Keywords
INTRODUCTION
Cardiac resynchronization therapy (CRT) by atrio-biventricular pacing has become a promising treatment for patients with advanced heart failure, severe systolic ventricular dysfunction and intra- and interventricular conduction defects caused principally by left bundle branch block.1
Several studies have shown that CRT can improve functional class and peak oxygen uptake, reduce the number of admissions to hospital due to decompensation and increase left ventricular ejection fraction.2-5 However, up to 30% of the patients enrolled in these studies did not respond to CRT, perhaps because of poor patient selection or an inappropriate site for the left ventricular electrode.6
Potential candidates for this therapy are currently selected by clinical criteria (New York Heart Association [NYHA] functional class III or IV) and electrocardiographic findings (intraventricular conduction disorder, particularly left bundle branch block evidenced by QRS complex duration >120 ms).7 These electrocardiographic criteria are applied because it is thought that intra- and interventricular electromechanical delay, and therefore ventricular dyssynchrony, are caused by left bundle branch block.8 Nevertheless, the duration of the QRS complex does not correlate clearly with the degree of dyssynchrony and is certainly not a strong predictor of favorable response. Thus, other more specific diagnostic techniques are being investigated to assess inter- and intraventricular dyssynchrony. Various types of echocardiography have shown promising results for diagnosis of dyssynchrony and prediction of a favorable response to CRT, though echocardiographic criteria for diagnosis still await widespread validation. Importantly, echocardiography is non-invasive and can be repeated several times during the follow-up of a given patient.9
The choice of left ventricular electrode site for best resynchronization is often difficult because of the characteristics of the coronary venous anatomy, unacceptable electrical parameters in the target area or other technical difficulties.10 The lateral or post-lateral part of the left ventricle has been identified as the site that provides greatest acute hemodynamic benefit, particularly for dp/dt,11-13 but the effect of left ventricular electrode site on echocardiographic parameters after resynchronization has yet to be determined.
The objective of the present study was to assess intra- and interventricular dyssynchrony with Doppler echocardiography before and at 3 months after implantation of an atrio-biventricular pacing device and to quantify how the degree of resynchronization varies according to site of left ventricular pacing in patients with advanced heart failure who underwent CRT.
PATIENTS AND METHODS
Study Population
From a series of 49 patients who underwent CRT, we selected the first 13 with lateral left ventricular implantation of the electrode and the first 12 with the electrode implanted in the anterior position. All had chronic heart failure of NYHA functional class III or IV despite maximum tolerated medication, left ventricular dysfunction (ejection fraction <35%), sinus rhythm and left bundle branch block with a QRS complex duration >120 ms. Patients with acute heart failure, valve disease or ischemic heart disease treatable by surgery, those indicated for conventional pacemakers, and those with permanent atrial fibrillation were excluded from the study.
Study Procedures
Clinical history, a 12-lead electrocardiogram (ECG) and a Doppler echocardiogram were recorded, and a physical examination was performed for all patients before implantation of the device. All patients underwent the same evaluations after 3 months. The echocardiograms were recorded with and without resynchronization. The maximum duration of the QRS complex was measured in leads II, V1 and V6 of the ECG.
Echocardiographic Study
The echocardiographic study (Sonos 5500, Hewlett-Packard, 3 MHz probe) was performed in left lateral decubitus before implantation of the resynchronization device (baseline conditions) and after 3 months with atrio-biventricular pacing (device connected) and without pacing (device disconnected). Images were taken in the parasternal long- and short-axis views, two- and four- chamber apical views, and subxiphoid view with continuous electrocardiographic monitoring during the examination.
The ejection fraction and left ventricular end-diastolic diameter were determined with the Teichholtz formula.
Interventricular dyssynchrony was defined as the difference in electromechanical delay in the right ventricle (time in milliseconds from onset of the QRS complex until onset of pulmonary flow) and left ventricle (time in milliseconds from onset of the QRS complex until onset of aortic flow).
Intraventricular dyssynchrony was assessed with the following parameters:
- Septal-to-posterior-wall motion delay (SPWMD): the delay (difference in milliseconds) between maximum displacement of the septum and of the posterior wall, measured in M-mode along the parasternal axis.
- Septal-to-lateral-wall motion delay (SLWMD): the difference (in milliseconds)--assessed by tissue echo-Doppler evaluation--between onset of the QRS complex and the maximum systolic velocity of each ventricular region along the 4-chamber apical axis.
The following parameters were also assessed: aortic flow velocity integral (measured by pulsed echo-Doppler technique at the outflow tract of the left ventricle), the mitral filling pattern by pulsed echo-Doppler technique (E and A waves) and tissue echo-Doppler evaluation of the mitral annulus velocity (E' and A' waves) and the E/E' ratio.
All echocardiographic studies were performed by a cardiologist who had no other part in the study and who was blinded to the device programming. The final value was the mean of three measurements of different cycles.
Device Implantation
Implantation was performed in an electrophysiology laboratory equipped with a Hicor digital angiography system (Siemens). The procedure was performed under local anesthetic with normal venous approaches (subclavian and/or cephalic vein) using previously described techniques.14
All patients underwent angiography of the coronary venous system, and the left ventricular electrode was implanted preferably in the lateral region. An anterior site was chosen as a last resort if lateral implantation was not possible or if a lateral site produced unacceptable pacing thresholds.
The atrioventricular interval was optimized by echo-Doppler technique and by previously described methods15 in all patients. We checked that the generator was functioning correctly on discharge from hospital by measurement of thresholds and radioscopic control of the electrodes.
An observer, who did not participate in any other way in the study, analyzed the difference between maximum width of the QRS complex during pacing and width of the complex at baseline in each patient.
Site of Left Ventricular Electrode
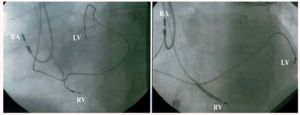
The anterior zone was defined as lying between 10 and 1 o'clock and the lateral zone as lying between 2 and 5 o'clock in the 45º left anterior oblique projection (Figure 1).
Fig. 1. 45º left anterior oblique x-ray image. Left: anterior left ventricular electrode. Right: lateral left ventricular electrode. RA indicates right atrial electrode; RV, right ventricular electrode; LV, left ventricular electrode.
The first 13 consecutive patients with the ventricular electrode in the lateral or posterolateral position and the first 12 consecutive patients with the electrode in the anterior position were included.
The parameters at baseline and after resynchronization were first compared for all 25 patients of the overall population and then analyzed by the final left ventricular pacing site.
Statistical Analysis
The numerical results from the study are expressed in the study as mean±standard deviation. The Kolmogorov-Smirnov test, with the Lillefors correction, was used to evaluate how well the data fitted a normal distribution. The differences in percentages between groups were compared with the exact test (PEPI statistics package, Abramson and Gahlinger, 1993-2000). The differences between different variables were compared by the Student t-test for dependent and independent samples as appropriate. Statistical significance was set at P<.05.
RESULTS
All devices were pacing correctly when the patients were discharged from hospital and after 3 months. The demographic and clinical characteristics of the overall patient group are presented in Table 1.
Twenty patients received a Guidant TR 1241 resynchronization device and 5 an automatic defibrillator with biventricular pacing capability (Guidant H135 and 1823). The mean acute pacing threshold was 1.4±0.6 volts, with mean left ventricular electrode impedance of 1.082±307 Ohms and amplitude of 11.1±5.7 millivolts.
At 3 months, an improvement in ejection fraction (23.7±6.5 at baseline vs 27.8±5.5% after 3 months) and a decrease in end-diastolic diameter of the left ventricle were observed after CRT (72.9±8.2 mm at baseline vs 65.6±6.6 mm at 3 months). This was associated with an increase in the aortic flow velocity integral (19.6±4.3 at baseline vs 21.3±4.9 at 3 months) and with a decrease in the ratio of mitral flow to mitral annulus velocity (E/E') (15.7±5.7 at baseline vs 10.7±5.3 at 3 months). The ejection fraction and aortic flow velocity integral improved significantly only in the group of patients with lateral pacing, whereas end-diastolic diameter and E/E' ratio improved with both lateral pacing and anterior pacing (Table 2).
Electrocardiographic Parameters
The mean duration of the baseline QRS complex was 159.9±29.4 ms. After biventricular stimulation, this duration was 157.8±20.2 ms (P=.77).
No statistically significant differences were seen in the width of the QRS complex before and after implantation of the resynchronization device regardless of whether pacing was lateral (156.0±33.8 ms vs 154.7±18.2 ms; P=.58) or anterior (161.9±25.3 ms vs 160.9±22.6 ms; P=.23).
Interventricular Dyssynchrony
The electromechanical delay between left and right ventricle was similar on comparison of baseline with 3 months pacing regardless of whether the device was connected (38.3±22.3 ms at baseline vs 31.1±24.2 ms after 3 months; P=.35) or disconnected (38.3±22.3 ms at baseline vs 37.8±21.8 ms after 3 months; P=.42). Similarly, when the same parameters were compared according to anterior or lateral pacing, no statistically significant differences were observed (Table 3).
Intraventricular Dyssynchrony
Septal-posterior-wall motion delay was significantly lower after 3 months of pacing when measured with the pacing device connected (86.6±35.4 ms at 3 months; Figure 2) compared to measurement with the device disconnected (218.3±44.7 ms; Figure 2) in the overall patient population (P<.001). When the device was disconnected, baseline SPWMD and SPWMD at 3 months showed no significant differences (218.3±44.7 vs 211.6±34.7 ms; P=.56).
Fig. 2. M-mode parasternal echocardiograph showing motion delay between septum and posterior wall with pacing device connected (right) and disconnected (left). SWMD indicates septum wall motion delay; CRT "off," pacing device disconnected; CRT "on," pacing device connected.
Similarly, resynchronization was also associated with a statistically significant decrease (P=.007) in SLWMD at baseline (111.4±30.9 ms) compared to SLWMD at 3 months with the device connected (61.9±21.3 ms; Figure 3). Baseline SLWMD was similar to SLWMD with the device disconnected at the end of follow-up (111.4±30.9 vs 115.3±28.6; P=.58).
Fig. 3. Tissue Doppler quantification of septal-to-lateral-wall motion delay. Top: before resynchronization, delay of 340 ms. Bottom: after resynchronization, improvement of 100 ms. TDLW indicates tissue Doppler of lateral wall; TDS, tissue Doppler of the septum. CRT, cardiac resynchronization therapy.
Both SPWMD and SLWMD were significantly smaller in patients with lateral pacing compared to those with anterior pacing (Table 3).
Events During Follow-Up
Of the 25 patients, 3 (16%) were considered non-responders to treatment because their NYHA functional class remained unchanged and/or the number of meters covered in the 6-minute walk test did not increase by >10%. All 3 non-responders had anterior pacing of the left ventricle.
DISCUSSION
The tissue echo-Doppler technique in M-mode used in this study shows that SPWMD and SLWMD return to normal after implantation of a cardiac resynchronization device. These findings agree with other studies in which intraventricular electromechanical delay (SPWMD and SLWMD) improves after resynchronization, and such parameters may even by useful for predicting benefit from CRT.16,17 Maximum systolic velocity of the septum and the left ventricular free wall and the delay in contraction between these areas were assessed by tissue echo-Doppler techniques. The specificity and sensitivity of the delay was approximately 90% for prediction of clinical improvement after CRT.18
This study specifically shows the influence of the left ventricular electrode site on the correction of intraventricular dyssynchrony. We are not aware of any studies that assess medium-term response to CRT by site of ventricular stimulation. The parameters that were measured in our population improved after 3 months with both lateral and anterior pacing. For lateral pacing, the improvement was large and statistically highly sign ificant, in contrast to the small improvements with anterior pacing that were not statistically significant. Likewise, the improvement in ejection fraction and aortic flow velocity integral was only significant for lateral pacing, which reinforces the results for resynchronization. The effectiveness of resynchronization may be greater when the electrode is in a lateral position because there is a larger distance between the right and left ventricular electrodes. The two fronts of activation are therefore further apart, in contrast to anterior implantation, which places the left ventricular electrode much closer to the right ventricular electrode. Similarly, our findings might explain the improved hemodynamic effects (indicated by dp/dt) for lateral implantation of the electrode compared to anterior implantation.19 A recent observational study found a similar clinical effect for patients with lateral and anterior implantation of the electrode,20 but there are no randomized studies to compare the effect on medium-term clinical outcome of the two sites in patients who undergo resynchronization.
The importance of the site of the electrode in determining which patients are non-responders remains to be clarified. The 3 non-responders in our patient population had anterior pacing. Our conclusions are necessarily limited by the low number of patients, but these results do suggest that it is advisable to choose lateral implantation of the left ventricular electrode whenever possible despite the greater technical difficulties.21 Unfortunately, lateral implantation may not be possible in more than 30% of the patients because of unfavorable venous anatomy, high pacing thresholds, instability of the electrode, or the presence of phrenic pacing.20
No differences in interventricular dyssynchrony were found and device implantation seemed to have little effect, possibly because of the low degree of interventricular dyssynchrony observed in our patients. These results agree with some studies,22 but others find a clear improvement in interventricular dyssynchrony during CRT.23 This discrepancy might arise because the difference in the electromechanical delays of the 2 ventricles does not fully correlate with the dyssynchrony of the left ventricular free wall.24
In agreement with other studies, CRT was associated with an improvement in ejection fraction and a reduction in end-diastolic diameter at 3 months in patients with lateral pacing, suggesting that regression of ventricular remodeling may occur in these patients.25 The same dyssynchrony persists at 3 months with the device disconnected. We might expect improvement in dyssynchrony after a positive change in ventricular remodeling, but the persistence of conduction disorders may counteract the improved ventricular remodeling so the degree of dyssynchrony remains unchanged. We also found that CRT decreased the ratio of E/E'. Values for this ratio above 15 have been associated with increased ventricular filling pressures as assessed by echocardiography.26
Currently, the main criterion for diagnosis of dyssynchrony is left bundle branch block as evidenced by QRS complex duration >120 ms. However, left bundle branch block does not always lead to alterations in ventricular synchrony and may be the result of other anomalies such as actual conduction defects or slowing of propagation of the electrical impulse in ischemic or necrotic tissue.27 In fact, our study showed no differences in the width of the QRS complex before and after resynchronization. This width does not correlate with the degree of resynchronization after implantation even though a width of around 150 ms has been shown to discriminate between responders and non-responders to CRT.28 Our findings agree with other studies which show little association between the number of responders and narrowing of the QRS complex with biventricular pacing.29 Given that the width of the QRS complex, the degree of dyssynchrony and response to CRT are poorly correlated, the echo-Doppler technique may prove a useful tool for diagnosis of dyssynchrony in order to reduce the proportion of non-responders. This potential application should be investigated in prospectively designed studies.
Study Limitations
The pacing site was not randomly assigned but rather chosen according to accessibility and electrical parameters. We therefore cannot separate the effects of the site of electrode implantation from other factors (whether anatomical or of some other kind) that prevented implantation in the lateral site.
The electromechanical and ejection delays of the different chambers were recorded from different heart cycles and so were inevitably subject to beat-to-beat variations.
The methods available at present for quantifying dyssynchrony are limited in a number of ways. First, tissue Doppler techniques do not differentiate between passive and active movement of the myocardial wall. Deformation measures known as the strain rate would be needed for better quantification. Second, for the measurement of SPWMD in M-mode, 2 small areas of the myocardium were compared on the assumption that they were representative of the entire wall movement. A further source of error may lie in the assumption that exactly the same wall segment was used for with and without resynchronization. Finally, the limitations of the calculation of the ejection fraction with the Teichholtz formula, particularly in patients with segmental contraction disorders, are well known.
CONCLUSIONS
Lateral pacing of the left ventricle is associated with greater intraventricular resynchronization than anterior pacing after implantation of a cardiac resynchronization device. Measurement of SPWMD and SLWMD by echocardiography is useful for diagnosis of intraventricular dyssynchrony, can help assess changes in synchrony according to the pacing site and may contribute to an evaluation of the effectiveness of CRT.
Correspondence: Dr. I. García-Bolao.
Departamento de Cardiología y Cirugía Cardiovascular.
Clínica Universitaria de Navarra.
Avda. Pío XII, s/n. 31008 Pamplona. Navarra, España.
E-mail: igarciab@unav.es