Severe tricuspid regurgitation (TR) is associated with poor prognosis when left untreated, and a growing number of studies on transcatheter tricuspid valve repair (TTVr) have been published over the last few months.
MethodsWe performed a comprehensive systematic review of published literature providing clinical data on TTVr for patients with significant TR. Early and mid-term clinical and echocardiographic outcomes were evaluated. Risk ratios (RR) or mean differences (MD) were obtained when comparing pre- and postprocedural data. A sensitivity analysis was also performed according to the main approach for repair (edge-to-edge vs annuloplasty).
ResultsA total of 19 studies (all observational or single-arm trials) and 991 patients who underwent isolated TTVr were included. Thirty-day mortality and stroke rates were 2.8% and 0.2%, respectively. Pooled random-effects resulted in a significant reduction of ≥ severe TR (RR, 0.33; 95%CI, 0.26-0.42; P < .001), vena contracta width (MD, 5.9mm; 95%CI, 4-7.9; P <.001), right ventricular end-diastolic diameter (MD, 3.5mm; 95%CI, 2.5-4.5; P <.001), and New York Heart Association (NYHA) class III or IV at last follow-up (RR, 0.32; 95%CI, 0.27-0.37; P <.001). Bleeding complications and residual ≥ severe TR were numerically higher in the annuloplasty-like group compared with edge-to-edge repair (13.3% vs 3.8% for bleeding and 40.4% vs 27.9% for residual severe TR).
ConclusionsAmong 991 patients comprising the early experience for several TTVr devices, there was a statistically significant reduction in ≥ severe TR, NYHA class III-IV, vena contracta width and right ventricular end-diastolic diameter after TTVr. Thus far, the edge-to-edge approach seems to be associated with a better safety profile.
Keywords
Tricuspid regurgitation (TR) is the second most frequent regurgitant valvular heart disease in the United States, only surpassed in prevalence by mitral regurgitation.1 Additionally, due to the increase in life expectancy for patients with left valvular heart disease and in those with right and/or left ventricular dysfunction, there will likely be an increment in the prevalence of TR in the upcoming decades.
The prognosis of untreated TR remains poor2,3 and, if left significant, it may lead to gradual annular and right ventricular (RV) dilatation and intractable RV heart failure. However, isolated tricuspid valve surgery is rarely performed, as it is associated with the highest surgical risk among all valve procedures in contemporary practice, with mortality rates close to 10%.4,5 Indeed, the paucity of robust surgical data has led to a scarcity of tricuspid specific surgical risk score assessment (ie, Society of Thoracic Surgeons [STS]) compared with their mitral and aortic valve counterparts.
The surgical results, along with the large comorbidity burden of TR patients, has led to the implementation of less invasive transcatheter techniques aiming to repair the tricuspid valve, mainly by means of leaflet transcatheter edge-to-edge (TEER) approximation and percutaneous annuloplasty-like techniques. A growing number of studies on transcatheter tricuspid valve repair (TTVr) have been published over the last few months, and a summary of the main results seems necessary. In this systematic review and meta-analysis, we aimed to provide updated data on the clinical outcomes observed for patients with significant TR undergoing TTVr, providing pre- and postprocedural comparisons for clinical and echocardiographic features.
METHODSA comprehensive systematic review of published literature providing clinical data on TTVr for patients with significant TR was performed in accordance with the guidance and the reporting items specified on the Preferred Reported Items for Systematic Reviews and Meta-Analysis (PRISMA) statement6 and the guidance on conducting systematic reviews of observational studies.7 The original study protocol was registered on the PROSPERO platform.
A computerized search was performed of the PubMed and EMBASE databases to identify any relevant entry, as well as a manual search of the references in primary studies (backward snowballing). Reviews, meta-analyses, and editorials were also checked to identify potentially eligible studies.
The following keywords or terms were used: “tricuspid repair” and “tricuspid valve intervention”. The databases were last accessed on 21 April 2022, and the studies were included if they were published in English. Eligible studies were those of original design reporting on clinical outcomes after TTVr and including at least 5 patients. If the same patient population was included in several manuscripts, only the study with the largest sample size and longest available follow-up was included in the present analysis. For studies including patients undergoing simultaneous transcatheter mitral and tricuspid valve repair, only those reporting data separately for tricuspid repair recipients were included in the main manuscript. A subanalysis of studies reporting on simultaneous mitral and tricuspid repair is available in the . Studies reporting on devices that are no longer under clinical use or evaluation were also excluded.
Data were extracted using a standardized data abstraction form. Clinical characteristics, as well as in-hospital and/or 30-day and mid-term outcomes were collected as reported by authors. Two investigators (A.A and I.P.) conducted the literature search, selection, and data extraction in duplicate. Any discrepancies between them were resolved by a third investigator (P.A.).
No approval by an ethics committee was needed to perform this study.
EndpointsThe outcomes evaluated in the meta-analysis were as follows: a) in-hospital/30-day complications (all-cause mortality, stroke, life-threatening/major bleeding, conversion to surgery), b) technical success, postprocedural rate of ≥ severe TR, postprocedural reduction in vena contracta width and 30-day changes in RV end-diastolic diameter; and c) mid-term outcomes (mortality, heart failure hospitalization, and New York Heart Association [NYHA] functional class). Pooled estimates comparing outcomes before and 30-days after intervention were performed for TR severity (TR ≥ severe and vena contracta width), functional class (NYHA class III or IV), and RV remodeling (basal RV diameter).
Statistical analysisDescriptive characteristics are presented as mean (standard deviation) for continuous variables and frequencies and percentages for categorical variables, as reported by authors. Risk ratio (RR) or mean difference (MD) and 95% confidence intervals (95%CI) were obtained for the following endpoints comparing pre- and postprocedural 30-day data: ≥ severe TR grade, NYHA class III-IV, vena contracta width, and RV end-diastolic diameter. Consistency across studies was assessed with the I2 index, which takes values between 0% and 100%, with values of 25% typically suggesting low, 50% moderate, and 75% wide heterogeneity.8 A random-effects model was performed to obtain pooled estimates. Publication bias assessment was carried out with the Egger regression for all endpoints, as well as funnel plot visual inspection.
For the remaining characteristics and study outcomes, global values are reported as weighted means (95%CI) or frequencies (percentages). The formula derived from9 was used to calculate means and standard deviation when medians and interquartile ranges were provided. Weighted means were calculated according to the total number of patients in each study (weight=n).
A subanalysis of TTVr systems according to the mechanism of valve repair (transcatheter edge-to-edge repair and annuloplasty-based systems) was performed, as well as a subanalysis of studies including patients undergoing concomitant percutaneous mitral valve repair. The analyses were performed using STATA software (v14.0; StataCorp, Unites States) and Review Manager version 5.4 (The Nordic Cochrane Center, The Cochrane Collaboration, United States).
RESULTSStudy selectionThe PubMed and EMBASE searches identified 10 272 and 11 205 records, respectively, yielding 19 376 records whose titles and abstracts were reviewed after exclusion of duplicates. Of those, the full texts of 41 articles were selected and assessed. Finally, 19 studies fulfilled the inclusion criteria and were deemed eligible for the analysis: 14 for transcatheter edge-to-edge techniques,10–23 and 5 for annuloplasty-like systems.24–28 The PRISMA flow-diagram is shown in figure 1. All studies were observational or single-arm trials. The characteristics of the selected studies are summarized in table 1.
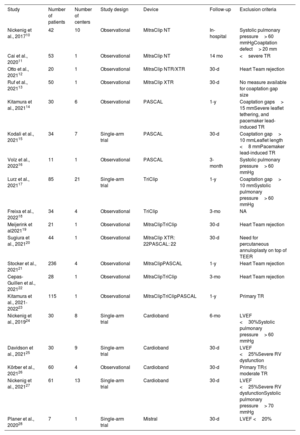
Characteristics of the included studies for transcatheter tricuspid valve repair
| Study | Number of patients | Number of centers | Study design | Device | Follow-up | Exclusion criteria |
|---|---|---|---|---|---|---|
| Nickenig et al., 201710 | 42 | 10 | Observational | MitraClip NT | In-hospital | Systolic pulmonary pressure> 60 mmHgCoaptation defect> 20 mm |
| Cai et al., 202011 | 53 | 1 | Observational | MitraClip NT | 14 mo | <severe TR |
| Otto et al., 202112 | 20 | 1 | Observational | MitraClip NTR/XTR | 30-d | Heart Team rejection |
| Ruf et al., 202113 | 50 | 1 | Observational | MitraClip XTR | 30-d | No measure available for coaptation gap size |
| Kitamura et al., 202114 | 30 | 6 | Observational | PASCAL | 1-y | Coaptation gaps> 15 mmSevere leaflet tethering, and pacemaker lead-induced TR |
| Kodali et al., 202115 | 34 | 7 | Single-arm trial | PASCAL | 30-d | Coaptation gap> 10 mmLeaflet length <8 mmPacemaker lead-induced TR |
| Volz et al., 202216 | 11 | 1 | Observational | PASCAL | 3-month | Systolic pulmonary pressure> 60 mmHg |
| Lurz et al., 202117 | 85 | 21 | Single-arm trial | TriClip | 1-y | Coaptation gap> 10 mmSystolic pulmonary pressure> 60 mmHg |
| Freixa et al., 202218 | 34 | 4 | Observational | TriClip | 3-mo | NA |
| Meijerink et al202119 | 21 | 1 | Observational | MitraClipTriClip | 30-d | Heart Team rejection |
| Sugiura et al., 202120 | 44 | 1 | Observational | MitraClip XTR: 22PASCAL: 22 | 30-d | Need for percutaneous annuloplasty on top of TEER |
| Stocker et al., 202121 | 236 | 4 | Observational | MitraClipPASCAL | 1-y | Heart Team rejection |
| Cepas-Guillen et al., 202122 | 28 | 1 | Observational | MitraClipTriClip | 3-mo | Heart Team rejection |
| Kitamura et al., 2021-202223 | 115 | 1 | Observational | MitraClipTriClipPASCAL | 1-y | Primary TR |
| Nickenig et al., 201924 | 30 | 8 | Single-arm trial | Cardioband | 6-mo | LVEF <30%Systolic pulmonary pressure> 60 mmHg |
| Davidson et al., 202125 | 30 | 9 | Single-arm trial | Cardioband | 30-d | LVEF <25%Severe RV dysfunction |
| Körber et al., 202126 | 60 | 4 | Observational | Cardioband | 30-d | Primary TR≤ moderate TR |
| Nickenig et al., 202127 | 61 | 13 | Single-arm trial | Cardioband | 30-d | LVEF <25%Severe RV dysfunctionSystolic pulmonary pressure> 70 mmHg |
| Planer et al., 202028 | 7 | 1 | Single-arm trial | Mistral | 30-d | LVEF <20% |
LVEF, left ventriclar ejection fraction; NA, not available; TEER, transcatheter edge-to-edge repair; TR, tricuspid regurgitation.
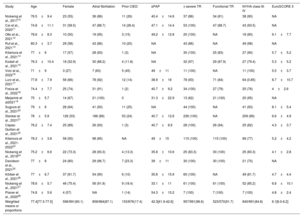
The main clinical and baseline characteristics across studies are summarized in table 2. A total of 991 patients were included. The weighted mean age was 77.4 years, and 596 (60.1%) were female. Most of the patients exhibited at least severe TR (96.6%), and 840 patients (84.8%) had advanced heart failure symptoms (NYHA class III or IV). Most patients had a functional mechanism of TR (91.7%).
Clinical characteristics of patients from selected studies
| Study | Age | Female | Atrial fibrillation | Prior CIED | sPAP | ≥ severe TR | Functional TR | NYHA class III-IV | EuroSCORE II |
|---|---|---|---|---|---|---|---|---|---|
| Nickenig et al., 201710 | 76.5±9.4 | 23 (55) | 36 (86) | 11 (26) | 40.4±14.6 | 37 (86) | 34 (81) | 38 (90) | NA |
| Cai et al., 202011 | 74.8±11.1 | 31 (58.5) | 47 (88.7) | 14 (26.4) | 47.1±14.4 | 53 (100) | 47 (88.7) | 43 (93.5) | NA |
| Otto et al., 202112 | 78.6±8.3 | 10 (50) | 19 (95) | 3 (15) | 49.2±12.8 | 20 (100) | NA | 18 (90) | 9.1±7.7 |
| Ruf et al., 202113 | 80.3±3.7 | 29 (58) | 43 (86) | 10 (20) | NA | 43 (86) | NA | 49 (98) | NA |
| Kitamura et al., 202114 | 77±6 | 17 (57) | 28 (93) | 1 (3) | NA | 30 (100) | 25 (83) | 27 (90) | 5.7±5.2 |
| Kodali et al., 202115 | 76.3±10.4 | 18 (52.9) | 30 (88.2) | 4 (11.8) | NA | 32 (97) | 29 (87.9) | 27 (79.4) | 5.3±5.2 |
| Volz et al., 202216 | 71±9 | 3 (27) | 7 (63) | 5 (45) | 49±11 | 11 (100) | NA | 11 (100) | 5.5±3.7 |
| Lurz et al., 202117 | 77.8±7.9 | 56 (66) | 78 (92) | 12 (14) | 38.9±16 | 78 (93) | 71 (84) | 64 (0.85) | 8.7±10.7 |
| Freixa et al., 202218 | 74.4±7.7 | 25 (74) | 31 (91) | 1 (2) | 40.7±9.2 | 34 (100) | 27 (79) | 25 (76) | 4±2.6 |
| Meijerink et al202119 | 75±5.7 | 14 (67) | 21 (100) | 0 | 31.3±22.9 | 13 (62) | 21 (100) | 20 (95) | NA |
| Sugiura et al., 202120 | 79±6 | 28 (64) | 41 (93) | 11 (25) | NA | 44 (100) | NA | 41 (93) | 8.1±5.4 |
| Stocker et al., 202121 | 78±5.9 | 126 (53) | 186 (89) | 53 (24) | 40.7±12.6 | 236 (100) | NA | 209 (89) | 6.6±4.9 |
| Cepas-Guillen et al., 202122 | 76.2±7.4 | 25 (89) | 26 (93) | 1 (3) | 40.7±8.9 | 28 (100) | 26 (94) | 25 (82) | 4.3±3.7 |
| Kitamura et al., 2021-202223 | 78.3±3.8 | 58 (50) | 98 (85) | NA | 49±15 | 115 (100) | 115 (100) | 89 (77) | 5.2±4.2 |
| Nickenig et al., 201924 | 75.2±6.6 | 22 (73.3) | 28 (93.3) | 4 (13.3) | 35.8±10.6 | 25 (83.3) | 30 (100) | 25 (83.3) | 4.1±2.8 |
| Davidson et al., 202125 | 77±8 | 24 (80) | 29 (96.7) | 7 (23.3) | 39±11 | 30 (100) | 30 (100) | 21 (70) | NA |
| Körber et al., 202126 | 77±6.7 | 37 (61.7) | 54 (90) | 6 (10) | 35.8±15.9 | 60 (100) | NA | 49 (81.7) | 4.7±4.4 |
| Nickenig et al., 202127 | 78.6±5.7 | 46 (75.4) | 56 (91.8) | 9 (18.4) | 33.1±11 | 61 (100) | 61 (100) | 52 (85.2) | 6.8±10.1 |
| Planer et al., 202028 | 74.8±5.6 | 4 (57) | NA | 1 (14) | 54.3±15.2 | 7 (100) | 7 (100) | 7 (100) | 4.8±2.4 |
| Weighted means or proportions | 77.4[77.3-77.5] | 596/991(60.1) | 858/984(87.1) | 153/876(17.4) | 42.3[41.9-42.6] | 957/991(96.6) | 523/570(91.7) | 840/991(84.8) | 6.1[6.0-6.2] |
CIED, cardiovascular implantable electronic device; NA, not available; sPAP, systolic pulmonary artery pressure; TR, tricuspid regurgitation.
The data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
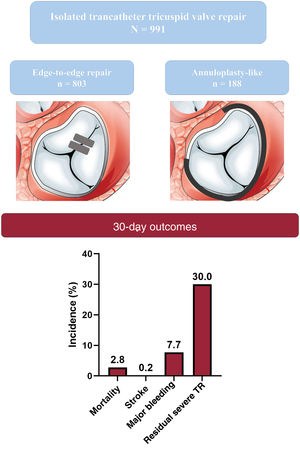
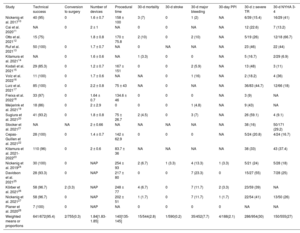
The main procedural and early outcomes are summarized in table 3. The overall technical success rate was 95.4% (641/672 patients), with a very low rate of conversion to open heart surgery (0.3%). Thirty-day mortality and stroke rates were 2.8% (15/544) and 0.2% (1/590), respectively. For patients with available 30-day functional class data (n=555), the rate of poor functional class (NYHA III or IV) at 30 days was 27%.
Procedural and early outcomes from the studies included of patients undergoing transcatheter tricuspid valve repair
| Study | Technical success | Conversion to surgery | Number of devices | Procedural time | 30-d mortality | 30-d stroke | 30-d major bleeding | 30-day PPI | 30-d ≥ severe TR | 30-d NYHA 3-4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Nickenig et al. 201710 | 40 (95) | 0 | 1.6 ± 0.7 | 158 ± 100 | 3 (7) | 0 | 1 (2) | NA | 6/39 (15.4) | 16/29 (41) |
| Cai et al. 202011 | NA | 0 | 2 ± 1 | NA | 0 | 0 | NA | NA | 12 (22.6) | 7 (13.2) |
| Otto et al. 202112 | 15 (75) | 1.8 ± 0.8 | 170 ± 75.8 | 2 (10) | 0 | 2 (10) | NA | 5/19 (26) | 12/18 (66.7) | |
| Ruf et al. 202113 | 50 (100) | 0 | 1.7 ± 0.7 | NA | 0 | NA | NA | NA | 23 (46) | 22 (44) |
| Kitamura et al. 202114 | NA | 0 | 1.6 ± 0.6 | NA | 1 (3.3) | 0 | 0 | NA | 5 (16.7) | 2/29 (6.9) |
| Kodali et al. 202115 | 29 (85.3) | 0 | 1.2 ± 0.7 | 167 ± 151 | 0 | 0 | 2 (5.9) | NA | 13 (48) | 3 (11) |
| Volz et al. 202216 | 11 (100) | 0 | 1.7 ± 0.6 | NA | NA | 0 | 1 (16) | NA | 2 (18.2) | 4 (36) |
| Lurz et al. 202117 | 85 (100) | 0 | 2.2 ± 0.8 | 75 ± 43 | NA | 0 | NA | NA | 36/83 (44.7) | 12/66 (18) |
| Freixa et al. 202218 | 33 (97) | 0 | 1.64 ± 0.7 | 134.6 ± 46 | 0 | 0 | 0 | NA | 3 (9) | NA |
| Meijerink et al. 202119 | 18 (86) | 0 | 2 ± 2.9 | 0 | 0 | 0 | 1 (4.8) | NA | 9 (43) | NA |
| Sugiura et al. 202120 | 41 (93.2) | 0 | 1.8 ± 0.8 | 75 ± 26.7 | 2 (4.5) | 0 | 3 (7) | NA | 26 (59.1) | 4 (9.1) |
| Stocker et al. 202121 | NA | NA | 2 ± 0.66 | NA | NA | NA | NA | NA | 38 (16) | 50/171 (29.2) |
| Cepas-Guillen et al. 202122 | 28 (100) | 0 | 1.4 ± 0.7 | 142 ± 62.9 | 0 | 0 | 0 | NA | 5/24 (20.8) | 4/24 (16.7) |
| Kitamura et al. 2021-202223 | 110 (96) | 0 | 2 ± 0.6 | 83.7 ± 36 | NA | NA | NA | NA | 38 (33) | 43 (37.4) |
| Nickenig et al. 201924 | 30 (100) | 0 | NAP | 254 ± 93 | 2 (6.7) | 1 (3.3) | 4 (13.3) | 1 (3.3) | 5/21 (24) | 5/28 (18) |
| Davidson et al. 202125 | 28 (93.3) | 0 | NAP | 217 ± 80 | 0 | 0 | 7 (23.3) | 0 | 15/27 (55) | 7/28 (25) |
| Körber et al. 202126 | 58 (96.7) | 2 (3.3) | NAP | 248 ± 77 | 4 (6.7) | 0 | 7 (11.7) | 2 (3.3) | 23/59 (39) | NA |
| Nickenig et al. 202127 | 58 (96.7) | 0 | NAP | 202 ± 51 | 1 (1.7) | 0 | 7 (11.7) | 1 (1.7) | 22/54 (41) | 13/50 (26) |
| Planer et al. 202028 | 7 (100) | 0 | NAP | NA | 0 | 0 | 0 | 0 | NA | NA |
| Weighted means or proportions | 641/672(95.4) | 2/755(0.3) | 1.84[1.83-1.85] | 140[135-145] | 15/544(2.8) | 1/590(0.2) | 35/452(7.7) | 4/188(2.1) | 286/954(30) | 150/555(27) |
NA, not available; NAP, not applicable; NYHA, New York Heart Association; PPI, permanent pacemaker implantation; TR, tricuspid regurgitation.
The data are expressed as No. (%), mean ± standard deviation, or median [interquartile range].
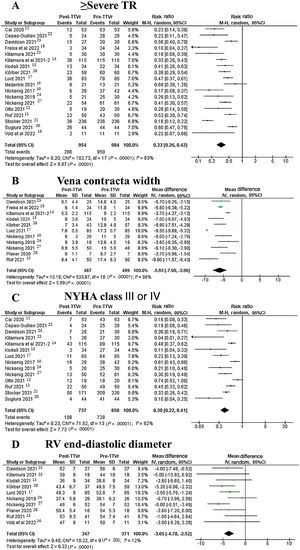
At 30-days, 286 out of 954 patients (30%) exhibited ≥ severe TR, compared with 957 out of 991 (96.6%) at baseline. Pooled random-effects resulted in a significant reduction of ≥ severe TR after the intervention (RR, 0.33; 95%CI, 0.26-0.42; P <.001) (figure 2A).10–15,17,19–22,24–27
Vena contracta width significantly decreased after TTVr from 13.2±0.9mm to 7.3±0.5mm, yielding a pooled random-effects MD estimate of 5.9mm (95%CI, 4-7.9; P <.001) (figure 2B).10,13,15,17,18,23–28
At 30 days, 150 out of 555 patients (27%) exhibited functional class NYHA III or IV compared with 840 out of 991 (84.8%) at baseline. Pooled random-effects resulted in a significant reduction of poor functional class after the intervention (RR, 0.32; 95%CI, 0.24-0.43; P <.001) (figure 2C).10–15,17,20–25,27
RV end-diastolic diameter significantly decreased after TTVr from 49.1±2mm to 45.5±2mm, yielding a pooled random-effects MD estimate of 3.7mm (95%CI, 2.6-4.7; P <.001) (figure 2D).13–17,24–28
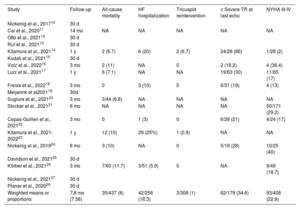
Mid-term outcomesA total of 10 studies reported clinical data beyond the first month after the procedure (table 4). The weighted mean follow-up was 7.8 months (95%CI, 7.5-8 months). The rates of all-cause mortality and heart failure rehospitalization were 8% (35 out of 437) and 16.3% (42 out of 258), respectively. Among the 6 studies reporting survival data at 3 to 6 months of follow-up (table 4), all-cause mortality was 7.25% (15 out of 207 patients) whereas among studies reporting survival rates at 1 year of follow-up, all-cause mortality was 8.7% (20 out of 230 patients), as shown in table 4.
Mid-term outcomes for studies reporting data beyond 30-day follow-up
| Study | Follow-up | All-cause mortality | HF hospitalization | Tricuspid reintervention | ≥ Severe TR at last echo | NYHA III-IV |
|---|---|---|---|---|---|---|
| Nickenig et al., 201710 | 30 d | |||||
| Cai et al., 202011 | 14 mo | NA | NA | NA | NA | NA |
| Otto et al., 202112 | 30 d | |||||
| Ruf et al., 202113 | 30 d | |||||
| Kitamura et al., 202114 | 1 y | 2 (6.7) | 6 (20) | 2 (6.7) | 24/28 (86) | 1/28 (2) |
| Kodali et al., 202115 | 30 d | |||||
| Volz et al., 202216 | 3 mo | 2 (11) | NA | 0 | 2 (18.2) | 4 (36.4) |
| Lurz et al., 202117 | 1 y | 6 (7.1) | NA | NA | 19/63 (30) | 11/65 (17) |
| Freixa et al., 202218 | 3 mo | 0 | 3 (10) | 0 | 6/31 (19) | 4 (13) |
| Meijerink et al202119 | 30d | |||||
| Sugiura et al., 202120 | 3 mo | 3/44 (6.8) | NA | NA | NA | NA |
| Stocker et al., 202121 | 6 mo | NA | NA | NA | NA | 50/171 (29.2) |
| Cepas-Guillen et al., 202122 | 3 mo | 0 | 1 (3) | 0 | 6/28 (21) | 4/24 (17) |
| Kitamura et al., 2021-202223 | 1 y | 12 (10) | 29 (25%) | 1 (0.9) | NA | NA |
| Nickenig et al., 201924 | 6 mo | 3 (10) | NA | 0 | 5/18 (28) | 10/25 (40) |
| Davidson et al., 202125 | 30 d | |||||
| Körber et al., 202126 | 3 mo | 7/60 (11.7) | 3/51 (5.9) | 0 | NA | 9/48 (18.7) |
| Nickenig et al., 202127 | 30 d | |||||
| Planer et al., 202028 | 30 d | |||||
| Weighted means or proportions | 7.8 mo (7.58) | 35/437 (8) | 42/258 (16.3) | 3/308 (1) | 62/179 (34.6) | 93/406 (22.9) |
HF, heart failure; NA, not available; NYHA, New York Heart Association; TR, tricuspid regurgitation.
The data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
A total of 308 patients had available data on the need for tricuspid valve reintervention at follow-up, with 3 patients needing either surgical or percutaneous reintervention (1%). The rate of ≥ severe TR on the last available echocardiogram was 34.6% (62 out of 179).
At last follow-up, a total of 93 of 406 patients (22.9%) exhibited functional class NYHA III or IV.
Subanalysis for edge-to-edge vs annuloplasty-like repair techniquesIn the edge-to-edge repair subgroup, a weighted mean of 1.84 devices were implanted per patient. The most frequent location for device grasping was between the anterior and septal leaflets (438/526; 83.3%), followed by the posterior and septal leaflets (112/526; 21.3%), and the anterior and posterior leaflets (10/526 devices; 1.9%).
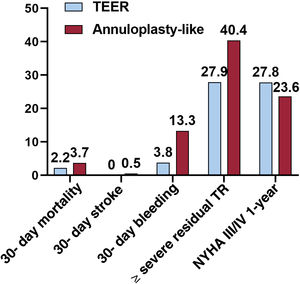
Technical success rates were almost similar for the 2 techniques (95% TEER vs 96.2% annuloplasty-like systems), whereas TEER recipients showed slightly lower rates in terms of 30-day mortality (2.2% vs 3.7%) and 30-day stroke (0% vs 0.5%). Bleeding complications and residual ≥ severe TR were higher in the annuloplasty-like subgroup (13.3% vs 3.8% for bleeding and 40.4% vs 27.9% for residual severe TR, respectively). In the meta-regression analysis, the use of edge-to-edge devices vs annuloplasty-like systems did not reach statistical significance for the endpoint of residual ≥ severe TR (beta, −0.31; 95%CI, −0.91-0.30; P=.26). Main outcomes according to the percutaneous repair technique are displayed in figure 3.
Regarding specific procedural complications, a total of 27 out of 512 (5.3%) patients undergoing edge-to-edge repair had a single leaflet device attachment. In the annuloplasty-like repair subgroup, the rate of right coronary artery related complications was 10.9% (18/165 patients), and the rate of conduction disturbances leading to permanent pacemaker implantation was 2.1% (4/188 patients).
Subanalysis of studies including concomitant mitral valve repairA total of 5 studies included patients undergoing concomitant mitral and tricuspid transcatheter repair, yielding a total of 213 out of 510 patients (41.8%) who received both procedures (). The main clinical characteristics and early outcomes in studies including concomitant transcatheter mitral valve repair are displayed in . The rate of 30-day stroke was numerically higher in patients undergoing combined therapy when compared with patients receiving tricuspid repair exclusively (0.7% vs 0.2%). The rates of 30-day mortality (2.4% vs 2.8%), residual ≥ severe TR (26.5% vs 30%), and major bleeding (6% vs 7.7%) were fairly similar between the 2 groups.
DISCUSSIONThe main findings of this meta-analysis can be summarized as follows (see figure 4): a) among 991 patients comprising the early experience of several TTVr techniques in isolated TR, the rate of technical success was high (95.4%), whereas early mortality and stroke rates were low (2.8% and 0.2%, respectively); b) there was a statistically significant reduction in ≥ severe TR, NYHA class III-IV, vena contracta width and RV end-diastolic diameter after TTVr; and c) patients receiving tricuspid TEER compared with their annuloplasty-like counterparts had numerically lower rates of severe bleeding and residual ≥ severe TR.
In the early experience of transcatheter tricuspid valve repair techniques, the patient population ultimately undergoing these procedures was highly comorbid and symptomatic: mean age was close to 80 years, the prevalence of atrial fibrillation was> 85%, and close to 90% of the patients had advanced functional class at the time of the procedure. Given these comorbidities and the EuroSCORE 2 calculated for these patients across the several studies included, the surgical risk would have been intermediate-high for a potential valvular surgical procedure.
Early outcomesThe weighted early mortality rate (2.8%) might be considered as relatively low given the comorbidity of the patients ultimately treated, who showed a predictive risk for early mortality according to the EuroSCORE-2 close to 7%. However, and in contrast to mitral repair or aortic replacement procedures where cardiac surgery has demonstrated highly positive outcomes, the poor results observed with open heart surgery in this clinical setting (isolated tricuspid valve repair) may likely favor the expansion of the transcatheter tricuspid repair approach to lower risk and less comorbid populations. This fact, along with refinements in procedural techniques and growing operator experience are critical to improve the mortality rates in the future.
Stroke and cerebrovascular events have been one of the most feared complications in the interventional cardiology field regardless of the targeted valve. The rates of stroke have been systematically lower when percutaneously repairing the tricuspid valve compared with mitral valve repair, in which periprocedural stroke rates were estimated to be around 1%.29 This may be mainly explained by the lack of transseptal puncture and left atrial navigation and manipulation, thus allowing for safer procedures in terms of cerebrovascular complications. This low stroke rate is of the upmost importance in a frail, elderly, and comorbid population in which a large percentage of patients need chronic oral anticoagulation and exhibit prior cerebrovascular disease. Indeed, in our subanalysis we observed that the stroke rates increased nearly 2-fold in studies including concomitant mitral repair procedures in addition to the tricuspid intervention. However, both rates are much lower when compared with the stroke rate observed in patients undergoing isolated tricuspid valve surgery (both <1% vs 2.6% in open heart surgery).4
The use of large bore catheters for structural valve interventions as well as the manipulation of large device delivery systems within the cardiac chambers favor the occurrence of bleeding complications. The rate of major bleeding complications in the field of TTVr has been relatively low thus far (6%), considering that most of the patients were old, highly comorbid and on oral anticoagulants due to atrial fibrillation. In addition, we found that annuloplasty-like procedures accounted for most of these complications, whereas TEER receivers had an early major bleeding rate of just 4% when evaluated separately. The greater experience with edge-to-edge techniques both in the mitral and tricuspid position among operators may have accounted for some of these differences. Future studies are needed to better inform on this issue, although the safety profile seems to favor TEER so far.
The amount of TR reduction remains as one of the major caveats to be improved in the upcoming years in this field. It should be highlighted that close to 30% of the patients exhibited at least a severe degree of TR despite repair, and this rate was maintained when we assessed the last available echocardiographic data. However, it must be acknowledged that this constituted the very early experience with percutaneous tricuspid repair systems for most of the centers and operators, and these results are expected to improve in the upcoming years. For instance, the rate of single leaflet attachment for TEER recipients (> 5%) was more in line with the early experience of TEER for the mitral position30 than with current available data.31 In addition, patient selection may have played a role in efficacy outcomes, as very large coaptation gaps were not systematically excluded from the selected studies. Refinement in patient selection from an anatomic standpoint may also help to improve overall results.
Future prospectsDespite the positive initial findings, future studies comparing TTVr with optimal medical therapy are needed to further assess the clinical utility of this technique. Therefore, primary outcomes to be evaluated in these potential trials might combine both hard clinical outcomes (eg, mortality) and functional class and quality of life improvement. The pivotal clinical trials TRILUMINATE (NCT03904147) and CLASP II TR (NCT04097145) will randomize patients to TTVr with edge-to-edge devices vs medical therapy, and their results will shed more light in this setting in the upcoming years. In the meanwhile, optimal patient selection considering RV function, pulmonary pressure, and multiorgan involvement must be a cornerstone to prevent futility. So far, the low overall rates of early complications and the improvement in TR grade and functional class presented seem promising. In addition, improvement in walking distance after TTVr has also been demonstrated in a prior review.32 Therefore, TTVr might be progressively included among the therapeutic armamentarium to improve symptoms in patients with severe TR.
Of note, the combination of TEER and annuloplasty-like techniques for the tricuspid location was not assessed in an important cohort of patients, and the potential benefit of this approach remains to be further studied. Finally, several devices are currently under clinical evaluation for transcatheter tricuspid valve replacement, demonstrating promising early results. Recent data have shown the absence of early mortality, conversion to surgery, and stroke in 25 patients receiving a transcatheter tricuspid replacement device under a compassionate use program, and more than 90% of the patients exhibited postprocedural TR between none and mild.33 Therefore, whether to percutaneously repair or replace the tricuspid valve may become a matter of debate in the near future.
LimitationsOur study has some limitations. All studies included were single-arm trials or observational studies with no comparator group, and therefore the benefit of transcatheter tricuspid repair vs optimal medical therapy remains to be assessed in future randomized studies. Most studies included did not have independent echocardiographic and clinical committees for endpoint assignment, and there was a substantial lack of data even for some early outcomes. Clinical trials are warranted to obtain higher quality data. Heterogeneity across studies, although low for RV dimension, was high for other pooled results. Although there was no statistically significant publication bias according to Egger's regression (), the funnel plot for TR severity suggests a potential publication bias ().
CONCLUSIONSThe early experience with transcatheter tricuspid valve repair systems has yielded a high rate of technical success with relatively low rates of early mortality, stroke, and bleeding events. There was a clinical improvement in functional class early after the repair and at 1 year of follow-up, although the rates of postprocedural and mid-term residual> moderate TR need further improvement.
- -
Transcatheter tricuspid valve repair has increasingly grown over the last few years, and individual data from the studies reporting on the early experience has demonstrated the favorable safety profile of the technique.
- -
The degree of TR, RV diameters, as well as the rate of patients in poor functional class, have decreased significantly after the intervention, but the rates of residual severe TR are still high (> 27%).
- -
The edge-to-edge repair technique seems to offer a better safety profile than annuloplasty-like systems.
No funding was received for this study.
AUTHORS’ CONTRIBUTIONA. Alperi, I. Pascual, P. Avanzas: concept and design; literature search and statistical analysis; article drafting. All authors participated in the analysis and interpretation, critical revision of the article, and final approval of the article.
CONFLICTS OF INTERESTP. Avanzas is associate editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed. The remaining authors have not reported any potential conflict of interest with respect to the content of this article.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.06.004