Percutaneous transluminal septal ablation is an alternative treatment in patients with hypertrophic obstructive cardiomyopathy. However, due to the relatively new introduction of this technique, there is no information on its very long term results (>10 years).
MethodsThe present study included consecutive patients treated in 5 centers between 1998 and 2003. We analyzed clinical, hemodynamic, and echocardiographic data at baseline and follow-up.
ResultsA total of 45 patients were included; there were 31 (69%) women, the mean age was 62.4 (14) years, and 39 patients (86.6%) showed functional class III or IV. Septal thickness was 21.8 (3.5) mm, the peak resting gradient on echocardiography was 77 (39) mmHg, and mitral regurgitation was at least moderate in 22 patients (48.8%). During hospitalization, permanent pacemaker implantation was required in 3 patients and ventricular perforation (by pacing lead) occurred in 1 patient, requiring surgery. After a follow-up of 12.3 years (11.0-13.5 years), 2 patients (4.4%) died from cardiac causes (heart failure and posttransplantation), 3 patients required an implantable cardioverter-defibrillator (1 for primary prevention and 2 due to sustained ventricular tachycardia after cardiac surgery), and 2 underwent cardiac surgery (due to endocarditis and mitral regurgitation). In the last clinical review, functional class was I-II in 39 patients (86.6%) (P<.0001), the peak resting gradient was 16 (23) mmHg (P<.0001), and mitral regurgitation was absent or mild in 34 patients (75.5%) (P<.03).
ConclusionsThe results of this study suggest that septal ablation is safe and effective in the very long term. The procedure was not associated with a significant incidence of sudden death or symptomatic ventricular arrhythmias.
Keywords
In the last few years, percutaneous transluminal septal ablation (PTSA) has been developed as an alternative to surgery in patients with hypertrophic obstructive cardiomyopathy and inadequate response to pharmacological treatment.1–3 Several studies have shown symptom improvement and gradient reduction with excellent short- and medium-term survival, but the available long-term data are limited, with no published series reporting results beyond a mean follow-up of 8 years.4–8
The aim of the present multicenter study was to retrospectively evaluate the clinical and echocardiographic outcomes in patients with hypertrophic obstructive cardiomyopathy treated with PTSA and followed-up for more than 10 years.
METHODSThe present study included all consecutive patients diagnosed with hypertrophic obstructive cardiomyopathy who underwent PTSA in 5 Spanish centers before 2003. The criteria for performing the technique were not expressly agreed on by consensus among the centers for obvious reasons (retrospective study). However, in general, the technique was indicated in patients diagnosed with hypertrophic obstructive cardiomyopathy and with persistent symptoms despite optimal medical therapy and who had a resting or provocable left ventricular outflow tract gradient greater than 50mmHg.
In all patients, the decision to perform the procedure was taken by the treating cardiologist after evaluation and discussion with the patient and after determining that medical treatment would provide no further benefit. In all patients, the decision to perform PTSA rather than the surgical alternative was agreed by consensus, after discussion in sessions with clinical cardiologists, interventional cardiologists, and surgeons. All patients provided informed consent.
The procedure was carried out using the previously-described, conventional technique.6 This technique consisted of the introduction and inflation of a 2-2.5mm balloon catheter in a basal perforating artery, usually a branch of the anterior descending coronary artery. Angiographic contrast material was injected through the lumen of the inflated balloon to confirm the absence of reflux to the anterior descending artery and to detect potential communication between this septal branch and the posterior interventricular branch or other branches of other myocardial territories.
Two-dimensional transthoracic echocardiography and injection of echo-contrast medium through the balloon catheter were carried out to confirm that the territory irrigated by the selected septal branch corresponded to the basal segment of the septum, the area generating maximal obstruction, and did not affect another myocardial territory (for example, the papillary muscle). Alcohol was subsequently injected and the presence and degree of obstruction was constantly evaluated through hemodynamic and echo-Doppler monitoring. The initial dose of alcohol was 1-2mL, which could be repeated at 5 to 10min until there was a significant and sustained reduction in gradient.
In patients without a definitive pacemaker, the procedure was carried out by introducing a temporary pacemaker into the right ventricle through the femoral vein; the pacemaker was kept in place for the first 48h. After undergoing ablation, the patients were admitted to the coronary unit for the first 48 to 72h for electrocardiographic monitoring to detect any arrhythmias.
Contrast echocardiography started to be systematically used in 2000. The protocols for the procedure may have differed somewhat among centers but, in general, the protocols were highly similar and differences were minor.
The clinical follow-up strategy, the type of noninvasive tests, and their time of application varied somewhat among centers. In general, patients were followed up in the outpatient setting every 6 to 12 months. To determine the patients’ clinical course and outcome at the end of follow-up, clinical records and electrocardiographic and echocardiographic recordings were reviewed and contact was made with the patient or family.
We report the clinical and echocardiographic variables that were gathered by all participating centers. These variables consisted of survival status, functional class, symptoms, electrocardiographic recordings, and echocardiographic recordings. The latter included information on ejection fraction, mitral regurgitation, peak and mean subaortic gradients at rest and peak and mean subaortic gradients under provocable conditions. The results of tests performed occasionally (magnetic resonance or Holter monitoring) were not reported, except when they formed part of the evaluation a particular patient with a specific indication for these tests.
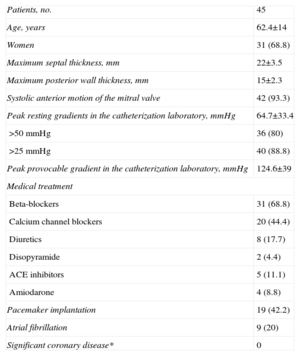
RESULTSBetween February 1998 and March 2003, 45 PTSA procedures were carried out in 45 patients with hypertrophic obstructive myocardiopathy in 5 centers. Thirty-six patients underwent isolated myectomy in the same period. The patients’ baseline characteristics are shown in Table 1. The mean age was around 62 years and, as is usually the case in such series, most patients were women.
Baseline Clinical Characteristics
| Patients, no. | 45 |
| Age, years | 62.4±14 |
| Women | 31 (68.8) |
| Maximum septal thickness, mm | 22±3.5 |
| Maximum posterior wall thickness, mm | 15±2.3 |
| Systolic anterior motion of the mitral valve | 42 (93.3) |
| Peak resting gradients in the catheterization laboratory, mmHg | 64.7±33.4 |
| >50mmHg | 36 (80) |
| >25 mmHg | 40 (88.8) |
| Peak provocable gradient in the catheterization laboratory, mmHg | 124.6±39 |
| Medical treatment | |
| Beta-blockers | 31 (68.8) |
| Calcium channel blockers | 20 (44.4) |
| Diuretics | 8 (17.7) |
| Disopyramide | 2 (4.4) |
| ACE inhibitors | 5 (11.1) |
| Amiodarone | 4 (8.8) |
| Pacemaker implantation | 19 (42.2) |
| Atrial fibrillation | 9 (20) |
| Significant coronary disease* | 0 |
ACE, angiotensin converting-enzyme.
Data are expressed no. (%) or mean±standard deviation.
*Coronary lesions with greater than 50% stenosis.
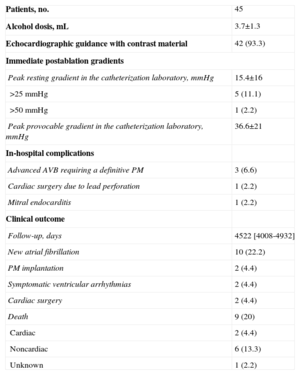
The mean volume of alcohol injected was 3.7mL (2-7mL). These volumes were typical of initial experience with this procedure and have been progressively reduced. In this initial experience, only 1 branch per patient was ablated. The immediate and follow-up results are shown in Table 2. Immediately after the procedure, the gradient was drastically reduced both under resting and provocable conditions. Elevated gradients with hardly any modification persisted in only 1 patient.
Procedural Characteristics and Subsequent Clinical Outcome
| Patients, no. | 45 |
| Alcohol dosis, mL | 3.7±1.3 |
| Echocardiographic guidance with contrast material | 42 (93.3) |
| Immediate postablation gradients | |
| Peak resting gradient in the catheterization laboratory, mmHg | 15.4±16 |
| >25 mmHg | 5 (11.1) |
| >50 mmHg | 1 (2.2) |
| Peak provocable gradient in the catheterization laboratory, mmHg | 36.6±21 |
| In-hospital complications | |
| Advanced AVB requiring a definitive PM | 3 (6.6) |
| Cardiac surgery due to lead perforation | 1 (2.2) |
| Mitral endocarditis | 1 (2.2) |
| Clinical outcome | |
| Follow-up, days | 4522 [4008-4932] |
| New atrial fibrillation | 10 (22.2) |
| PM implantation | 2 (4.4) |
| Symptomatic ventricular arrhythmias | 2 (4.4) |
| Cardiac surgery | 2 (4.4) |
| Death | 9 (20) |
| Cardiac | 2 (4.4) |
| Noncardiac | 6 (13.3) |
| Unknown | 1 (2.2) |
AVB, atrioventricular block; PM: pacemaker.
Data are expressed as no. (%), no. [range] or mean±standard deviation.
Complications during the procedure and hospital stay consisted of the following: 3 patients (6.6%) had advanced grades of atrioventricular block (AVB) and required definitive pacemaker implantation; 1 patient experienced right ventricular perforation by the lead of a prophylactic pacemaker at 48h and required surgery; 10 days later, the same patient had an episode of sustained ventricular tachycardia causing presyncope and underwent implantation of an implantable cardioverter defibrillator; another patient developed endocarditis around the lead of a previously implanted pacemaker, leading to aortic valve involvement that required aortic valve replacement.
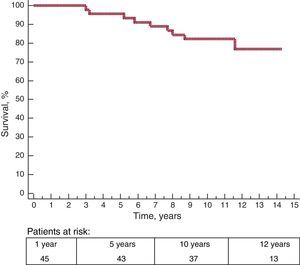
During a mean follow-up of 12.3 years, 9 patients died (20%), 6 from noncardiac causes, 2 from cardiac causes, and 1 from unknown cause. Ten-year survival was 82% (5.8%) (Figure 1). Of the 2 deaths from cardiac causes, 1 patient died from progressive heart failure 5 years after PTSA. The other patient required mitral valve replacement due to severe mitral regurgitation and subsequently experienced 3 episodes of recurrent prosthetic valve thrombosis; this patient died after cardiac transplantation 4 years after the PTSA procedure. The cause of death was unknown in 1 patient; this death occurred in the out-of-hospital setting in an octogenarian 7 years after the procedure.
Among patients who died from any cause, echocardiographic follow-up 1 to 2 years before death showed slight gradients (< 15mmHg) in 7 patients, a moderate gradient (45mmHg) in 1 patient, and a severely elevated gradient only in the patient who died after cardiac transplantation and who did not initially respond to PTSA.
During follow-up, 2 patients underwent cardiac surgery, but none required myectomy. The first was the above-mentioned patient who had developed endocarditis and, 11 years after PTSA, showed severe mitral and tricuspid regurgitation, requiring mitral valve replacement and tricuspid valve annuloplasty. The second was the previously-mentioned patient who required mitral valve replacement and who developed prosthetic valve thrombosis and finally underwent cardiac transplantation.
During follow-up, 2 patients required pacemaker implantation due to advanced block and a further 3 patients required an implantable cardioverter-defibrillator. Of the latter, 1 was indicated for primary prevention. Another was implanted in the above-mentioned patient who experienced right ventricular perforation by a pacemaker lead and who required defibrillator implantation due to sustained ventricular tachycardia; this patient has received no shocks due to ventricular arrhythmia during subsequent follow-up. The third patient was the patient with endocarditis who underwent implantation of an implantable cardioverter-defibrillator 5 years after the PTSA procedure due to sustained ventricular tachycardia associated with complete AVB. New atrial fibrillation after the procedure developed in 10 patients.
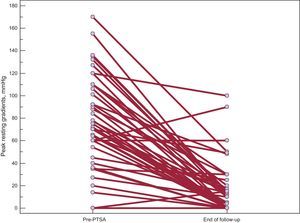
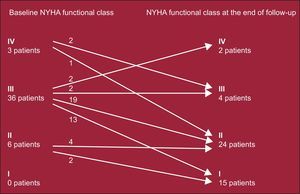
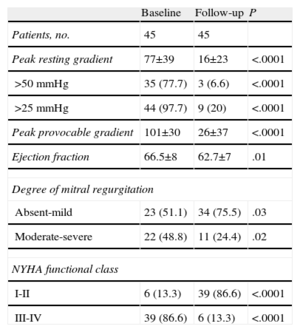
Table 3 compares the echocardiographic and clinical data at baseline and during follow-up. Figures 2 and 3 show the changes in the gradient and functional class, respectively. Grouping patients as a whole revealed a highly significant and sustained reduction in peak gradients on echocardiography, both under resting and provocable conditions. Similarly, the degree of mitral regurgitation was maintained at grades significantly lower than that at baseline. In line with these findings, the patients’ functional class at the end of follow-up was significantly better than that before the procedure.
Comparison of Echocardiographic Parameters and Functional Class Before the Procedure and at the Last Follow-up Evaluation
| Baseline | Follow-up | P | |
| Patients, no. | 45 | 45 | |
| Peak resting gradient | 77±39 | 16±23 | <.0001 |
| >50mmHg | 35 (77.7) | 3 (6.6) | <.0001 |
| >25 mmHg | 44 (97.7) | 9 (20) | <.0001 |
| Peak provocable gradient | 101±30 | 26±37 | <.0001 |
| Ejection fraction | 66.5±8 | 62.7±7 | .01 |
| Degree of mitral regurgitation | |||
| Absent-mild | 23 (51.1) | 34 (75.5) | .03 |
| Moderate-severe | 22 (48.8) | 11 (24.4) | .02 |
| NYHA functional class | |||
| I-II | 6 (13.3) | 39 (86.6) | <.0001 |
| III-IV | 39 (86.6) | 6 (13.3) | <.0001 |
NYHA, New York Heart Association.
Data are expressed as no. (%) or mean±standard deviation.
Ten years after the procedure, only 7 patients had shown 1 of the following events: death from cardiac or undetermined cause (3 patients), reintervention with PTSA (0 patients), surgical myectomy (0 patients), symptomatic ventricular arrhythmia (2 patients), or advanced symptomatic recurrence (New York Heart Association functional class greater than II) associated with subaortic gradients greater than 25mmHg under resting or provocable conditions (2 patients). Ten-year survival free of these events was 84.1% (5.5%).
The results were highly comparable among centers. Ten-year survival was within the range of 80% to 85% and New York Heart Association functional class was I or II at the end of follow-up in 85% to 90% of patients.
DISCUSSIONIn this series, the only series with a follow-up of more than 10 years in all patients treated with PTSA, most patients showed a sustained reduction in gradient and degree of mitral regurgitation, which was reflected in sustained clinical improvement in these patients. Importantly, in this series, PTSA was not associated with a significant incidence of sudden death or symptomatic ventricular arrhythmias. The 2 patients with sustained ventricular tachycardia experienced these episodes after prior cardiac surgery. Holter monitoring was not systematically carried out during follow-up, which is unsurprising in an observational study. Holter monitoring was indicated exceptionally in a few symptomatic patients. Therefore, given the complete clinical follow-up of all patients, the arrhythmic events that could have gone undetected would be silent or subclinical events. Sudden death and symptomatic ventricular arrhythmias were detected.
The effectiveness and safety of PTSA have previously been demonstrated in the short- and medium-term. Two of the centers involved in the present study have reported their pioneering experience in Spain, publishing the first case report,9 and the first large series.4
The advantage of PTSA over surgery is its lesser aggressiveness. Two meta-analyses have been published that compared surgery and PTSA with short- and medium-term follow-up.10,11 Both meta-analyses concluded that there were no differences in mortality; however, after adjusting for patient characteristics, 1 of these meta-analyses detected that rates of all-cause mortality and sudden cardiac death were lower with ablation,10 while the other reported a higher frequency of conduction disorders and residual gradient with the percutaneous technique.11 A recently published study of 177 patients reported a survival of 5.7 years, similar to that in a surgical cohort matched by age and sex.8
Importantly, the results of these techniques are closely linked to operator experience and therefore to the volume of activity. Given the limited casuistics of this disease, it would be desirable to concentrate experience in specific referral centers for this type of intervention. In fact, the 2011 US guidelines on hypertrophic myocardiopathy recommend (class I, level C) that septal reduction techniques be carried out by operators that individually have experience of more than 20 cases per year or by operators working within teams with a dedicated program and a cumulative series of more than 50 interventions.12 In the same guidelines, PTSA is indicated (class IIa, level B) in patients with high surgical risk for their age and comorbidities. In patients without elevated risk, the technique is assigned a class IIb indication (level B). This technique is not generally recommended in patients with hypertrophy greater than 30mm and should be avoided in young persons or those with other cardiac diseases with surgical indication. While not providing detailed recommendations, the 2003 European guidelines adopt a similar position, recommending surgery as the first-line alternative except in specific situations (very high risk) and stressing that the technique should be performed by experienced teams.2
Septal reduction through PTSA is not free of potential complications, which may be serious, and consequently this technique continues to be reserved for patients at high surgical risk, especially in centers with extensive surgical experience. Periprocedural mortality is low, around 1%, in the more experienced centers.13 The most frequent complication is AVB requiring permanent pacemaker implantation (7%-20%). These rates may have been reduced by the use of echocardiographic contrast material and lower doses of alcohol.13 Nevertheless, a randomized study did not show that the alcohol dose influenced complications or medium- to long-term clinical outcomes.7
In our series, 1 patient had sustained ventricular tachycardia 12 days after the procedure, after undergoing surgical repair of a perforation by a pacemaker lead a few days previously. In another patient, sustained ventricular tachycardia associated with AVB was detected 5 years after the procedure and after valvular surgery. The cause of the arrhythmia was not well defined in either of the patients.
One of the advantages of surgery is knowledge of its very long-term results, which is not feasible with PTSA. It is important to elucidate the potential arrhythmogenic impact of the myocardial scar caused by this procedure and the possible loss of effectiveness, with the reappearance of the obstruction. In the last few years, a certain degree concern has arisen regarding the possibility that PTSA may create an arrhythmogenic substrate. The results of some studies have indicated that this procedure has potentially unwanted long-term effects,14,15 but there is currently no solid evidence of a higher incidence of sudden death and ventricular arrhythmias after PTSA. Moreover, the disease itself creates an unfavorable arrhythmogenic context.
Therefore, long-term data are crucial to ensure that PTSA is an appropriate alternative to surgery, especially in patients without high surgical risk.
The long-term data on this technique are highly limited, with the largest series having 3.7 years of follow-up and 2 smaller series with a mean of 7 and 8 years of follow-up.5–8 These studies did not analyze subgroups with more than 10 years of follow-up, because of the very small number of patients. In the largest study, with 279 patients, only 4 were followed-up for more than 10 years.6 In the next study, with a large sample (177 patients), the survival data were limited to 8 years with 40 patients at risk at that time.8 Finally, the study with the longest mean-follow-up, 8 years (1 year) included only 55 patients in total.5
These studies reported satisfactory clinical outcomes, with sustained symptom improvement and gradient reduction. The only predictor of mortality was age.6
Study LimitationsThis study analyzed a small series of patients, although the follow-up was the longest reported in the literature. Nevertheless, consideration of the sample size should include the following factors: the prevalence of hypertrophic obstructive cardiomyopathy in Spain, the satisfactory disease control achieved with drug therapy in most patients, surgical remission, and—in particular—the very small number of patients undergoing PTSA, especially in the study period.
Another limitation is the absence of a comparable surgical group, which would be difficult to achieve given that the patients selected for one or other technique are very different; those selected for surgery are generally younger, with more severe hypertrophy, and not infrequently have another significant associated heart disease (coronary or valvular). The clinical course of this disease is closely linked to age at diagnosis and any attempt at statistical pairing, with such small series, would yield results that would be very difficult to evaluate.
The aim of this study was to determine the outcomes of patients undergoing PTSA with more than 10 years of follow-up. This was an observational, multicenter, and retrospective study. Consequently, noninvasive imaging tests, such as magnetic resonance, were not systematically applied nor was there a protocol for obtaining specific or predefined variables on echocardiographic evaluation. Similarly, Holter monitoring was not systematically performed and was only used in patients with a clinical indication. Nevertheless, given that all patients had complete clinical follow-up, the arrhythmic events that could have gone undetected would be silent or subclinical. Sudden death and symptomatic ventricular arrhythmias were detected.
CONCLUSIONSThe results beyond 10 years suggest the very long-term effectiveness and safety of PTSA with alcohol, with a sustained reduction in gradients, mitral regurgitation, and functional class. The technique was not associated with a significant incidence of symptomatic ventricular arrhythmia or sudden death. Nevertheless, larger series are required to confirm these results.
CONFLICTS OF INTERESTNone declared.