment. Although there were no significant differences between the dynamic HAS-BLED at 4 years and the baseline score, the dynamic HAS-BLED showed a slight improvement in sensitivity, and the overall clinical usefulness and net benefit of the dynamic HAS-BLED scores were still higher.
Overall, our results reinforce those of previous studies since the use of the dynamic CHA2DS2-VASc and HAS-BLED scores was associated with a higher net benefit and therefore with increased clinical usefulness than the baseline scores. Even though dynamic evaluation and reassessment of stroke and bleeding risks are currently widely accepted, many international clinical practice guidelines for AF patients do not as yet include clear and concise recommendations on how to perform and address this dynamic risk monitoring. The 2021 Asia Pacific Heart Rhythm Society AF guidelines provide recommendations on the dynamic nature of risk in patients with AF, whereby frequent reassessment of patients with AF is suggested, using the CHA2DS2-VASc and HAS-BLED scores.2,25
In our study, we show that changes in the overall score of CHA2DS2-VASc also corresponded to variations in the risk category of AF patients, showing an evolutionary increase in the proportion of patients classified within the high-risk group, to the detriment of a decrease in the proportion of low- and moderate-risk patients. For this reason, periodic reassessment of stroke risk is of particular interest in patients classified as low risk by a baseline CHA2DS2-VASc score (ie, 0 in men; 1 in women) given that OAC is not required in these patients but the progression of aging and new risk factors/comorbidities would change the overall CHA2DS2-VASc score, and therefore OAC may become indicated.15,26,27 Although there is special clinical interest in reassessment of the CHA2DS2-VASc score to reconsider the decision to initiate OAC in these patients initially classified as low-risk, it is also important to reevaluate the risk of stroke in high-risk AF patients already receiving OAC. Indeed, stroke rates can vary significantly between patients with a CHA2DS2-VASc from 3 to 9, even though all of them are in the same high-risk category, and not all stroke risk factors have the same impact.28 Additionally, several risk factors for stroke are also risk factors for bleeding, hence modifiable risk factors could be identified to be managed and reduced appropriately in high-risk patients.
LimitationsThis study has some limitations. First, it is limited by its Caucasian-based population and single-center design. Second, although patients with prosthetic valves were excluded, no data were available on other valvular diseases that may have an impact on adverse events in these patients with AF. Third, during the previous 6 months after entry, all patients were stable with vitamin K antagonists (INR 2.0-3.0) and had no adverse events or hemodynamic instability to ensure homogeneity, since these factors may have an impact on baseline risk and subsequent clinical outcomes. As a result, our cohort may have a lower baseline thromboembolic and hemorrhagic risk. This may have interfered with the baseline CHA2DS2-VASc and HAS-BLED scores, underestimating their predictive ability in comparison with the dynamic estimation of risk. These strict selection criteria may not reflect typical clinical practice, but we believe that this initial homogenization of the population limits the possibility that certain variables that generate instability acted as confounding factors, and the long follow-up under standard care make this cohort suitable. Some ischemic strokes that occurred during follow-up may be caused by noncardioembolic reasons, and these have not been investigated in detail. However, participants were carefully followed up and all events (even very early events) were recorded.
We acknowledge that all together, these factors limit the generalizability of the findings to broader and more diverse populations, even those patients under direct-acting OACs, and that the cohort might not be representative of the broader population of AF patients, especially those with higher risk.
Although our dataset was collected prospectively, the baseline assessment and reassessment of risk scores were performed post hoc, which might introduce potential biases. Thus, our results should be interpreted with caution and as hypothesis-generating only. Finally, we were not able to explore the change (Delta’) CHA2DS2-VASc or the Delta HAS-BLED, given that the sample size is limited, and the various (relative short) periods of observation did not allow an adequate evaluation of this metric.
CONCLUSIONSIn AF patients, stroke and bleeding risks are dynamic and change over time with aging and incident comorbidities. The CHA2DS2-VASc and HAS-BLED scores (and clinical risk profiles) should be regularly reassessed, which is particularly necessary for appropriate stroke and bleeding risk prediction.
FUNDINGThis work was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, through the Instituto de Salud Carlos III after independent peer review (research grant: PI17/01375 cofinanced by the European Regional Development Fund) and group CB16/11/00385 from CIBERCV.
ETHICAL CONSIDERATIONSThe study protocol was approved by the Ethics Committee of Hospital General Universitario Morales Meseguer and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All patients gave informed consent for participation. The possible variables of sex and gender have been taken into account in this work in accordance with the SAGER guidelines. In fact, the study is balanced between male and female participants (48.7% men, 51.3% women).
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this work.
AUTHORS’ CONTRIBUTIONSM.J. Serna, J.M. Rivera-Caravaca, and V. Roldán contributed to data collection, performed statistical analyses, and drafted the manuscript. E. Soler-Espejo and R. López-Gálvez critically revised the manuscript. G.Y.H. Lip and F. Marín conceived and supervised the study and critically revised the manuscript. All authors read and approved the final version of the manuscript.
CONFLICTS OF INTERESTG.Y.H. Lip is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally.
J.M. Rivera-Caravaca is a consultant for Idorsia Pharmaceuticals LTD.
The remaining authors have nothing to disclose.
- -
Atrial fibrillation (AF) is associated with a high risk of stroke and thromboembolism.
- -
Risk assessment in AF is usually performed at baseline, as a one-off evaluation considering risk as a static process.
- -
Stroke and bleeding risk are dynamic, which may increase the initial CHA2DS2-VASc and HAS-BLED scores.
- -
Both the CHA2DS2-VASc and HAS-BLED scores were significantly higher at 2 and 4 years.
- -
The CHA2DS2-VASc score recalculated at 2 and 4 years had significantly higher predictive ability than the baseline score for ischemic stroke/TIA during the periods from 2 to 4 years and from 4 to 6 years.
- -
The HAS-BLED recalculated at 2-years showed significantly higher predictive ability than the baseline score for major bleeding during the period from 2 to 4-years.
- -
The dynamic CHA2DS2-VASc and HAS-BLED scores were clinically useful and provided an overall improvement in the net benefit for the prediction of ischemic stroke/TIA and major bleeding.
- -
The CHA2DS2-VASc and HAS-BLED scores should be regularly reassessed.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2024.02.011