Several studies have linked the presence of atrial fibrillation (AF) with reduced estimated glomerular filtration rate (eGFR). Our objective was to compare changes in eGFR in patients with AF after pulmonary vein (PV) ablation depending on the success of the technique, as well as to examine the relationship between eGFR and several biomarkers.
MethodsProspective cohort of patients with AF referred to our center for PV ablation with a 1-year follow-up. We estimated eGFR using the Chronic Kidney Disease Epidemiology Collaboration formula at baseline and at 3 and 12 months. Biomarkers (B-type natriuretic peptide, corin, and galectin-3) were measured before ablation and at 12 months.
ResultsWe studied 124 patients (age 55±10 years, 69.4% men). Seventy-five had paroxysmal AF (60.5%). The mean baseline eGFR was 90.8 [77.8-100.0] mL/min/1.73 m2. The eGFR increased at the end of follow-up, with a statistically significant difference between patients with recurrence at 12 months and those without (−1.1 [-6.0 to 8.8] mL/min/1.73 m2 vs 7.1 [−0.6 to 14.2] mL/min/1.73 m2, P=.017). The improvement in eGFR at 12 months was inversely proportional to baseline eGFR. B-type natriuretic peptide and corin levels improved at 12 months, while galectin-3 levels worsened, which was unrelated to eGFR.
ConclusionsIn patients with AF treated with PV ablation, an overall improvement in eGFR was observed, which was more marked in the subgroup without recurrences, although without significant differences on multivariate analysis.
Keywords
Renal and cardiac functions are closely linked because the 2-way interaction between the kidneys and heart hinges on complex neurohumoral mechanisms, which are widely recognized in fields such as heart failure.1 However, the relationship between renal function and arrhythmias such as atrial fibrillation (AF) is less well understood. Various studies have found a significant association between AF prevalence and a reduced estimated glomerular filtration rate (eGFR).2,3 In addition, renal function deterioration is a powerful marker of AF onset in hypertensive patients4 and of AF recurrence after electrical cardioversion5,6 and catheter ablation.7,8
Only 1 prospective study9 has analyzed the relationship between AF ablation and temporal changes in renal function. In patients without arrhythmia recurrence, the eGFR was higher at 1-year follow-up; in contrast, in patients with recurrence, the eGFR was lower. A retrospective study also found eGFR improvement during follow-up, particularly in patients who maintained sinus rhythm.10 Another retrospective study found that patients with arrhythmia recurrence after AF ablation had a worse eGFR, both at baseline and at 1 year of follow-up, vs patients without recurrence.11
The objective of the present study was to further our understanding of the relationship between renal function and AF by evaluating the changes in eGFR at 1-year of follow-up after pulmonary vein (PV) ablation in a European population and with the formula currently recommended by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) in the KDIGO guidelines.12 This work is valuable because the only other prospective study evaluated a Japanese population with a formula specifically formulated for that population9 and the results are thus not applicable to other population groups. In addition, the secondary objective included an analysis of biomarkers (B-type natriuretic peptide [BNP], corin, and galectin-3) as possible predictors of changes over time in renal function and of recurrence after PV ablation.
METHODSStudy design and populationThis prospective cohort study consecutively enrolled patients with paroxysmal or persistent AF referred to our center according to routine clinical practice to undergo PV ablation from June 2015 to February 2017. Patient follow-up was performed for 12 months. All patients signed written informed consent for the study, which had previously been approved by the local research ethics committee.
The exclusion criteria were as follows: a) patient refusal to sign the informed consent; b) an inability to perform clinical follow-up or biological sample collection; c) a personal history of severe renal impairment (eGFR < 30mL/min/1.73 m2); d) patients on hemodialysis; e) acute myocardial infarction or percutaneous coronary intervention in the 6 months before the ablation; and f) AF ablation in the 6 months before the performance of the study.
Renal function and atrial fibrillation markersThe analysis included BNP, corin, and galectin-3 measurements at baseline and 1 year of follow-up. Blood samples for analysis of corin and galectin-3 were centrifuged and frozen at −80°C after extraction until their analysis.
Catheter ablationThe ablation procedure was performed as described in the supplementary data and as illustrated in .
Follow-upAfter the ablation, patients continued their antiarrhythmic therapy and oral anticoagulation for the first 3 months, considered the window period. At the 3-month follow-up, Holter monitoring was performed for 4 to 7 days, as well as blood and urine analysis. At 12 months, a 24-hour Holter electrocardiogram was performed, as well as urine and blood biomarker analysis. Any atrial arrhythmia (AF, flutter, or atrial tachycardia) lasting ≥ 30seconds beyond the window period was considered recurrence. Because no event recorder was available, patients who experienced palpitations were directed to go to their health care center or emergency department in an effort to document possible recurrences.
Renal function evaluationThe eGFR was calculated according to the 2012 KDIGO guidelines,12 which recommend its estimation using the CKD-EPI formula. The eGFR was measured at baseline and 3 and 12 months after the ablation. Any increase during follow-up vs baseline was considered an eGFR improvement. In patients who received iodinated contrast during the procedure for rotational angiography, a 24-hour analysis was performed to rule out contrast nephropathy, defined as an absolute increase in serum creatinine ≥ 0.5mg/dL or a relative increase ≥ 25% within 24hours after contrast exposure.13
Statistical analysisNumerical variables are expressed as mean ± standard deviation or, for nonnormally distributed variables, as median [interquartile range]. Categorical variables are expressed as absolute and relative frequencies. To compare the possible differences in categorical variables between the groups according to type of AF and eGFR group, the Pearson chi-square test was used or the Fisher exact test, as appropriate. For numerical variables, the t test and ANOVA were used for independent samples and the Mann-Whitney and Kruskal-Wallis for nonparametric variables. Variable normality was assessed with the Shapiro-Wilk test. The repeated measures general linear model was applied to compare the changes over time in the different follow-up parameters, considering time as an individual factor and the comparison group (eg, the different cardiovascular risk factors and treatments) as an interindividual factor. The McNemar test was used to compare qualitative parameters at different times. A mixed-effects linear regression model was performed to analyze the variables influencing the changes in the eGFR. It was adjusted for time, recurrence, and the remaining possible confounding variables, and the patients were considered a random effect in the model. First, the null model was adjusted to consider patient variability alone (model I); then, it was adjusted by the time variable (model II); finally, it was adjusted by the other covariables (model III). All comparisons were considered significant at P <.05. Data were analyzed with SPSS 19.0 software (SPSS Inc; Chicago, Illinois, United States).
RESULTSBaseline characteristicsOut of 174 patients who underwent PV ablation during the study inclusion period, 124 were enrolled. The reasons for patient exclusion were as follows: 1 due to severe renal impairment (2%; eGFR, 23mL/min); 5 due to ablation within the previous 6 months (10%); 6 due to their participation in another study that was incompatible with ours (12%); 26 due to patient refusal or impossibility of follow-up (52%); and 12 because biomarker sampling could not be performed (24%).
The mean patient age was 55 ± 10 (range, 22-75) years and 86 were men (69.4%). The PV ablation was performed using radiofrequency in 98 patients (79%) and using cryoablation in 26 (21%). In addition, 75 patients had paroxysmal AF (60.5%) and 49 had persistent AF (39.5%). The ablation procedure was the first in 96 patients (77.4%) but a previous ablation procedure had been performed in 28 (22.6%). The baseline eGFR was 90.8 [73.8-90.8] mL/min/1.73 m2 and the baseline creatinine was 0.88 [0.78-1.03] mg/dL. Patients were divided into 3 groups according to their baseline eGFR: group 1 (67 patients, 54%) with a normal eGFR (≥ 90mL/min/1.73 m2); group 2 (48 patients, 38.7%) with a slightly reduced eGFR (60-89mL/min/1.73 m2); and group 3 (9 patients, 7.3%) with a moderately reduced eGFR (30-59mL/min/1.73 m2). The 9 patients with an eGFR <60mL/min/1.73 m2 had no recorded history of renal impairment but all were hypertensive, 2 were diabetic, and 1 had systemic lupus erythematosus as possible causes of the renal impairment deterioration. No patients had an eGFR <30mL/min/1.73 m2 because it was an exclusion criterion. No contrast nephropathy occurred within 24hours after the ablation.
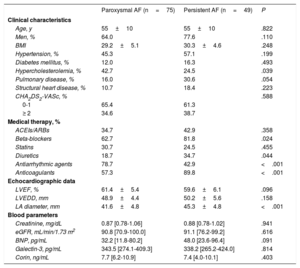
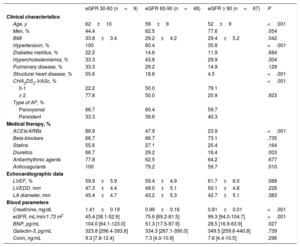
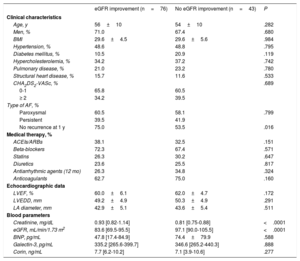
The clinical, echocardiographic, and blood parameter characteristics of the patients according to AF type and baseline eGFR are shown in table 1 and table 2, respectively. Patients with persistent AF were more frequently treated with diuretics, beta-blockers, and anticoagulants than those with paroxysmal AF; they also had a larger left atrial diameter. In contrast, patients with paroxysmal AF had a higher frequency of hypercholesterolemia and a greater use of antiarrhythmic drugs. There were no differences between the 2 groups in baseline eGFR, baseline creatinine, or biomarker levels. When patients were grouped by baseline eGFR, those with worse eGFR (groups 2 and 3) were older, were more likely to have hypertension and structural heart disease, and had a higher body mass index, greater use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, diuretics, and anticoagulants, and a higher baseline BNP concentration.
Baseline characteristics according to type of atrial fibrillation
| Paroxysmal AF (n=75) | Persistent AF (n=49) | P | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, y | 55±10 | 55±10 | .822 |
| Men, % | 64.0 | 77.6 | .110 |
| BMI | 29.2±5.1 | 30.3±4.6 | .248 |
| Hypertension, % | 45.3 | 57.1 | .199 |
| Diabetes mellitus, % | 12.0 | 16.3 | .493 |
| Hypercholesterolemia, % | 42.7 | 24.5 | .039 |
| Pulmonary disease, % | 16.0 | 30.6 | .054 |
| Structural heart disease, % | 10.7 | 18.4 | .223 |
| CHA2DS2-VASc, % | .588 | ||
| 0-1 | 65.4 | 61.3 | |
| ≥ 2 | 34.6 | 38.7 | |
| Medical therapy, % | |||
| ACEIs/ARBs | 34.7 | 42.9 | .358 |
| Beta-blockers | 62.7 | 81.8 | .024 |
| Statins | 30.7 | 24.5 | .455 |
| Diuretics | 18.7 | 34.7 | .044 |
| Antiarrhythmic agents | 78.7 | 42.9 | <.001 |
| Anticoagulants | 57.3 | 89.8 | <.001 |
| Echocardiographic data | |||
| LVEF, % | 61.4±5.4 | 59.6±6.1 | .096 |
| LVEDD, mm | 48.9±4.4 | 50.2±5.6 | .158 |
| LA diameter, mm | 41.6±4.8 | 45.3±4.8 | <.001 |
| Blood parameters | |||
| Creatinine, mg/dL | 0.87 [0.78-1.06] | 0.88 [0.78-1.02] | .941 |
| eGFR, mL/min/1.73 m2 | 90.8 [70.9-100.0] | 91.1 [76.2-99.2] | .616 |
| BNP, pg/mL | 32.2 [11.8-80.2] | 48.0 [23.6-96.4] | .091 |
| Galectin-3, pg/mL | 343.5 [274.1-409.3] | 338.2 [265.2-424.0] | .814 |
| Corin, ng/mL | 7.7 [6.2-10.9] | 7.4 [4.0-10.1] | .403 |
ACEIs, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARBs, angiotensin II receptor blockers; BMI, body mass index; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
Variables with a normal distribution are presented as mean ± standard deviation, whereas those with a nonnormal distribution are presented as median [interquartile range].
Baseline characteristics grouped according to estimated glomerular filtration rate
| eGFR 30-60 (n=9) | eGFR 60-90 (n=48) | eGFR ≥ 90 (n=67) | P | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 62±10 | 59±9 | 52±9 | <.001 |
| Men, % | 44.4 | 62.5 | 77.6 | .054 |
| BMI | 33.8±3.4 | 29.2±4.2 | 29.4±5.2 | .042 |
| Hypertension, % | 100 | 60.4 | 35.8 | <.001 |
| Diabetes mellitus, % | 22.2 | 14.6 | 11.9 | .684 |
| Hypercholesterolemia, % | 33.3 | 43.8 | 29.9 | .304 |
| Pulmonary disease, % | 33.3 | 29.2 | 14.9 | .129 |
| Structural heart disease, % | 55.6 | 18.8 | 4.5 | <.001 |
| CHA2DS2-VASc, % | <.001 | |||
| 0-1 | 22.2 | 50.0 | 79.1 | |
| ≥ 2 | 77.8 | 50.0 | 20.9 | .923 |
| Type of AF, % | ||||
| Paroxysmal | 66.7 | 60.4 | 59.7 | |
| Persistent | 33.3 | 39.6 | 40.3 | |
| Medical therapy, % | ||||
| ACEIs/ARBs | 88.9 | 47.9 | 23.9 | <.001 |
| Beta-blockers | 66.7 | 66.7 | 73.1 | .735 |
| Statins | 55.6 | 27.1 | 25.4 | .164 |
| Diuretics | 66.7 | 29.2 | 16.4 | .003 |
| Antiarrhythmic agents | 77.8 | 62.5 | 64.2 | .677 |
| Anticoagulants | 100 | 79.2 | 59.7 | .010 |
| Echocardiographic data | ||||
| LVEF, % | 59.9±5.9 | 59.4±4.9 | 61.7±6.0 | .088 |
| LVEDD, mm | 47.3±4.4 | 49.0±5.1 | 50.1±4.8 | .226 |
| LA diameter, mm | 45.4±4.7 | 43.2±5.3 | 42.7±5.1 | .383 |
| Blood parameters | ||||
| Creatinine, mg/dL | 1.41±0.19 | 0.99±0.16 | 0.81±0.01 | <.001 |
| eGFR, mL/min/1.73 m2 | 45.4 [38.1-52.9] | 75.6 [69.2-81.5] | 99.3 [94.0-104.7] | <.001 |
| BNP, pg/mL | 104.0 [64.1-123.0] | 51.3 [17.5-97.9] | 28.5 [16.9-63.9] | .027 |
| Galectin-3, pg/mL | 323.8 [296.4-393.8] | 334.3 [267.1-390.0] | 349.5 [259.6-440.8] | .739 |
| Corin, ng/mL | 9.3 [7.8-12.4] | 7.3 [4.0-10.9] | 7.6 [4.4-10.5] | .296 |
ACEIs, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARBs, angiotensin II receptor blockers; BMI, body mass index; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
Variables with a normal distribution are presented as mean ± standard deviation, whereas those with a nonnormal distribution are presented as median [interquartile range].
Circumferential PV isolation was performed in all patients. Isolation of all targeted veins was achieved in 121 patients (97.6%). A second ablation procedure was performed in 9 patients (7%) during the first postprocedural year. This procedure was performed with radiofrequency in all patients; these cases were considered recurrences. At 12 months, 84 patients (67.7%) were recurrence free; of these, 57 had paroxysmal AF (76%) and 27 had persistent AF (55%) (P=.013). Of the 40 patients with recurrence, the recurrence was paroxysmal AF in 28 (22.6%), persistent AF in 8 (6.5%), and atypical flutter in 4 (3.2%). Recurrences were documented in 16 patients (12.9%) using electrocardiography performed during emergency department treatment, in 20 (16.1%) using electrocardiography or Holter monitoring performed during clinical follow-up, and in 4 (3.3%) using both methods. Of the 84 patients without 12-month recurrence, 11 (13%) were taking antiarrhythmic drugs at the end of follow-up vs 25 patients with recurrence (62.5%) (P <.0001). No relationship was found between the biomarkers and recurrence; on multivariate analysis, the only variable associated with recurrence was the presence of persistent AF (odds ratio=2.3; 95% confidence interval [95%CI], 0.9-5.7).
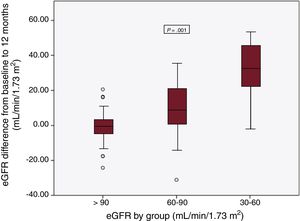
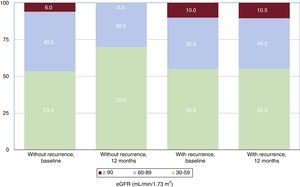
Changes in glomerular filtrationAt the 1-year follow-up and in the overall patient group, the eGFR increased from 90.8 [77.8-100.0] to 95.6 [84.8-103.3] mL/min/1.73 m2 (P <.0001) and the creatinine level decreased from 0.88 [0.78-1.03] to 0.82 [0.76-0.92] mg/dL (P <.0001). There was no difference in the eGFR improvement according to recurrence during the window period (P=.095). However, there were differences among the baseline, 3-month, and 12-month eGFRs. These differences were due to time, not group, although there was an interaction between time and group (table 3). The eGFR increased until the end of follow-up by 2.9 [–2.7 to 11.8] mL/min/1.73 m2 on average, with significant differences between patients with 12-month recurrence and those without (–1.1 [–6.0 to 8.8] vs 7.1 [–0.6 to 14.2] mL/min/1.73 m2; P=.017). The 12-month improvement in eGFR was inversely proportional to the baseline eGFR. Thus, patients with an eGFR 30-59mL/min/1.73 m2 showed the largest increase in eGFR (group 1, –0.2 [–4.3 to 3.8] mL/min/1.73 m2; group 2, 9.1 [0.9 to 21.5] mL/min/1.73 m2; group 3, 32.9 [19.4 to 47.6] mL/min/1.73 m2; P=.001) (figure 1). Of patients without 12-month recurrence, 5% were in group 3 and 41.2% were in group 2 at baseline, whereas they numbered 0% and 30%, respectively, at 12 months (P <.0001) (figure 2). In contrast, patients with recurrence comprised the same percentage of all patients in each group at baseline and at 12 months (P=.006). The clinical, echocardiographic, and blood parameter characteristics were similar in patients with an eGFR improvement at the end of follow-up and in those without such an improvement, although the group with an eGFR improvement showed a tendency for a higher number of diabetic patients, better left ventricular ejection fraction, greater use of anticoagulants, and lower use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers. The only variable associated with an eGFR improvement was recurrence presence/absence (table 4).
Repeated measures general linear model for analyzing the eGFR at baseline and 3 and 12 months according to recurrence
| Without recurrence | With recurrence | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 mo | 12 mo | Baseline | 3 mo | 12 mo | Group | Time | Group × Time | |
| eGFR | 88.2±15.4 | 90.2±13.9 | 93.9±12.6 | 86.6±20.4 | 92.7±16.7 | 87.6±19.9 | .556 | .026 | .006 |
eGFR, estimated glomerular filtration rate.
Data are presented as mean ± standard deviation.
Baseline characteristics grouped according to eGFR at 1 year of follow-up
| eGFR improvement (n=76) | No eGFR improvement (n=43) | P | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, y | 56±10 | 54±10 | .282 |
| Men, % | 71.0 | 67.4 | .680 |
| BMI | 29.6±4.5 | 29.6±5.6 | .984 |
| Hypertension, % | 48.6 | 48.8 | .795 |
| Diabetes mellitus, % | 10.5 | 20.9 | .119 |
| Hypercholesterolemia, % | 34.2 | 37.2 | .742 |
| Pulmonary disease, % | 21.0 | 23.2 | .780 |
| Structural heart disease, % | 15.7 | 11.6 | .533 |
| CHA2DS2-VASc, % | .689 | ||
| 0-1 | 65.8 | 60.5 | |
| ≥ 2 | 34.2 | 39.5 | |
| Type of AF, % | |||
| Paroxysmal | 60.5 | 58.1 | .799 |
| Persistent | 39.5 | 41.9 | |
| No recurrence at 1 y | 75.0 | 53.5 | .016 |
| Medical therapy, % | |||
| ACEIs/ARBs | 38.1 | 32.5 | .151 |
| Beta-blockers | 72.3 | 67.4 | .571 |
| Statins | 26.3 | 30.2 | .647 |
| Diuretics | 23.6 | 25.5 | .817 |
| Antiarrhythmic agents (12 mo) | 26.3 | 34.8 | .324 |
| Anticoagulants | 62.7 | 75.0 | .160 |
| Echocardiographic data | |||
| LVEF, % | 60.0±6.1 | 62.0±4.7 | .172 |
| LVEDD, mm | 49.2±4.9 | 50.3±4.9 | .291 |
| LA diameter, mm | 42.9±5.1 | 43.6±5.4 | .511 |
| Blood parameters | |||
| Creatinine, mg/dL | 0.93 [0.82-1.14] | 0.81 [0.75-0.88] | <.0001 |
| eGFR, mL/min/1.73 m2 | 83.6 [69.5-95.5] | 97.1 [90.0-105.5] | <.0001 |
| BNP, pg/mL | 47.8 [17.4-84.9] | 74.4±79.9 | .588 |
| Galectin-3, pg/mL | 335.2 [265.6-399.7] | 346.6 [265.2-440.3] | .888 |
| Corin, ng/mL | 7.7 [6.2-10.2] | 7.1 [3.9-10.6] | .277 |
ACEIs, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARBs, angiotensin II receptor blockers; BMI, body mass index; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
Variables with a normal distribution are presented as mean ± standard deviation, whereas those with a nonnormal distribution are presented as median [interquartile range].
Patients without 12-month recurrence had a baseline eGFR similar to that of patients with recurrence (90.8 [75.7-100.0] vs 90.9 [69.8-100.3] mL/min/1.73 m2; P=.708). There were also no significant differences in recurrence according to baseline eGFR group (32.8% in group 1 vs 29.2% in group 2 vs 44.4% in group 3; P=.660).
Biomarker changesBNP levels decreased from 47.8 [17.5-85.7] to 32.6 [13.1-69.4] pg/mL at 1 year of follow-up (P=.013), although there were no significant differences between patients with and without recurrence at 1 year (P=.465). No relationships were found either between BNP and any of the other variables studied. Corin levels increased from 7.63 [4.72-10.64] to 10.60 [9.00-12.23] ng/mL at 12 months (P <.001), without differences according to recurrence (P=.461). Corin levels were higher at 12 months in the persistent AF group than in the paroxysmal AF group (P=.011); there were no differences in the other variables studied. Galectin-3 levels increased from 343.5 [266.4-411.7] pg/mL at baseline to 371.7 [310.8-470.5] pg/mL at 12 months (P=.025), but no associations were found with any of the variables studied.
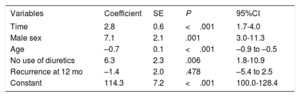
Variables associated with renal function improvementThe mixed-effects multivariate model showed a significant increase in the eGFR over time, with an average increase of 2.8 (95%CI, 1.7-4.0) mL/min/1.73 m2 during follow-up. Patients with recurrence had a nonsignificantly lower eGFR (–1.4; 95%CI, –5.4 to 2.5mL/min/1.73 m2). Male sex was directly associated with an eGFR increase of 7.1 (95%CI, 3.0-11.3) mL/min/1.73 m2; the rate improved by 6.3 (95%CI, 1.8-10.9) mL/min/1.73 m2 in patients not taking diuretics, whereas age was inversely associated with an eGFR deterioration of –0.71 (95%CI, –0.9 to –0.5) mL/min/1.73 m2 per year (table 5). None of the biomarkers studied was associated with the eGFR improvement.
Mixed-effects model for the analysis of variable affecting the changes over time in eGFR
| Variables | Coefficient | SE | P | 95%CI |
|---|---|---|---|---|
| Time | 2.8 | 0.6 | <.001 | 1.7-4.0 |
| Male sex | 7.1 | 2.1 | .001 | 3.0-11.3 |
| Age | –0.7 | 0.1 | <.001 | –0.9 to –0.5 |
| No use of diuretics | 6.3 | 2.3 | .006 | 1.8-10.9 |
| Recurrence at 12 mo | –1.4 | 2.0 | .478 | –5.4 to 2.5 |
| Constant | 114.3 | 7.2 | <.001 | 100.0-128.4 |
95%CI, 95% confidence interval; eGFR, estimated glomerular filtration rate; SE, standard error.
Our results show that patients treated with PV ablation exhibited improved renal function, as represented by eGFR. Of these patients, those without recurrence beyond the window period had a higher eGFR than those with recurrence, although the difference was not statistically significant in the multivariate model. This result is similar to that found in previous studies.9,10 In a study by Kornej et al.,11 there was no improvement in the eGFR after ablation but patients with recurrence had a higher probability of worse eGFR at the end of follow-up. These findings strengthen the hypothesis that renal function is influenced by the presence of AF.
As in the studies by Takahashi et al.9 and Navaravong et al.,10 our results showed that the eGFR improvement at 1 year of follow-up was inversely proportional to patients’ baseline eGFR, with a higher increase in patients initially in the lowest eGFR quartile. This association is important because it is considered more important in routine clinical practice to improve the eGFR of patients with a worse initial eGFR than to improve that of those with a normal eGFR. According to cardiovascular prevention guidelines,14 patients with moderate (eGFR, 30-59mL/min/1.73 m2) or severe (eGFR, <30mL/min/1.73 m2) chronic kidney disease should be included in high or very high cardiovascular risk categories, respectively. Thus, our patients whose eGFR increased from 30-59mL/min/1.73 m2 at baseline to > 60mL/min/1.73 m2 were moved to the high cardiovascular risk category.
Neither our work nor that of Takahashi et al.9 or Navaravong et al.10 included patients with an eGFR <30mL/min/1.73 m2, and Kornej et al.11 enrolled only 6 patients in this group (0.5% of the study population). Accordingly, it would be interesting to directly and specifically study this population. However, although our study and that by Navaravong et al.10 failed to find an association between worse baseline renal function and a higher rate of recurrences, other authors have reported this relationship.8,11,15–17
In the multivariate analysis, male patients had a higher eGFR while older patients had a worse eGF possibly because both variables are considered in the CKD-EPI formula. This makes it difficult to determine whether the relationship identified would remain if they were not included in this formula. Patients under treatment with diuretics had worse eGFR, probably because they have worse functional class or more difficult-to-control hypertension or due to the effects of the diuretics themselves on renal function.
The main reason for indicating a patient for AF ablation is to improve symptoms and, hence, quality of life; other potential objectives are withdrawal of antiarrhythmic drugs in the case of successful ablation or improved ventricular function in patients with tachycardia-induced cardiomyopathy. In light of our data, an improved renal function can be considered another possible benefit of ablation, although larger studies are needed to confirm this improvement.
The concentration of N-terminal pro-B type natriuretic peptide (NT-proBNP) is significantly elevated in patients with paroxysmal or persistent AF vs patients in sinus rhythm.18 Nonetheless, few studies have prospectively analyzed the association between NT-proBNP levels and the incidence of renal failure. In a study of 125 patients with heart failure followed up for 18 months, the risk of renal failure was significantly higher in patients with an elevated NT-proBNP (incidence rate ratio=3.6; 95%CI, 1.9-7.0).19 In the MESA study,20 NT-proBNP levels were significantly associated with the risk of atrial fibrillation (hazard ratio=2.2; 95%CI, 1.9-2.5). In our study, the BNP was lower at 1 year of follow-up in the overall sample, but no association was found with the eGFR or presence of recurrence. This information is relevant because a possible pathophysiological explanation for the renal function improvement after ablation is improved cardiac output and, consequently, renal perfusion pressure, due to effective maintenance of sinus rhythm. Confirmation of this finding requires specific studies.
Corin is a transmembrane protein that converts proatrial natriuretic peptide and pro-BNP into their active forms and plays an important role in regulating the salt-water balance, blood pressure, and cardiac function.21 Low levels of corin have been associated with worse New York Heart Association functional class, higher NT-proBNP concentrations, and lower left ventricular ejection fraction and eGFR; in addition, low serum levels of corin are an independent prognostic factor for major cardiovascular events in patients with heart failure.22 Lower concentrations of corin have also been found in patients with paroxysmal AF vs those with persistent AF.23 In our study, corin levels were increased during follow-up, which might be related to the improved cardiac function after ablation. In addition, galectin-3 is a profibrotic protein associated with hepatic, renal, pulmonary, and cardiac fibrosis. Fibrosis and electrical remodeling in the left atrium could be the end result of an intracellular signaling cascade (with a possible essential role played by galectin-3) that would help to stabilize and perpetuate the arrhythmia.24 Studies have correlated the presence of AF with elevated levels of galectin-3.25 At the same time, inhibition of galectin-3 might one day represent a therapeutic target for preventing myocardial fibrosis-associated cardiac remodeling.26 In contrast to the results of Takemoto et al.,27 our study indicated that galectin-3 levels can predict recurrence after AF ablation. The lack of an association of changes over time in biomarkers with the eGFR improvement, as well as with recurrence, could be because our study did not consider arrhythmia burden.
The patients included in our study were derived from those who underwent PV ablation according to routine clinical practice. Our results would thus be generalizable to a large portion of this population. Although patients with an eGFR <30mL/min/1.73 m2 were excluded, such patients are rarely referred for PV ablation, as indicated by the exclusion of only 1 patient from our study for this reason. Nonetheless, it would be beneficial to perform multicenter studies with a larger sample size that do not exclude any patients based on renal function. It would also be desirable to perform studies that include quantification of the arrhythmia burden because the lack of statistically significant differences between patients with and without recurrence beyond the window period could be due to the significantly lower arrhythmia burden of these patients than before ablation, despite their recurrence. This would explain why these patients also show an eGFR improvement, although less pronounced.
LimitationsAlthough most factors possibly associated with renal function were studied, there may be other confounding factors that could explain the changes in the eGFR. The detection of recurrences in our study could have been improved through the use of event recorders and it would also have been useful to quantify the AF burden. The eGFR increase in all of the patients with recurrence was small and its relevance in routine clinical practice needs to be determined.
CONCLUSIONSIn our cohort of patients with AF and without severe baseline renal impairment, treated using PV ablation, there was general improvement in eGFR at 1 year of follow-up. Patients without recurrence beyond the window period had a higher eGFR than those with recurrence, although the difference was not statistically significant in the multivariate model. The BNP level was lower and the corin concentration higher. Although both parameters are indicators of good cardiac function, it was not possible to prove an association with the eGFR improvement observed.
FUNDINGThe present work was partially funded by a collaboration with Bayer for the purchase of cardiac biomarker reagents. Bayer had no other participation in the study.
CONFLICTS OF INTERESTM. Álvarez-López has received personal fees from Johnson & Johnson.
- –
Several studies have found an association between AF and a lower eGFR.
- –
The causal relationship between AF and worse renal function is unclear.
- –
Few studies have compared the changes over time in renal function in patients treated with AF ablation according to arrhythmia recurrence.
- –
PV ablation was associated with an eGFR improvement at 1 year of follow-up.
- –
A greater improvement in eGFR was seen in patients with a lower baseline eGFR.
- –
The strategy for controlling AF rhythm could be considered a factor to be taken into account to avoid renal function deterioration in patients with a history of AF.
To Manuela Expósito for her help with the statistical analysis and to Laura Jáimez and María Molina for their invaluable assistance.