Stent implantation is an effective therapy for aortic coarctation and recoarctation. However, in adolescents and adults, aortic wall rupture and dissection can occur, as well as aneurysms during follow-up. In order to reduce these complications, we electively implant covered stents.
MethodsSince 2005, we have performed the procedure using femoral access in 17 patients (2 adolescents and 15 adults), 16 electively and 1 as a rescue procedure. We used the Mullins technique in all cases, implanting a NuMED® covered stent.
ResultsGood stent apposition was achieved in all 17 procedures; 8 patients required a distal flare. Gradient was reduced from 40 (16) mmHg to 2 (2) mmHg (P<.001) and lumen diameter increased from 4 (2) mm to 19 (3) mm (P<.001). Two exceptional cases are discussed: one patient with aortic wall rupture who underwent a rescue procedure using a stent within a covered stent and another patient with total obstruction and intercostal aneurysm in whom the outcome was fatal at 48 h postprocedure (autopsy is shown). Four-year clinical follow-up included Doppler echocardiography; an additional imaging technique was required in 13 patients. All patients recovered well and there were no complications.
ConclusionsCovered stents are effective in treating coarctation and recoarctation in adolescents and adults, are the treatment of choice in patients with complex anatomy, and must be available in the operating room as a rescue device when implanting a conventional stent.
Keywords
.
INTRODUCTIONStent implantation is an alternative to traditional surgery in older children, adolescents, and adults with native coarctation and recoarctation.1–9 However, in complicated, very severe, tortuous or eccentric coarctation, and in adolescent and adult patients, who often have cystic medial degeneration and disruption of the aortic wall, complications may occur such as aortic aneurysm, aortic pseudoaneurysm, aortic dissection, and the dreaded rupture of the aorta, which can be immediately fatal after conventional angioplasty or stenting.8–16
Having observed the case of aortic rupture described below, and with the aim of reducing these complications in patients who have had coarctation and recoarctation since their youth, we decided to electively implant a NuMED® (Hopkinton, New York, United States) expanded polytetrafluoroethylene (ePTFE) covered stent (CS). This stent is mounted on a balloon catheter and protects the vascular wall when expanded.6,15–19
METHODSBetween November 2005 and January 2012, we implanted ePTFE CS in 17 patients (2 adolescents and 15 adults) with coarctation and recoarctation.
The NuMED® CS is composed of platinum (90%) and heat-treated iridium (10%) wire arranged in a zigzag pattern, laser-welded at each joint and gold-brazed.6,15,18 The number of zigs in each row can vary, affecting radial strength, expanded diameter, and degree of shortening; the number of rows determines the length prior to expansion.
The 8-zig CS for coarctation has a PTFE covering that expands along with the stent and protects vascular tissue. Stent length ranges from 16mm to 45mm and the stent can be expanded up to a diameter of 24mm or 28mm in some cases. In the largest-diameter stents, shortening ranges from 33% to 40% of their total length.
The first 2 patients underwent surgical resection of the femoral artery, under general anesthesia and receiving heparin for anticoagulation; the remaining patients had previously received a ProStar® XL 10 Fr (Abbot Vascular Devices; Redwood City, California, United States) device. Angiographic control was achieved using the transradial approach to the aortic arch. We measured the diameter of the aorta above, at, and below the coarctation points, as well as the length of the segment.
To expand the stent, we used Z-Med balloons (NuMED®) or BIB Balloons (NuMED®) of the same diameter as the aorta either slightly before the coarctation point or at the terminal portion of the arch. If needed, the stent was re-expanded and flared using a second balloon with a larger diameter (Table 1). Depending on balloon diameter, access was achieved using 12 Fr to 16 Fr Mullins sheaths and the stent was expanded using the Mullins technique, ie, by withdrawing the sheath and exposing the stent at the middle portion of the coarctation.
In cases of complete obstruction, Hi-Torque Cross-It 200 or Asahi Confianza (Abbott) coronary guide wires were used to gain access from the upper portion for later capture using an Amplatz GooseNeck™ snare (ev3 Inc.; Plymouth, Minnesota, United States) in the distal portion to establish a radial-femoral arterial line as previously described.5,20
The stent should be mounted on the balloon with continuous saline irrigation, using a rotational motion and with the balloon well-folded. This assembly is inserted into the loader while still being irrigated and rotated, and once inside, the loader is inserted into the Mullins sheath and the valve released.
After the procedure, the patients were monitored in the coronary care unit, where antiplatelet treatment was initiated and continued for 6 months. Antibiotic prophylaxis was also administered, as 3 intravenous 1.5 g doses of cefuroxime.
Qualitative variables were expressed as frequencies and percentages and quantitative variables as mean (standard deviation). Before-and-after comparisons of two continuous variables were done by Wilcoxon nonparametric test due to the small sample size. Correlation between balloon diameter and aortic diameter before the coarctation was tested using Spearman's correlation coefficient. Significance was considered as P<.05. The SPSS v.19.0 software package was used for statistical analysis.
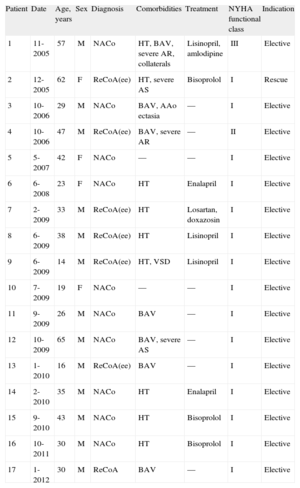
RESULTSOver a period of 7 years, we conducted 17 CS procedures in 13 men and 4 women with a mean age of 35 (15) years (range 14-65 years). Seven patients had a bicuspid aortic valve and 1 patient had a small muscular ventricular septal defect (patient 9, Table 1). Five patients had a second cardiac surgery pending: 2 for aortic regurgitation, 2 for stenosis, and 1 for annuloaortic ectasia. Nine patients were treated for hypertension and 15 patients were in New York Heart Association (NYHA) class I, 1 was in NYHA class II, and 1 was in NYHA class III (Table 1).
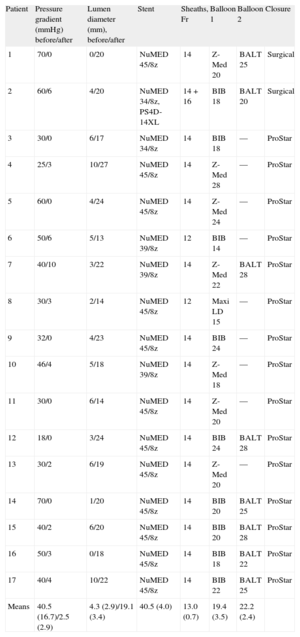
Eleven patients had native coarctation and 6 patients had recoarctation, which was secondary to end-to-end anastomosis in 5 patients and patch enlargement in 1 patient. The CS was electively implanted in 16 patients, whereas 1 patient underwent emergency implantation due to aortic rupture after expansion of a conventional stent11 (patient 2, Tables 1 and 2). The CS used was 40 (4) mm long, mounted on 19 (3) mm-diameter balloons, which correlated well with aortic diameter (18 [4] mm) before the coarctation point. In 8 patients, the distal portion was redilated with a larger balloon (25 [3] mm diameter). Stents were implanted directly except when there was total occlusion or very severe stenosis, in which case, in addition to the special technique described, the vessel was previously dilated using 8-10mm diameter balloons (patient 1 and patient 16, Tables 1 and 2).
Clinical Characteristics of Patients (Mean Age 35.0 [15.2] Years)
| Patient | Date | Age, years | Sex | Diagnosis | Comorbidities | Treatment | NYHA functional class | Indication |
| 1 | 11-2005 | 57 | M | NACo | HT, BAV, severe AR, collaterals | Lisinopril, amlodipine | III | Elective |
| 2 | 12-2005 | 62 | F | ReCoA(ee) | HT, severe AS | Bisoprolol | I | Rescue |
| 3 | 10-2006 | 29 | M | NACo | BAV, AAo ectasia | — | I | Elective |
| 4 | 10-2006 | 47 | M | ReCoA(ee) | BAV, severe AR | — | II | Elective |
| 5 | 5-2007 | 42 | F | NACo | — | — | I | Elective |
| 6 | 6-2008 | 23 | F | NACo | HT | Enalapril | I | Elective |
| 7 | 2-2009 | 33 | M | ReCoA(ee) | HT | Losartan, doxazosin | I | Elective |
| 8 | 6-2009 | 38 | M | ReCoA(ee) | HT | Lisinopril | I | Elective |
| 9 | 6-2009 | 14 | M | ReCoA(ee) | HT, VSD | Lisinopril | I | Elective |
| 10 | 7-2009 | 19 | F | NACo | — | — | I | Elective |
| 11 | 9-2009 | 26 | M | NACo | BAV | — | I | Elective |
| 12 | 10-2009 | 65 | M | NACo | BAV, severe AS | — | I | Elective |
| 13 | 1-2010 | 16 | M | ReCoA(ee) | BAV | — | I | Elective |
| 14 | 2-2010 | 35 | M | NACo | HT | Enalapril | I | Elective |
| 15 | 9-2010 | 43 | M | NACo | HT | Bisoprolol | I | Elective |
| 16 | 10-2011 | 30 | M | NACo | HT | Bisoprolol | I | Elective |
| 17 | 1-2012 | 30 | M | ReCoA | BAV | — | I | Elective |
AAo, ascending aorta; AR, aortic regurgitation; AS, aortic stenosis; BAV, bicuspid aortic valve; F, female; HT, hypertension; M, male; NACo, native aortic coarctation; NYHA, New York Heart Association; ReCoA(ee), recoarctation of the aorta with previous end-to-end anastomosis; VSD, ventricular septal defect.
Procedural Characteristics
| Patient | Pressure gradient (mmHg) before/after | Lumen diameter (mm), before/after | Stent | Sheaths, Fr | Balloon 1 | Balloon 2 | Closure |
| 1 | 70/0 | 0/20 | NuMED 45/8z | 14 | Z-Med 20 | BALT 25 | Surgical |
| 2 | 60/6 | 4/20 | NuMED 34/8z, PS4D-14XL | 14+16 | BIB 18 | BALT 20 | Surgical |
| 3 | 30/0 | 6/17 | NuMED 34/8z | 14 | BIB 18 | — | ProStar |
| 4 | 25/3 | 10/27 | NuMED 45/8z | 14 | Z-Med 28 | — | ProStar |
| 5 | 60/0 | 4/24 | NuMED 45/8z | 14 | Z-Med 24 | — | ProStar |
| 6 | 50/6 | 5/13 | NuMED 39/8z | 12 | BIB 14 | — | ProStar |
| 7 | 40/10 | 3/22 | NuMED 39/8z | 14 | Z-Med 22 | BALT 28 | ProStar |
| 8 | 30/3 | 2/14 | NuMED 45/8z | 12 | Maxi LD 15 | — | ProStar |
| 9 | 32/0 | 4/23 | NuMED 45/8z | 14 | BIB 24 | — | ProStar |
| 10 | 46/4 | 5/18 | NuMED 39/8z | 14 | Z-Med 18 | — | ProStar |
| 11 | 30/0 | 6/14 | NuMED 45/8z | 14 | Z-Med 20 | — | ProStar |
| 12 | 18/0 | 3/24 | NuMED 45/8z | 14 | BIB 24 | BALT 28 | ProStar |
| 13 | 30/2 | 6/19 | NuMED 45/8z | 14 | Z-Med 20 | — | ProStar |
| 14 | 70/0 | 1/20 | NuMED 45/8z | 14 | BIB 20 | BALT 25 | ProStar |
| 15 | 40/2 | 6/20 | NuMED 45/8z | 14 | BIB 20 | BALT 28 | ProStar |
| 16 | 50/3 | 0/18 | NuMED 45/8z | 14 | BIB 18 | BALT 22 | ProStar |
| 17 | 40/4 | 10/22 | NuMED 45/8z | 14 | BIB 22 | BALT 25 | ProStar |
| Means | 40.5 (16.7)/2.5 (2.9) | 4.3 (2.9)/19.1 (3.4) | 40.5 (4.0) | 13.0 (0.7) | 19.4 (3.5) | 22.2 (2.4) |
After the procedure, we observed a decrease in pressure gradient from 40 (16) mm to 2 (2) mmHg (P<.001) and an increase in lumen diameter from 4 (2) mm to 19 (3) mm (P<.001). Finally, aortography was performed and the femoral artery closed, surgically or by using the ProStar XL system. No local complications occurred, except in patient 12, who had a hematoma that resolved spontaneously. No patient had any complication at the iliac-femoral level that required stenting.
There were 2 exceptional cases in the series:
- •
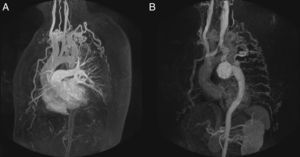
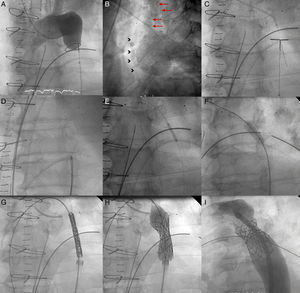
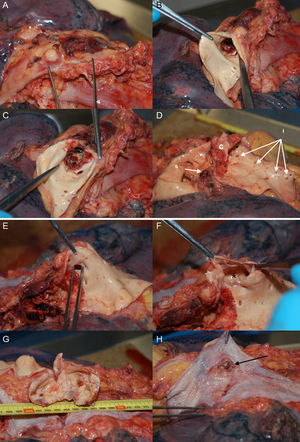
Patient 1, a 57-year-old man in NYHA class III with severe aortic regurgitation and dyspnea, required percutaneous coronary intervention before surgery. Numerous collateral vessels had complete obstruction of the aorta and a giant intercostal aneurysm below the coarctation point (Fig. 1). The aortic obstruction was treated using the technique described and a CS was implanted to exclude the aneurysm. The final outcome was very good; the gradient disappeared and there was a large increase in lumen diameter. We also performed redilation and obtained good apposition of the distal stent edge. The aneurysm could be visualized very faintly following the final injection (Fig. 2). Two days later, the end was tragic when the patient suffered a massive hematemesis and died. Autopsy showed that although the coarctation was well repaired and the stent did not have its cover, leaving the aneurysm in communication with the descending aorta. We believe that the large increase in systolic blood pressure from 70mmHg to 150mmHg after the coarctation point led to a high-pressure retrograde flow into the aneurysm. In the following hours, the aneurysmal bulge ruptured into the esophagus, with fatal gastrointestinal bleeding (Fig. 3).
Figure 2.Coarctation with complete aortic obstruction and giant intercostal aneurysm (patient 1). A, aortography of the arch showing the complete obstruction with the snare in position; B, in the late phases, in 40° left anterior oblique view, large collateral vessel (arrows) that provides circulation into the descending aorta contrasted with a large intercostal aneurysm (arrowheads); C, perforation with Crossit 300 guide wire and insertion into the GooseNeck™ snare; D, guide wire captured with the snare and extraction establishing the radial-femoral line; E, expansion with 2mm×20mm Maverick coronary balloon catheter; F, inversion of the loop, positioning of the 0.035 guide wire, and expansion of the 8mm×20mm Balt balloon catheter; G, positioning the covered stent; H, expansion; I, outcome with good stent apposition to the wall.
(0.66MB).Figure 3.Patient 1, autopsy. A, the isthmic portion of the aorta; B and C, opening of the aorta and bare stent, without the polytetrafluoroethylene covering; D, stent extraction showing obstructive point of coarctation; (c), the left subclavian orifice (s) the orifices of the intercostal arteries, (i) the largest intercostal artery compared to the aneurysm; E, tweezers indicate the intercostal orifice; F, the opening of the orifice showing the aneurysm (*); G, opening of the aneurysm, measuring 7cm; H, esophagus with area of chronic inflammation due to the aneurysmal bulge (the arrow indicates a gap that connects to the aneurysm, found at autopsy).
(0.47MB). - •
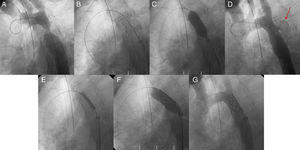
Patient 2 was a woman with a long-standing recoarctation that was treated using a Palmaz stent. The mid-portion of the stent was underexpanded and had to be redilated using a high-pressure Mullins balloon. Aortic rupture occurred with massive thoracic hemorrhage. This led to hypotension, extreme bradycardia, and cardiac arrest; a CS was immediately implanted in the stent and the patient recovered (Fig. 4). Subsequently, the hemothorax was drained and the outcome was favorable.
Figure 4.Patient 2, ruptured aorta. A, aortography showing a large recoarctation; B, underexpansion of the Palmaz stent. C, post-expansion to 9atm using the Mullins balloon; D, aortic rupture and contrast leaking into the chest (arrow); E, covered stent in the stent prior to expansion; F, expansion of the covered stent in the stent; G, completely sealed aortic rupture.
(0.33MB).
Clinical and Doppler echocardiographic follow-up was conducted over 4 (2.5) years. In all cases, the good initial outcome persisted without any signs of reobstruction. Patients 2, 3, 4, and 12 underwent intervention between 4 months and 8 months after our procedure. Two patients were implanted with mechanical valves, 1 patient received a biological valve, and the ascending aorta was replaced with a Dacron tube in the fourth patient. All 4 patients made good progress. The patient with a small muscular ventricular septal defect, did not require its closure.
Thirteen of the 17 patients underwent imaging study; no aneurysms, dissections, and/or obstructive processes were observed. Medication for hypertension was reduced in 5 patients and in 2 patients could not be discontinued.
DISCUSSIONOur consecutive series of patients with coarctation and recoarctation included 17 patients (2 adolescents and 15 adults) who were implanted with ePTFE CS (including patient 2 who received a stent within a stent). The procedures were conducted over 7 years and are comparable to those described in the literature.16–19 The immediate results were very good and similar to those obtained using conventional stents.5
The choice of CS for all consecutive patients from adolescence onward, with the particular aim of attempting to reduce severe complications, is the most interesting aspect of this series. We draw attention to the degenerative changes due to cystic medial necrosis in the wall of the aorta adjacent to the coarctation. With the passage of time, polysaccharides are deposited and medial elastic fibers become fractured, leading to fibrosis and collagen proliferation that weaken its structure.12,14
Although the incidence of aneurysms is higher in infants with hypoplasia and coarctation,21 this complication also occurs after conventional stent implantation in approximately 5% of adults.4,8,18,22,23 The risk of rupture of these aneurysms during follow-up is not negligible and intervention involves high-risk surgery.24,25
Rupture is another serious complication of aortic intervention that is infrequent but may also be under-reported.4,11,13,16 As in our case,11 it is dramatic when it occurs and in half of the cases reported it causes death in the operating room. In our patient, who had a longstanding recoarctation, we prepared the CS and all the necessary materials to rapidly implant the stent as a rescue procedure. Although in this case we used the CS as a rescue device, in the rest of the series they were indicated as a first choice option and for elective implantation.
The mechanism of aortic stenting involves controlled damage to the diseased wall and acute remodeling of the vessel. The scaffolding creates a lesion in the wall followed by healing, which in most patients is favorable, without the occurrence of aneurysms, dissections, and/or restenosis. However, in unfavorable complex coarctation, the risk of uncontrolled damage is greater, leading to acute and late complications.
Thus, the incidence of rupture, dissection, and aortic aneurysms is greater with conventional stenting in the following situations:
- •
In adults with strongly reduced aortic distensibility before above and after below the coarctation, where both rupture and dissection can easily occur.14
- •
In cases of complete obstruction (misnamed as atresia) in which there is a huge increase in lumen size, possibly leading to significant wall damage.10,17
- •
In cases of distorted or angulated coarctation due to unequal radial force during stent expansion.10
- •
In cases of severe degeneration of the wall where calcification occurs.10
- •
In adults with long-term recoarctation, as in patient 2, particularly after end-to-end anastomosis. This produces a circumferential scar that cannot expand, requiring the use of a high-pressure balloon; expanding the stent may rupture the aorta.11,13,14,16
- •
In cases of previous aneurysm, native, postoperative, or after percutaneous intervention.
Although CS use has undeniable advantages and particularly offers increased safety, they also have certain disadvantages compared to conventional stents:
- •
They need femoral arteries must be able to accept 12 Fr to 16 Fr sheaths; these sheaths involve an increased risk of bleeding. Therefore proficiency in the use of the ProStar XL system is essential. In younger patients in whom access is difficult due to their size, the possibility has been raised of using a smaller stent, 8 Fr to 11 Fr, such as the Advanta™ V12 Covered Stent (Atrium Medical; Hudson, New Hampshire, United States), which is premounted on 12-mm, 14-mm, and 16-mm diameter balloons and can be expanded to 22mm.26 Bruckheimer et al. developed an ingenious method of stent implantation, describing 9 cases of implantation using a small balloon for redilation to the reference diameter during a second step or procedure. The net result is a decrease in the thickness of the catheter; however, this technique might increase the number of displacements and embolizations.27
- •
Use of CS increases the risk of obstructing important aortic branch vessels, such as the carotid, subclavian, etc. Obstruction of the left subclavian artery appears to be well tolerated, but occasionally requires a carotid-subclavian bypass. To prevent ischemia in the right upper extremity, Tsai et al.28 described stent perforation via radial-subclavian access with effective perforation and expansion of the mesh. Recently, Lampropoulos et al.,29 described the double-wire kissing-balloon technique for cases in which the subclavian artery is very close to the coarctation.
We must draw attention to the fatal complication in patient 1 so that it does not happen again. This case highlights the importance of proficiency in the technique and the difficulties that can occur when passing the balloon-mounted CS from the cannula to the sheath; this step will not be required for the next implant, which will be similar to that of the Melody® percutaneous pulmonary valve (Medtronic; Minneapolis, Minnesota, United States).30
Intercostal aneurysms are a well-known entity that occur in coarctation with an incidence of 3% to 10%31,32 and can be true33 or false.34 They form due to the aforementioned weakness of the arterial medial layer and hyperflow through the collateral circulation and are often mistakenly considered aneurysms of the aortic wall. These aneurysms alone add further risk to coarctation surgery.35–37
Having reviewed the literature, we believe that this is the first case of CS use in a patient with complete obstruction and a giant intercostal aneurysm in which the progress was catastrophic and ended in the patient's death.
LimitationsThe basic limitations of this study are that it is retrospective and observational, and that there was no control group of patients receiving conventional stents. Although all patients underwent clinical follow-up, this did not include an imaging study in all cases, and so we cannot determine with certainty the incidence of potential aneurysms. The death of patient 1 due to a technical problem does not in any way detract from the merits and safety of CS.
CONCLUSIONSThe ePTFE CS is effective in the treatment of coarctation and recoarctation in adults. We consider it the stent of choice in patients at risk, with complete or very severe obstruction, distorted, angulated, or calcified aortas, longstanding recoarctation, and when associated with aneurysmal formation.
The ePTFE CS must be available in the operating room as a rescue device when implanting a conventional stent; it could have a life-saving function in acute complications, such as rupture and/or aortic dissection.
CONFLICTS OF INTERESTNone declared.
We would like to extend our thanks to product specialist Alberto Martinez Albalat, the nursing team of the cardiac catheterization laboratory (Asunción Ocariz, Javier Hernando, M. Antonia Pereiro, M. Isabel Peña, M. Victoria Izquierdo and Fernando Gómez), the auxiliary nurses Josefina Garcia, Felicidad Gutierrez and M. Ángeles Bañuelos, and the administrative assistant M. Teresa Vivas.