Heart failure (HF) is associated with a high rate of readmissions within 30 days postdischarge. Strategies to lower readmission rates generally show modest results. To reduce readmission rates, we developed a STructured multidisciplinary outpatient clinic for Old and frail Postdischarge patients hospitalized for HF (STOP-HF-Clinic).
MethodsThis prospective all-comers study enrolled patients discharged from internal medicine or geriatric wards after HF hospitalization. The intervention involved a face-to-face early visit (within 7 days), HF nurse education, treatment titration, and intravenous medication when needed. Thirty-day readmission risk was calculated using the CORE-HF risk score. We also studied the impact of 30-day readmission burden on regional health care by comparing the readmission rate in the STOP-HF-Clinic Referral Area (∼250000 people) with that of the rest of the Catalan Health Service (CatSalut) (∼7.5 million people) during the pre–STOP-HF-Clinic (2012-2013) and post–STOP-HF-Clinic (2014-2015) time periods.
ResultsFrom February 2014 to June 2016, 518 consecutive patients were included (age, 82 years; Barthel score, 70; Charlson index, 5.6, CORE-HF 30-day readmission risk, 26.5%). The observed all-cause 30-day readmission rate was 13.9% (47.5% relative risk reduction) and the observed HF-related 30-day readmission rate was 7.5%. The CatSalut registry included 65131 index HF admissions, with 9267 all-cause and 6686 HF-related 30-day readmissions. The 30-day readmission rate was significantly reduced in the STOP-HF-Clinic Referral Area in 2014-2015 compared with 2012-2013 (P < .001), mainly driven by fewer HF-related readmissions.
ConclusionsThe STOP-HF-Clinic, an approach that could be promptly implemented elsewhere, is a valuable intervention for reducing the global burden of early readmissions among elder and vulnerable patients with HF.
Keywords
Heart failure (HF) is the leading cause of hospital readmission in developed countries.1 It is a particular concern for patients ≥ 65 years of age, who comprise approximately 80% of the population with HF.2 The total annual cost of HF in the US is estimated to be $30.7 billion, with about two-thirds attributable to HF-related hospitalizations.3
Rates of rehospitalization within 30 days of discharge can reach 20%–30%.4 Early rehospitalization is attributed to underlying disease exacerbation5 and several other medical derangements.6 Up to 65% of 30-day readmissions are for diverse medical circumstances7 that often occur early in the hospital-to-home transition.8 Early postdischarge readmissions are strongly linked to the quality of inpatient care.9,10 Although they may be partly due to incomplete patient stabilization, they are frequently caused by poor discharge coordination and failure to ensure good quality postdischarge care. Indeed, up to 75% of such cases are considered to be preventable.11
Several interventions have been proposed and tested for reducing the social and economic burdens of early readmissions.12,13 However, previously tested interventions show only modest ability to reduce all-cause or unplanned 30-day postdischarge readmissions among patients hospitalized for HF, with absolute reductions of around 2%-3% (relative reductions of 10%-18%).12,14 Moreover, some studies exclude HF patients admitted to noncardiology wards, although these patients are usually elderly, frail, and have several comorbidities, and thus show the highest readmission rates.15
In the present study, we evaluated a STructured multidisciplinary outpatient clinic for Old and frail postdischarge patients hospitalized for HF (the “STOP-HF-Clinic”), which was established with the aim of reducing 30-day readmission rates and facilitating transition to primary care. We assessed the efficacy of this STOP-HF intervention in terms of the enrolled patients’ 30-day readmission rates compared with their readmission risk calculated using the CORE-HF risk score. As a secondary objective, we examined the impact of the STOP-HF-Clinic intervention within the official readmission data registry of the Catalan Health Service (CatSalut), which provides medical care to 7.5 million people in Catalonia, Spain.
METHODSStudy PopulationThis prospective single-center study was designed to include the most vulnerable patients admitted for acutely decompensated HF. We performed an all-comers, consecutive study of HF patients discharged from internal medicine and geriatric wards with a primary hospital diagnosis of HF according to the Framingham HF Criteria.16 Our study did not include patients discharged from the cardiology ward (n=106 during the study time period), who were generally younger (64 ± 12 years), male (76%), of ischemic etiology (51%), and with reduced left ventricular ejection fraction (30% ± 9%). Such patients received standard follow-up care by HF specialists.
At their first study visit, patients’ 30-day readmission risk was calculated using the Yale Center for Outcome Research and Evaluation score (CORE-HF).17 This calculator estimates risk using 20 demographic, clinical, and hemodynamic variables and is based on medical record chart models developed to allow the Centers for Medicare and Medicaid Services to validate publicly reported readmission measures.18 At the first study visit, we also obtained the Charlson comorbidity index and Barthel functional score and clinical, demographic, and treatment data. All participants provided written informed consent, and the study was approved by the local ethics committee. All study procedures were in accordance with the ethical standards outlined in the Helsinki Declaration of 1975, as revised in 2013.19
STOP-HF-Clinic InterventionSeven different interventions were applied in the STOP-HF-Clinic: a) within 7 days after discharge, an early postdischarge visit was scheduled for patients at a specialized HF faculty including a HF nurse and one or more clinic staff, such as a general practitioner, internist, geriatrician, or cardiologist; b) participants were examined for residual congestion and other reversible potentially decompensating conditions; c) a baseline blood sample was taken to test biomarker levels, including N-terminal pro–B-type natriuretic peptide (NT-proBNP), hemoglobin, and the estimated glomerular filtration rate; d) HF nurses performed a face-to-face educational intervention with both the patient and the caregiver. Advice was personalized and supported by an educational booklet and a telephone hotline number; e) a minimum of 3 visits were planned for drug adjustment during the 30-day period, with as many extra visits as required. Based on the first evaluation and treatment upon discharge, drug titration was individualized according to clinical guidelines. Diuretics were adjusted based on the patient's congestive state; f) intravenous treatments, such as furosemide, ferric carboxymaltose, and red blood cell transfusions, were administered as necessary; g) after 30 days, patients were transitioned to their general practitioner and/or specialist for an early follow-up via e-notification, and a written medical report and drug prescription information were uploaded to the electronic medical record.
The primary endpoints were all-cause and HF-related 30-day hospital readmissions among patients who attended the STOP-HF-Clinic. Outpatient medical visits and hospital admissions were reviewed using the electronic medical records.
Catalan Health Service RegistryTo evaluate the efficacy of the STOP-HF intervention in a real-world setting, we designed a population-based natural experiment including all patients admitted with HF in Catalonia, Spain, between 2012 and 2015. The STOP-HF-Clinic was conducted in the referral area for ∼250 000 inhabitants in the north Barcelona Metro Area (referred to as the STOP-HF Referral Area). CatSalut provides medical care to 7.5 million people in Catalonia, Spain.
Our analysis included all patients with HF who were consecutively admitted to any Catalan hospital and discharged alive between January 2012 and December 2015. We analyzed all-cause 30-day readmissions, which were classified as either HF-related (due to HF recurrence) or non–HF-related (readmission with a primary diagnosis of chronic disease not involving the circulatory system and with no external cause or due to a complication of the index admission). For the index admission and successive clinically related readmissions, we considered only unplanned acute admissions lasting longer than 24hours. The International Classification of Diseases-9-CM codes used for hospital admissions for HF were 398.91, 402.x1, 404.x1, 404.x3, 428.0, 428.1, 428.2x, 428.3x, and 428.4x. For the diagnosis of both HF and clinically related readmissions, we used the criteria recommended in the Chronic Condition Indicator of the Agency for Healthcare Research and Quality.20 The registry has an automatic data validation system, and an external audit is carried out periodically to ensure data quality and accuracy.
As a secondary endpoint, we evaluated the population-based impact on all-cause and HF-related 30-day readmissions of patients within the STOP-HF Referral Area before the program started (2012-2013) and after exposure to the STOP-HF-Clinic (2014-2015). The control group comprised all patients in the rest of the CatSalut area.
Statistical AnalysisContinuous variables are expressed as mean ± standard deviation or as median and [interquartile range, Q1-Q3], depending on data distribution (assessed by normal Q-Q plots). Categorical variables are expressed as number (percentage). Comparative analyses between variables were performed using the chi-square test, t test, or Mann-Whitney U test, depending on the variable type (dichotomous or continuous) and distribution type. Multivariable logistic regression analyses were performed to determine the variables associated with all-cause and HF-related 30-day readmissions. The model included variables determined to be significant in univariable analysis or considered potentially clinically relevant, such as age, sex, HF etiology, Barthel score, Charlson index, length of index hospital stay, CORE-HF risk prediction, and Framingham score and NT-proBNP at first visit. A conditional backward stepwise method was used to avoid over-adjustments. For NT-proBNP, we used its logarithmic function and a 1-standard deviation increase for odds ratio (OR) calculations. Actuarial HF readmission curves were obtained for the CatSalut area during the 2 studied periods and were compared with those of the STOP-HF Referral area using the Wilcoxon-Gehan test. Statistical analyses were performed using SPSS 15 (SPSS Inc, Chicago, IL, United States). P values of < .05 from 2-sided tests were considered to indicate statistical significance.
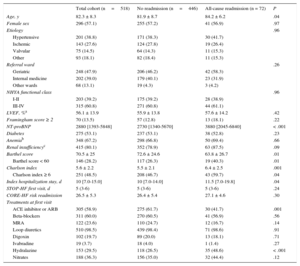
RESULTSA total of 518 patients attended the STOP-HF-Clinic from February 2014 to June 2016. These patients’ demographic and clinical characteristics are shown in Table 1. The mean patient age was 82.3 ± 8.3 years, 25% were ≥ 88 years old, and 57.1% were women. Common comorbidities included diabetes, anemia, and renal failure. Hemoglobin levels were < 10g/dL in 55 patients (10.6%) and < 9g/dL in 13 patients (2.5%). The median time from discharge to first STOP-HF-Clinic visit was 5 days (Q1-Q3, 3-6 days), and the mean number of visits per patient within 30 days was 3.1 ± 1.2.
Demographic and Clinical Characteristics
| Total cohort (n=518) | No readmission (n=446) | All-cause readmission (n = 72) | P | |
|---|---|---|---|---|
| Age, y | 82.3 ± 8.3 | 81.9 ± 8.7 | 84.2 ± 6.2 | .04 |
| Female sex | 296 (57.1) | 255 (57.2) | 41 (56.9) | .97 |
| Etiology | .96 | |||
| Hypertensive | 201 (38.8) | 171 (38.3) | 30 (41.7) | |
| Ischemic | 143 (27.6) | 124 (27.8) | 19 (26.4) | |
| Valvular | 75 (14.5) | 64 (14.3) | 11 (15.3) | |
| Other | 93 (18.1) | 82 (18.4) | 11 (15.3) | |
| Referral ward | .26 | |||
| Geriatric | 248 (47.9) | 206 (46.2) | 42 (58.3) | |
| Internal medicine | 202 (39.0) | 179 (40.1) | 23 (31.9) | |
| Other wards | 68 (13.1) | 19 (4.3) | 3 (4.2) | |
| NHYA functional class | .96 | |||
| I-II | 203 (39.2) | 175 (39.2) | 28 (38.9) | |
| III-IV | 315 (60.8) | 271 (60.8) | 44 (61.1) | |
| LVEF, %a | 56.1 ± 13.9 | 55.9 ± 13.8 | 57.6 ± 14.2 | .42 |
| Framingham score ≥ 2 | 70 (13.5) | 57 (12.8) | 13 (18.1) | .22 |
| NT-proBNP | 2880 [1393-5848] | 2730 [1340-5670] | 3880 [2045-6840] | < .001 |
| Diabetes | 275 (53.1) | 237 (53.1) | 38 (52.8) | .23 |
| Anemiab | 348 (67.2) | 298 (66.8) | 50 (69.4) | .66 |
| Renal insufficiencyc | 415 (80.1) | 352 (78.9) | 63 (87.5) | .09 |
| Barthel score | 70.5 ± 25 | 72.6 ± 24.6 | 63.8 ± 26.7 | .01 |
| Barthel score < 60 | 146 (28.2) | 117 (26.3) | 19 (40.3) | .01 |
| Charlson index | 5.6 ± 2.2 | 5.5 ± 2.1 | 6.4 ± 2.5 | .001 |
| Charlson index ≥ 6 | 251 (48.5) | 208 (46.7) | 43 (59.7) | .04 |
| Index hospitalization stay, d | 10 [7.0-15.0] | 10 [7.0-14.0] | 11.5 [7.0-19.8] | .04 |
| STOP-HF first visit, d | 5 (3-6) | 5 (3-6) | 5 (3-6) | .24 |
| CORE-HF risk readmission | 26.5 ± 5.3 | 26.4 ± 5.4 | 27.1 ± 4.6 | .30 |
| Treatments at first visit | ||||
| ACE inhibitor or ARB | 305 (58.9) | 275 (61.7) | 30 (41.7) | .001 |
| Beta-blockers | 311 (60.0) | 270 (60.5) | 41 (56.9) | .56 |
| MRA | 122 (23.6) | 110 (24.7) | 12 (16.7) | .14 |
| Loop diuretics | 510 (98.5) | 439 (98.4) | 71 (98.6) | .91 |
| Digoxin | 102 (19.7) | 89 (20.0) | 13 (18.1) | .71 |
| Ivabradine | 19 (3.7) | 18 (4.0) | 1 (1.4) | .27 |
| Hydralazine | 153 (29.5) | 118 (26.5) | 35 (48.6) | < .001 |
| Nitrates | 188 (36.3) | 156 (35.0) | 32 (44.4) | .12 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CORE-HF, Yale Center for Outcome Research and Evaluation score; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; STOP-HF, structured multidisciplinary outpatient clinic for old and frail postdischarge patients hospitalized for heart failure.
Data are expressed as no. (%), mean ± standard deviation or median [interquartile range, Q1-Q3].
The need for intravenous therapy and adjustment of HF treatment in the 30-day STOP-HF-Clinic are displayed in Table 2. One-third of patients required at least one infusion of 40mg intravenous furosemide. Infusions of intravenous furosemide were required once by 104 patients, twice by 39 patients, 3 times by 21 patients, 4 times by 11 patients, 5 times by 4 patients, and 6 times by 3 patients. One-sixth of patients required intravenous ferric carboxymaltose 1g, with up to 93 infusions needed. Twenty-one patients required red blood cell transfusion. Oral HF treatments were routinely initiated or adjusted in the STOP-HF-Clinic, with some patients requiring substantial dose uptitration. Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers were discontinued in 10.5% of patients due to renal worsening or hypotension. Those who continued treatment had a mean angiotensin-converting enzyme inhibitor/angiotensin receptor blocker dose increase of 15% after the 30-day follow-up. During the 30-day follow-up period, 12 patients (2.3%) died. At the end of the intervention, 54% of patients were transitioned to their general practitioner and 30% to a primary care specialist and 16% continued follow-up with hospital HF specialists.
Intravenous Treatment and Changes in Oral Heart Failure Drugs During STOP-HF-Clinic Follow-up
| Intravenous treatment | Patients, no. | Infusions, no. |
|---|---|---|
| Furosemide, 40 mg | 182 | 327 |
| Ferric carboxymaltose, 1 g | 86 | 93 |
| Red blood cell transfusion | 21 | 37 |
| Oral HF drug adjustments | Initiated/discontinued, %a | Dose uptitration, %b |
|---|---|---|
| ACE inhibitor/ARB | –10.5 | +15.0 |
| Beta-blockers | +4.7 | +11.2 |
| MRA | +29.8 | +2.0 |
| Loop diuretics | 0.0 | +35.5 |
| Digoxin | +11.4 | — |
| Ivabradine | +35.2 | — |
| Hydralazine | +4.2 | — |
| Nitrates | +9.0 | — |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; HF, heart failure; MRA, mineralocorticoid receptor antagonist; STOP-HF, structured multidisciplinary outpatient clinic for old and frail postdischarge patients hospitalized for heart failure.
The predicted CORE-HF all-cause 30-day readmission risk was 26.5% ± 5.3%, with 29.5% of patients having a CORE-HF risk of ≥ 30%. The observed all-cause 30-day readmission rate was 13.9% (n=72), and the observed HF-related 30-day readmission rate was 7.5% (n=39). Overall, the STOP-HF-Clinic intervention achieved a 47.5% relative reduction in all-cause 30-day readmission risk and an absolute reduction of 12.6% compared with the predicted CORE-HF readmission risk score.
Multivariable logistic regression analysis revealed that all-cause 30-day readmission was independently associated with age (OR, 1.04; 95% confidence interval [95%CI], 1.00-1.08; P=.03), Charlson index (OR, 1.19; 95%CI, 1.06-1.34; P=.005), length of index hospital stay (OR, 1.03; 95%CI, 1.00-1.06; P=.03), and NT-proBNP (OR, 1.34; 95%CI, 1.01-1.78; P=.045). On the other hand, HF-related 30-day readmission was independently associated with only the Charlson index (OR, 1.22; 95%CI, 1.05-1.41; P=.008) and NT-proBNP (OR, 1.43; 95%CI, 1.00-2.05; P=.05).
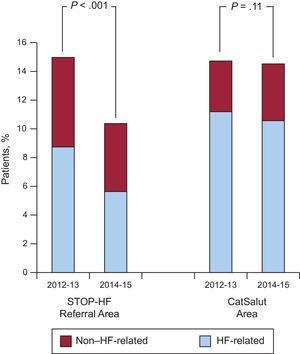
We next compared readmission rates within the STOP-HF Referral Area (∼250 000 people) with readmission rates within the entire CatSalut registry (∼7.5 million people) during 2 time periods: pre–STOP-HF (2012-2013) and post–STOP-HF (2014-2015) (). Within these 2 periods, 9267 all-cause and 6686 HF-related 30-day readmissions were documented after a total of 65 131 index HF-related admissions. Figure 1 illustrates the decline in 30-day readmission rates observed in the STOP-HF Referral Area during the 2014-2015 time period compared with the 2012-2013 time period (P < .001), with no significant changes in the rest of the CatSalut area (P=.11). The all-cause 30-day readmission rates during the 2012-2013 period in the 2 studied populations are shown in Table 3 (P=.88). On the other hand, during the 2014-2015 period, all-cause 30-day readmission rates were significantly lower in the STOP-HF Referral Area (P < .001), which was mainly driven by reduced HF-related readmissions.
Thirty-day readmission rates with and without the STOP-HF intervention. Thirty-day readmission rates in the STOP-HF Referral Area vs the CatSalut area before STOP-HF (2012-2013) and with STOP-HF (2014-2015). CatSalut, Catalan Health Service; HF-related, heart failure recurrence; non–HF-related: chronic noncirculatory disease and readmissions due to a complication of the index admission; STOP-HF, structured multidisciplinary outpatient clinic for old and frail postdischarge patients hospitalized for heart failure.
Catalan Health Service Population-based Data During the 2 Studied Periods
| 2012-2013 period | |||
|---|---|---|---|
| STOP-HF Referral Area | CatSalut | P | |
| Total HF admissions, no. | 1253 | 31 199 | |
| All-cause 30-day readmissions, no. (%) | 184 (14.7) | 4533 (14.5) | .88 |
| HF-relateda | 108 (8.6) | 3335 (10.7) | .02 |
| Non–HF-relatedb | 76 (6.1) | 1198 (3.8) | <.001 |
| 2014-2015 period | |||
|---|---|---|---|
| STOP-HF Referral Area | CatSalut | P | |
| Total HF admissions, no. | 1296 | 31 383 | |
| All-cause 30-day readmissions, no. (%) | 130 (10.0) | 4420 (14.1) | <.001 |
| HF-relateda | 71 (5.5) | 3172 (10.1) | <.001 |
| Non–HF-relatedb | 59 (4.5) | 1248 (4.0) | .30 |
CatSalut, Catalan Health Service; HF, heart failure; STOP-HF, structured multidisciplinary outpatient clinic for old and frail postdischarge patients hospitalized for heart failure.
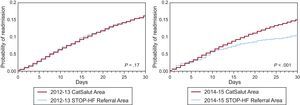
Actuarial curves of probability after HF hospitalization within the CatSalut area and the STOP-HF Referral Area are shown in Figure 2. Compared with the rest of the CatSalut area, the STOP-HF Referral Area showed a significant decline in HF readmissions during the 2014-2015 period, with the 2 curves following divergent paths after day 10.
Actuarial curves of the probability of 30-day readmission with and without STOP-HF. Probabilities of 30-day readmission in the STOP-HF Referral Area vs the CatSalut area before STOP-HF (2012-2013) and with STOP-HF (2014-2015). P values reflect comparisons between study groups. CatSalut, Catalan Health Service; STOP-HF, structured multidisciplinary outpatient clinic for old and frail postdischarge patients hospitalized for heart failure.
The concept that “hospitalization begets further hospitalization” is certainly applicable to HF.21 Because many HF readmissions are considered preventable, novel strategies are needed to reduce rehospitalizations. We have developed a comprehensive patient-centered model: the STOP-HF-Clinic. Our present data show that implementation of the STOP-HF-Clinic intervention was associated with a significant ∼50% reduction in all-cause 30-day readmission, mainly driven by reduced HF-related readmissions. Moreover, the STOP-HF intervention profoundly impacted all-cause readmission rates in the STOP-HF Referral Area compared with the CatSalut registry.
Rather than focusing on only one aspect of patient care, the STOP-HF-Clinic integrated a number of interventions, including quality of medical management, early reassessment, health literacy, and care transition. Despite growing interest in remote monitoring of these patients to reduce readmissions, structured telephone support interventions and home telemonitoring have not shown greater ability to reduce 30-day all-cause or HF-related readmission rates.22 Recent reports from the BEAT-HF trial, which aimed to explicitly adapt the care transition approach in combination with remote telemonitoring, failed to demonstrate reduced all-cause 30-day and 180-day readmission rates.23 On the other hand, our face-to-face early intervention reduced both all-cause and HF-related 30-day readmissions in our population.
Compared with most prior studies, our present real-life, prospective, all-comers study enrolled patients who were older and more frail and vulnerable in terms of medical complexity (mean age, 82 years; Barthel score, 70; Charlson index, 6; preserved left ventricular ejection fraction and high NT-proBNP).24–31 Previous studies have commonly excluded patients with advanced renal insufficiency or severe cardiovascular disease22 but elderly individuals with prevalent renal dysfunction and prominent congestion are most prone to early readmissions.32 The prospect of an increasingly aging frail population increases the need for solutions suitable for this patient group. Such measures will likely require the cooperation of multidisciplinary teams, such as in the STOP-HF-Clinic, rather than a focus on any one aspect of patient care.
It appears that early follow-up within 7 days of discharge is critical for reducing readmissions.21,33,34 In the STOP-HF-Clinic, we offered systematic medical contact at a median of 5 days postdischarge. During this transitional period, ineffective communication, low health literacy, and adherence issues contribute to readmissions. However, intervention programs often fail to act during this time frame,35 sometimes due to a lack of coordination with the medical provider, revealing a gap in transitional care. This period of postdischarge vulnerability has been described as “post-hospital syndrome”36 and is related to factors such as age, cognitive impairment, frailty, and polypharmacy. The STOP-HF intervention likely acted to prevent ‘post-hospital syndrome’.
The STOP-HF-Clinic provided a quick therapeutic intervention to promote disease stability. Indeed, the reduced readmission rate was likely affected by the high number of intravenous infusions of both furosemide and ferric carboxymaltose. Although intravenous loop diuretics are the standard-of-care for inpatient management in acutely decompensated HF, here we extended intravenous furosemide infusion to the outpatient setting during the 30-day postdischarge period among patients with refractory HF and congestion. This is not a widespread practice, although it has shown benefits as part of transitional care in some case series, promoting symptom improvement and avoidance of emergency department transfers and readmissions.37–40 Moreover, the CONFIRM-HF trial showed that iron replacement with ferric carboxymaltose significantly reduced the risk of hospitalization due to worsening heart failure, regardless of functional class severity, and had particular benefits in patients with diabetes or renal impairment,41 such as in our cohort, which comprised over 50% diabetic patients and 80% patients with renal insufficiency.
It seems clear that transition care interventions can reduce readmissions. As Comín-Colet et al. noted in a recent review article,42 a paradigm shift in the management of chronic diseases has taken place in recent years. This new approach is based on the development of a multidisciplinary model that provides integrated care to patients with HF throughout the duration of the disease, ensuring a successful follow-up and transition of care to different health care settings depending on the progress of the condition. In our case, the STOP-HF Clinic is included in a specialized HF unit that fulfills the standards recommended by the Spanish Society of Cardiology.43
Moreover, readmission risk assessment tools may help to appropriately target the delivery of these interventions to at-risk patients. Here, we predicted readmission risk using a validated risk score (the CORE-HF calculator). Although our cohort substantially differed from the population used to derive this score,18 we believe than the CORE-HF tool was appropriate given the high-risk population studied. However, it is notable that a reliable readmission predictive model for the current “real-life” population is not yet available.
Regarding our secondary endpoint, the present study was designed as a natural experiment rather than a conventional clinical trial. Our analysis included all patients within the STOP-HF Referral Area, regardless of their participation in the STOP-HF-Clinic. Their course was compared with that of a control group comprising the patients in the rest of the CatSalut area. This procedure minimized the characteristic selection bias of clinical trials, whose patient profile is often distinct from that of the general population and more pragmatically reflects the efficacy of the intervention. Our data revealed that the STOP-HF intervention had remarkable benefits during the post–STOP-HF period (2014-2015) compared with the pre–STOP-HF period (2012-2013) in terms of indicators relevant to the health care system and the patients (all-cause and HF-related 30-day readmissions).
LimitationsAlthough the current data reveal an association between the initiative implemented in our area and the improvement in clinical outcomes compared with the rest of the CatSalut area, population-based natural studies have limited ability to establish causality. Nevertheless, the inclusion of all patients admitted to hospitals in Catalonia for HF during the study period enabled us to avoid the selection bias inherent to clinical trials and to improve the generalizability of the results. Another limitation was our inability to determine the most effective component of the STOP-HF-Clinic design. Thus, the results of the STOP-HF-Clinic must be analyzed as a whole. While there is certainly room for improvement in our interventional design, our approach accounts for the multiple comorbidities encountered in the HF population and better reflects an intervention that, although focused on HF, was designed for the integrated care of old and frail patients in their transition from hospital to home.
ConclusionsThe STOP-HF-Clinic included early follow-up, HF nurses tasked with medication reconciliation, education and patient self-care empowerment, staff assigned to follow up on postdischarge test results, immediate availability of intravenous treatments and patient treatment titration, and partnerships with community physicians. This intervention resulted in an ∼50% reduction in the all-cause 30-day readmission rate after an index hospitalization for HF, which was mainly driven by a reduction in HF-related readmissions. Our results with the STOP-HF-Clinic in an elderly and frail comorbid population were better than with previously reported strategies. Our present data support the value of the STOP-HF-Clinic, an approach that could be implemented elsewhere to reduce the global burden of HF readmissions.
CONFLICTS OF INTERESTNone declared.
- –
Heart failure is associated with a high rate of readmissions within 30 days postdischarge.
- –
Old and frail patients are the most vulnerable and prone to require premature readmission after being hospitalized for HF.
- –
Strategies to lower these readmission rates have generally shown modest results.
- –
An early postdischarge multidisciplinary approach including face-to-face health literacy, intravenous therapy, and improved primary care transition significantly reduced 30-day readmission rates among discharged HF patients.
- –
This early multidisciplinary hospital-based intervention for the most vulnerable patients with HF reduced the global readmission burden, as shown in a population-based natural experiment including all HF readmissions in Catalonia, Spain, between 2012 and 2015.
We thank the nurses of the STOP-HF-Clinic—Roser Cabanes, Margarita Rodríguez, Carmen Rivas, Nuria Benito, and Alba Ros—for data collection and their invaluable work in the Clinic. This study was funded by the Red de Investigación Cardiovascular - RIC (RD12/0042/0047) and Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (FIS PI14/01682) projects as part of the Plan Nacional de I+D+I and was cofunded by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER).