Transcatheter aortic valve implantation (TAVI) is now the principal therapeutic option in patients with severe aortic stenosis deemed inoperable or at high surgical risk. Implementing TAVI in a lower risk profile population could be limited by relatively high cerebrovascular event rates related to the procedure. Diffusion-weighted magnetic resonance imaging studies have demonstrated the ubiquitous presence of silent embolic cerebral infarcts after TAVI, with some data relating these lesions to subsequent cognitive decline. Embolic protection devices provide a mechanical barrier against debris embolizing to the brain during TAVI. We review the current evidence and ongoing uncertainties faced with the 3 currently available devices (Embrella, TriGuard and Claret) in TAVI. Studies evaluated neurological damage at 3 levels: clinical, subclinical, and cognitive. Feasibility and safety were analyzed for the 3 devices. In terms of efficacy, all studies were exploratory, but none demonstrated significant reductions in clinical event rates. The Embrella and Claret devices demonstrated significant reductions of the total cerebral lesion volume on diffusion-weighted magnetic resonance imaging. Studies evaluating the effects on cognition were also somewhat inconclusive. In conclusion, despite embolic protection devices demonstrating reductions in the total cerebral lesion volume on diffusion-weighted magnetic resonance imaging, the clinical efficacy in terms of preventing stroke/cognitive decline requires confirmation in larger studies.
Keywords
During the last decade, transcatheter therapies have revolutionized the management of patients with valvulopathy.1 Currently, transcatheter aortic valve implantation (TAVI) is the principal therapeutic option in patients with severe aortic stenosis considered inoperable or at high risk for open-heart surgery,2–4 thus having progressively increased the rates of its use with this indication.5–7 Increasing evidence points toward the effectiveness of TAVI in patients considered at intermediate or even at low surgical risk.8–10 However, further implementation of TAVI in lower risk patients is limited by the relatively high incidence of neurological events related to TAVI. Despite significant device improvements achieved in recent times, clinical cerebrovascular events (CVEs) remain one of the most dreaded complications post-TAVI. Stroke represents an important source of morbidity and mortality, multiplying by more than 3.5-fold the risk of 30-day mortality and consumption of finite health resources.11,12
Earlier TAVI studies highlighted the relevance of neurological events during the peri-procedural period. The PARTNER trial demonstrated a greater incidence of 30-day stroke/transient ischemic attack (TIA) in the TAVI group compared with medically-treated (6.7 vs 1.7%, P = .03) and surgical groups (5.5 vs. 2.4%, P = .04).13,14 The U.S. Pivotal CoreValve trial reported a 30-day incidence of stroke/TIA of 4.9% with the CoreValve Self-expanding prosthesis.15 Eggebrecht et al.12 published a meta-analysis with more than 10 000 patients who underwent TAVI between 2004 and 2011. The overall 30-day stroke/TIA rate was 3.3%, with the majority being major strokes (2.9%). In addition, TAVI in the intermediate surgical risk population was not associated with a significant reduction in neurological event rates. The recently published PARTNER 2 trial showed the 30-day rate of stroke/TIA to be 6.4% with the use of a second generation SAPIEN XT balloon expandable valve.10
Timing of Cerebrovascular Events During TAVICerebrovascular events appear in 2 different scenarios in patients undergoing TAVI: a) during the acute phase related to the procedure, and (b) during the chronic phase, with a constant rate of new episodes during follow-up. Although the pathogenesis of the acute phase of stroke or TIA following TAVI is likely multifactorial, given the arterial distribution and temporal pattern of peri-procedural brain infarctions, embolization is likely to be the dominant mechanism.16–18 Nombela-Franco et al.17 found that 50% of acute phase stroke/TIAs appear within the first 24hours post-TAVI, suggesting that catheter manipulation in the setting of atherosclerotic-laden aortas and severely calcified aortic valves might play an important role. The remainder of strokes in the acute phase appears after the first 24hours of the procedure, with a vulnerable period of up to 2 months. In this setting, thromboembolism originating directly from the native-transcatheter heart valve complex per se, or as a result of chronic or new atrial fibrillation, likely further contributes to strokes early post-TAVI. Vascular complications, and an increased atherosclerotic burden are other proposed mechanisms. On the other hand, the stroke rate incidence during the chronic phase post-TAVI is similar in patients treated surgically or medically, suggesting that the risk of neurological events in this period is determined by the underlying baseline risk profile of patients with aortic stenosis rather than by factors associated with the procedure.18–22
DWI StudiesClinically overt stroke simply represents the tip of the iceberg in terms of cerebral embolization. The spectrum of cerebrovascular damage following TAVI also involves subclinical cerebral infarcts detected by diffuse weighted magnetic resonance imaging (DWI), which have been related to a potential cognitive decline in the long-term.23–31
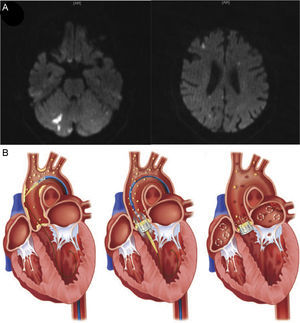
Cerebral DWI is a technique providing in vivo insights allowing the differentiation of acute from chronic stroke as well as nonspecific white-matter lesions. It is sensitive to changes in the mobility of water molecules from the extracellular to intracellular compartment seen in the early phases of the cascade of ischemic tissue changes. Therefore, DWI provides temporal information, because acute lesions become increasingly hyperintense during the first few days but then attenuate during the subsequent weeks (Figure 1). In addition, DWI has high sensitivity in identifying small ischemic lesions. Moreover, certain imaging patterns, such as multiple cortical or/and subcortical acute infarctions affecting both hemispheres, or anterior and posterior cerebral circulations, strongly suggest a cardiac or aortic source of embolism. However, it is important to keep in mind that such findings do not necessarily prove cerebral ischemia, given that other mechanisms, such as prothrombotic, inflammatory or infectious processes could also provide a similar distribution.23
A: Post-TAVI cerebral diffuse weighted magnetic resonance imaging in an 82-year-old patient following successful TAVI, demonstrating acute ischemic lesions (hyperintense images) distributed across both hemispheres and within the anterior and posterior territories, suggesting an embolic etiology. B: Origin of embolization following TAVI. Aortic plaque or valve disruption during catheter and guidewire passages, thrombus formation during the procedure, and subacute thromboembolism originating directly from the native-transcatheter heart valve complex per se, atherosclerotic burden, or caused by chronic or new atrial fibrillation, are the main sources of emboli associated with TAVI. TAVI, transcatheter aortic valve implantation. From Kahlert et al.27 and Fanning et al.18 with permission.
Several studies have demonstrated the near ubiquitous presence of subclinical or silent cerebral lesions detected by DWI following TAVI with a cerebral pattern highly suggestive of an embolic process (Table 1).24–30 Similarly, transcarotid Doppler studies have shown a high incidence of high-intensity transient signal (HITS) during TAVI, especially during valve positioning and implantation, thus suggesting the importance of embolization during these procedural steps.31,32 For these reasons, some authors have suggested a likely common origin of these cerebral DWI lesions with those generated by macroemboli that usually result in a clinically overt stroke. Importantly, the scanner potency used (ie, 1.5 T or 3 T), as well as the time point for performing DWI post-TAVI could affect the sensitivity for detecting silent cerebral infarcts, as these lesions tend to disappear over time, being totally absent at 30 days following the procedure.23,31–33 Therefore, in embolic protection device (EPD) studies, change in the cerebral DWI ischemic total lesion volume (TLV) has been proposed as a surrogate endpoint of clinical stroke.34,35 However, even though the biological plausibility and cost-rationale of this assumption, it should be confirmed in larger studies. In fact, the current American Heart Association guidelines do not recommend the use of silent cerebral infarcts detected on brain imaging as a surrogate endpoint for stroke studies, unless all study patients undergo standardized imaging at specific time points according to the study protocol.36,37
Transcatheter Aortic Valve Replacement Cerebral Diffusion-weighted Magnetic Resonance Imaging Studies
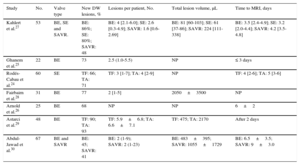
| Study | No. | Valve type | New DW lesions, % | Lesions per patient, No. | Total lesion volume, μL | Time to MRI, days |
|---|---|---|---|---|---|---|
| Kahlert et al.27 | 53 | BE, SE and SAVR. | BE: 86%; SE: 80%; SAVR: 48 | BE: 4 [2.1-6.0]; SE: 2.6 [0.3-4.9]; SAVR: 1.6 [0.6-2.69] | BE: 81 [60-103]; SE: 61 [37-86]; SAVR: 224 [111-338] | BE: 3.5 [2.4-4.9]; SE: 3.2 [2.0-4.4]; SAVR: 4.2 [3.5-4.8] |
| Ghanem et al.25 | 22 | BE | 73 | 2.5 (1.0-5.5) | NP | ≤ 3 days |
| Rodés-Cabau et al.24 | 60 | SE | TF: 66; TA: 71 | TF: 3 [1-7]; TA: 4 [2-9] | NP | TF: 4 [2-6]; TA: 5 [3-6] |
| Fairbairn et al.28 | 31 | BE | 77 | 2 [1-5] | 2050±3500 | NP |
| Arnold et al.26 | 25 | BE | 68 | NP | NP | 6±2 |
| Astarci et al.29 | 48 | BE | TF: 90; TA: 93 | TF: 5.9±6.8; TA: 6.6±7.1 | TF: 475; TA: 2170 | After 2 days |
| Abdul-Jawad et al.30 | 67 | BE and SAVR | BE: 45; SAVR: 41 | BE: 2 (1-9); SAVR: 2 (1-23) | BE: 483±395; SAVR: 1055±1729 | BE: 6.5±3.5; SAVR: 9±3.0 |
BE, balloon expandable prosthesis; DW, diffusion-weighted; MRI, magnetic resonance imaging; NP, not provided; SAVR, surgical aortic valve replacement; SE, self-expandable prosthesis; TA, transapical; TF, transfemoral.
Unless otherwise indicated, the data are expressed as mean±standard deviation, or median [interquartile range] or median (range).
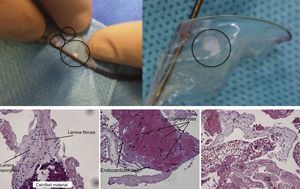
Gaseous embolization, an inflammatory state related to cardiac instrumentation, thrombus, or embolization of debris from the aortic wall or the aortic valve have been proposed as possible sources of silent DWI cerebral defects detected following cardiac procedures. Keeley et al.38 detected embolic debris in 51% of patients undergoing catheterization in a prospective study involving more than 1000 patients. Van Mirghem et al.19,39 studied the histopathological characteristics of debris traveling to the brain captured by the Claret filter in 81 patients undergoing TAVI. Thrombotic material was found in 74% of patients, and interestingly, tissue derived debris originating from the aortic wall, valve or myocardium was found in 63% of patients (Figure 2). These findings may relate to the still poorly understood pathophysiology of CVE after aortic valve manipulation. Disruption of the aortic intima, native valve, or myocardium following catheter manipulation during TAVI could lead to microthrombosis and consequent embolic complications and subsequent new silent cerebral lesions.40
Debris material travelling to the brain captured by the Claret embolic protection device during transcatheter aortic valve implantation. Histologic illustrations of captured debris of a degenerated aortic leaflet fragment, heart muscle, and arterial wall. Co, collagenous tissue; En, endothelial lining; Fi, fibrin. From Van Mieghem et al.19,39 with permission.
The relationship between subclinical cerebral infarcts and cognitive decline during the follow-up period post-TAVI remains uncertain. Recent studies performed in TAVI patients failed to find an association between new cerebral lesions and cognitive decline during follow-up.30,41 By contrast, some surgical series of patients undergoing aortic valve replacement found a relationship between new DWI cerebral lesions after the intervention and cognitive decline during follow-up.42 Similarly, silent brain infarcts detected with DWI in healthy patients are associated with a greater risk of dementia.43 Several issues need to be highlighted to better understand these apparent discrepancies. Firstly, there are currently no validated models for assessing neurocognitive status in patients undergoing TAVI. Interstudy differences could be partially explained by either different types of cognitive test battery or variations among definitions of cognitive decline. Secondly, a baseline degree of cognitive impairment is usually documented in a significant proportion of patients undergoing TAVI. In this situation, the floor effect (the incapacity to detect changes due to low baseline scores) needs to be taken into account when interpreting the results of the neurocognitive battery tests, especially in studies with negative results.44 In fact, part of the mild improvement in test scores observed in some studies that repeat the test at several time points may be related to a “learning effect” in the patients completing the test.32 Thirdly, cognitive assessment is affected by a high prevalence of inter- and intraobserver variability, and consequently the risk of bias is high in the absence of a well-matched control group.37
To avoid these prior concerns, some authors have proposed the use of normative test data well matched with the patient group of interest across demographic, health, and language normative domains, as well as the inclusion of study-specific well-matched controls and test-retest reliability adjustments for improving the generalizability and comparability of results across studies.30,32,44–46 Of note, none of these recommendations have yet to be followed in the published EPD studies.
EMBOLIC PROTECTION STRATEGIESThe majority of CVEs in patients undergoing TAVI have an embolic origin. Thus, EPDs should in theory reduce the amount of embolic material travelling to the brain, subsequently minimizing the extent of neurological insult. Essentially, embolic protection strategies harbor 2 chief objectives: to decrease the formation of thromboembolism or debris (using medications such as antithrombotic or anticoagulant agents, improving device performance, refining procedural technical techniques), and to protect the brain once debris or thrombus have been created (mechanically by using an EPD).
Regarding the first objective, there are currently several ongoing randomized clinical trials testing different antithrombotic strategies in patients undergoing TAVI that will provide valuable data in this field: the Aspirin Versus Aspirin + ClopidogRel Following Transcatheter Aortic Valve Implantation: the ARTE Trial (NCT01559298), comparing 2 different strategies of antiplatelet therapy (aspirin alone vs aspirin plus clopidogrel); the Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic Valve Implantation trial (POPular-TAVI trial, NCT02247128), testing different antiplatelet and antithrombotic strategies in patients with and without oral anticoagulation indication; and the Anti-Thrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis trial (ATLANTIS trial, NCT02664649), planning to test the superiority of a strategy of anticoagulation with apixaban compared with the current standard of care in more than 1500 patients, including patients with and without an indication for anticoagulation therapy.47
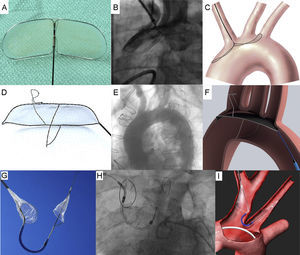
Mechanical barriers (or EPD) have been designed to capture or deflect emboli traveling to the brain during TAVI procedures (Figure 3),48–50 with the intention to protect the supra-aortic vessels from embolic debris using filters during the intervention. However, EPDs present several challenges. Firstly, similar devices designed for cardiac and carotid surgery have provided conflicting data in terms of preventing clinically apparent CVEs. In this regard, a multicenter randomized trial performed in 1289 patients undergoing cardiac surgery failed to demonstrate efficacy of the Embol-X-Filter to prevent clinical CVEs.51 In addition, a meta-analysis including 357 patients undergoing carotid artery stenting failed to find a benefit of the filter cerebral protection vs the use of proximal balloon occlusion technique for preventing the incidence of new ischemic brain lesions detected by DWI.52 Secondly, atherosclerotic plaques in the vicinity of the ostium of supra-aortic vessels hamper the implantation and positioning of EPDs, which in itself may even promote plaque disruption and consequently brain embolization. This scenario is not unusual in patients undergoing TAVI, in whom diffuse aortic atherosclerosis is common rather than the exception. Finally, the implementation of TAVI in a lower risk profile population, with a lower atherosclerotic burden, may be associated with a reduction in the incidence of neurological events during the peri-procedural period, thus making futile the use of these strategies in this population.37
Initial in-human experiences have shown the feasibility and safety of EPD during TAVI.32,49,50 Currently there are 3 devices available on the market with ongoing studies evaluating their efficacy.32,53,54 However, these studies are exploratory, with neither having the power to properly assess efficacy in terms of clinical stroke prevention (Table 2 and Table 3). In contrast, all of these trials have used findings on DWI to evaluate various surrogate endpoints of cerebral damage. The small sample size and the high rates of loss to follow-up in all these studies highlight the difficulties in conducting studies in this context, which require complex methodologies including 3 levels of assessment: clinical level (stroke/TIA prevention), subclinical (reduction in the rates of and ischemic lesion volumes on DWI), and a cognitive level (prevention of cognitive dysfunction at the follow-up).
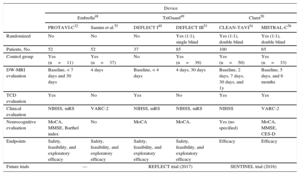
Characteristics of the Most Relevant Studies Evaluating Embolic Protection Device
| Device | ||||||
|---|---|---|---|---|---|---|
| Embrella48 | TriGuard49 | Claret50 | ||||
| PROTAVI-C32 | Samim et al.55 | DEFLECT I49 | DEFLECT III53 | CLEAN-TAVI54 | MISTRAL-C56 | |
| Randomized | No | No | No | Yes (1:1), single blind | Yes (1:1), double blind | Yes (1:1), double blind |
| Patients, No. | 52 | 52 | 37 | 85 | 100 | 65 |
| Control group | Yes (n=11) | Yes (n=37) | No | Yes (n=39) | Yes (n=50) | Yes (n=33) |
| DW-MRI evaluation | Baseline, < 7 days and 30 days | 4 days | Baseline, < 4 days | 4 days, 30 days | Baseline, 2 days, 7 days, 30 days, and 1y | Baseline, 5 days, and 6 months |
| TCD evaluation | Yes | No | Yes | No | Yes | Yes |
| Clinical evaluation | NIHSS, mRS | VARC-2 | NIHSS, mRS | NIHSS, mRS | NIHSS | VARC-2 |
| Neurocognitive evaluation | MoCA, MMSE, Barthel index | No | MoCA | MoCA. | Yes (no specified) | MoCA, MMSE, CES-D |
| Endpoints | Safety, feasibility, and exploratory efficacy | Safety, feasibility, and exploratory efficacy | Safety, feasibility, and exploratory efficacy | Safety, feasibility, and exploratory efficacy | Efficacy | Efficacy |
| Future trials | — | REFLECT trial (2017) | SENTINEL trial (2016) | |||
CES-D, Center for Epidemiologic Studies Depression scale; DW-MRI, diffusion-weighted magnetic resonance imaging; EPD, embolic protection device; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; TCD, trans-carotid Doppler; VARC-2, Valve Academy Research Consortium-2 criteria.
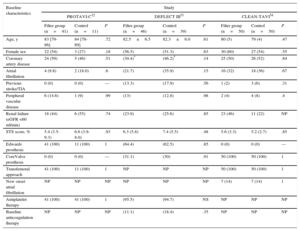
Device Performance and Feasibility of Embolic Protection Device
| Device performance | Embrella48 | TriGuard49 | Claret50 |
|---|---|---|---|
| Approach | Radial/brachial | Femoral | Radial |
| Position | Aorta | Aorta | Brachiocephalic and LCC |
| Mechanism | Deflection | Deflection | Capture |
| Delivery system size, Fr | 6 | 9 | 6 |
| Pore size, μm | 100 | 250a and 130b | 140 |
| Arteries covered | Brachiocephalic and LCC | Brachiocephalic, LCC and LSC | Brachiocephalic and LCC |
| Anatomic exclusion criteria | Significant RSC or brachiocephalic stenosis | Heavily calcified aortic arch or hostile aortic anatomy. Only TF-TAVI | LCC ≥ 5mm and brachiocephalic ≤ 9mm, without stenosis (≥ 70%) |
| EPD feasibility in studies | ProTAVI-C32 | Samim et al.55 | DEFLECT-I49 | DEFLECT-III53 | CLEAN-TAVI54 | MISTRAL-C5,6,c |
|---|---|---|---|---|---|---|
| Successful deployment | 40/41 (97.6) | 15 (93.3) | 33/37 (89.2) | 40/45 (88.9) | 48/50 (96) | 30/32 (94) |
| EPD successd | 40/41 (97.6) | 14 (93.3) | 28/35 (80.0) | 40/45 (88.9) | 47/50 (94) | NP |
| Device integrity at the end procedure | 41/41 (100) | 15 (100) | 41/41 (100) | 45/45 (100) | NP | NP |
| Vascular complications related EPD | 2/41 (4.9) | 0 (0.0) | 3/37 (8.1) | NP | NP | NP |
| More than 1 device used | 2/41 (4.9) | 0 (0.0) | 4/41 | 1/45 (2.2) | NP | NP |
| Time to complete deployment, min | 2 [1-3] | NP | 13.3 ± 11.6 | NP | NP | NP |
| Increase of radiation time, min | NP | 4.2 | NP | 10 | 2.7 ± 2.6 | NP |
| Increase of contrast, mL | NP | 15 | NP | 21 | −6.0 ± 4.0 | 13 |
EPD, embolic protection device; LCC, left common carotid artery; LSC, left subclavian artery; NP: not provided; RS: right subclavian artery; TF-TAVI: transfemoral-transcather aortic valve implantation.
Unless otherwise indicated, the data are expressed as no/No. (%), mean±standard deviation, median [interquartile range] or median (range).
The Embrella Embolic Deflector device (ED) (Edwards Lifesciences; Irvine, California, United States) is a mechanical system designed to deflect debris travelling to the brain originating from aortic valve or ascending aorta during TAVI. The ED is deployed into the aortic arch and is designed to cover the ostium of the brachiocephalic trunk and left common carotid artery, without systematic covering of the ostium of the left subclavian artery. The ED has 2 heparin coated polyurethane membranes with 100μm pore size called petals. The petals are mounted on a self-expandable nitinol frame attached to a 110cm long nitinol shaft. The petals are oriented in opposite directions to cover the supra-aortic arteries (the device extends over a length of 58mm with a width of 25mm). The system can be delivered through a 6 Fr delivery sheath introduced from the right radial or right brachial artery. Three radiopaque markers help fluoroscopic-guided deployment (Figure 3A, 3B, 3C and Table 3).48
Two studies have explored the efficacy of the ED; the ProTAVI-C study and a study performed by Samim et al. (Table 2).32,55 None of these studies were randomized. The ProTAVI-C was the first study to explore the efficacy of an EPD during TAVI, and to date is the most complete study evaluating this device. The study enrolled 52 patients undergoing TAVI across 6 hospitals. The ED was implanted in 41 patients while the remaining 11 patients comprised a control group. All events were adjudicated by an independent blinded core laboratory. Patients enrolled in this study were at intermediate preoperative risk, the median age was 83 years (range, 79-86 years) and patients with prior stroke/TIA were excluded (Table 4). The feasibility and safety of the ED was shown (Table 3). Patients undergoing TAVI with the ED did not present with significant additional complications. The ED was successfully deployed at the level of the greatest aortic curvature in all patients. Device success was achieved in all but 1 patient (97.6%), and the additional procedural time due to ED deployment was 2 min [interquartile range, 1-3 min]. There were no cases of membrane device rupture, and there were 2 complications related to the device procedure: 1 radial thrombosis without consequences and 1 brachial arterial pseudoaneurysm that required surgical repair. Transcarotid Doppler imaging was performed in all patients. The presence of HITS was detected during every step of the TAVI procedure, the most significant being while crossing the native aortic valve and positioning the transcatheter valve. Interestingly, the ED group showed a higher number of HITS than controls (632 [347-893] in ED group vs 279 [0-505] in the control group, P < .001), suggesting that ED manipulation may also represent a potential source of embolic debris. At a clinical level, there were 2 strokes and 1 TIA, all occurring within the ED group (the rate of clinical CVEs in ED group was 7.3%, all occurring after the procedure). The incidence of silent ischemic cerebral lesions detected by DWI was not reduced with the use of the device. All 40 patients who had baseline and postprocedure DWI (34 in the ED group and 6 in the control group) had new DWI defects, with most of these patients showing multiple defects. However, the use of an ED system appeared as a protective factor to reduce the ischemic TLV when compared with controls (Table 5). All but 1 of the neurocognitive tests failed to reveal any significant differences between treatment groups, with the Montreal Cognitive Assessment battery (MoCA) demonstrating a mild improvement in the ED group (score of 24 [21-27] in ED group vs score of 25 [23-28] in controls; P < .001). However the low number of patients, specifically in the control group, and the lack of randomization were the principal limitations of the study.
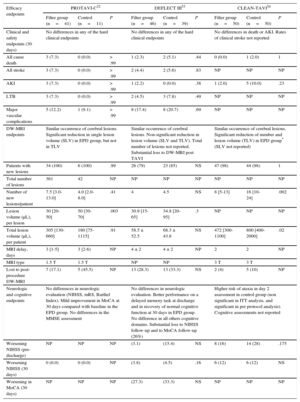
Population Characteristics of the Most Relevant Studies Evaluating Embolic Protection Device
| Baseline characteristics | Study | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PROTAVI-C32 | DEFLECT III53 | CLEAN-TAVI54 | |||||||
| Filter group (n=41) | Control (n=11) | P | Filter group (n=46) | Control (n=39) | P | Filter group (n=50) | Control (n=50) | P | |
| Age, y | 83 [79-86] | 84 [78-89] | .72 | 82.5±6.5 | 82.3±6.0 | .61 | 80 (5) | 79 (4) | .47 |
| Female sex | 22 (54) | 3 (27) | .18 | (56.5) | (51.3) | .63 | 30 (60) | 27 (54) | .55 |
| Coronary artery disease | 24 (59) | 5 (46) | .51 | (30.4)* | (46.2)* | .14 | 25 (50) | 26 (52) | .84 |
| Atrial fibrillation | 4 (9.8) | 2 (18.0) | .6 | (21.7) | (35.9) | .15 | 16 (32) | 18 (36) | .67 |
| Previous stroke/TIA | 0 (0) | 0 (0) | — | (13.3) | (17.9) | .56 | 1 (2) | 3 (6) | .31 |
| Peripheral vascular disease | 6 (14.6) | 1 (9) | .99 | (13) | (12.8) | .98 | 2 (4) | 4 (8) | .4 |
| Renal failure (eGFR <60 ml/min) | 18 (44) | 6 (55) | .74 | (23.9) | (25.6) | .85 | 23 (46) | 11 (22) | NP |
| STS score, % | 5.4 (3.5-9.3) | 6.6 (3.9-8.0) | .93 | 6.3 (5.8) | 7.4 (5.5) | .48 | 5.6 (3.3) | 5.2 (2.7) | .85 |
| Edwards prosthesis | 41 (100) | 11 (100) | 1 | (64.4) | (62.5) | .85 | 0 (0) | 0 (0) | — |
| CoreValve prosthesis | 0 (0) | 0 (0) | — | (31.1) | (30) | .91 | 50 (100) | 50 (100) | 1 |
| Transfemoral approach | 41 (100) | 11 (100) | 1 | NP | NP | NP | 50 (100) | 50 (100) | 1 |
| New onset atrial fibrillation | NP | NP | NP | NP | NP | NP | 7 (14) | 7 (14) | 1 |
| Antiplatelet therapy | 41 (100) | 41 (100) | 1 | (95.5) | (94.7) | NS | NP | NP | NP |
| Baseline anticoagulation therapy | NP | NP | NP | (11.1) | (18.4) | .35 | NP | NP | NP |
eGFR, estimated glomerular filtration rate; TIA, transient ischemic attack; STS, Society of Thoracic Surgeons predicted risk mortality score.
Unless otherwise indicated, the data are expressed as no. (%), mean±standard deviation or median [interquartile range].
Efficacy Endpoints of the Most Relevant Studies Evaluating Embolic Protection Devices
| Efficacy endpoints | PROTAVI-C32 | DEFLECT III53 | CLEAN-TAVI54 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Filter group (n=41) | Control (n=11) | P | Filter group (n=46) | Control (n=39) | P | Filter group (n=50) | Control (n=50) | P | |
| Clinical and safety endpoints (30 days) | No differences in any of the hard clinical endpoints | No differences in any of the hard clinical endpoints | No differences in death or AKI. Rates of clinical stroke not reported | ||||||
| All cause death | 3 (7.3) | 0 (0.0) | > .99 | 1 (2.3) | 2 (5.1) | .44 | 0 (0.0) | 1 (2.0) | 1 |
| All stroke | 3 (7.3) | 0 (0.0) | > .99 | 2 (4.4) | 2 (5.6) | .83 | NP | NP | NP |
| AKI | 3 (7.3) | 0 (0.0) | > .99 | 1 (2.2) | 0 (0.0) | .38 | 1 (2.0) | 5 (10.0) | .23 |
| LTB | 3 (7.3) | 0 (0.0) | > .99 | 2 (4.5) | 3 (7.8) | .49 | NP | NP | NP |
| Major vascular complications | 5 (12.2) | 1 (9.1) | > .99 | 8 (17.4) | 8 (20.7) | .69 | NP | NP | NP |
| DW-MRI endpoints | Similar occurrence of cerebral lesions. Significant reduction in single lesion volume (SLV) in EPD group, but not in TLV | Similar occurrence of cerebral lesions. Non-significant reduction in lesion volume (SLV and TLV). Total number of lesions not reported. Substantial loss to DW-MRI post TAVI | Similar occurrence of cerebral lesions. Significant reduction of number and lesion volume (TLV) in EPD group* (SLV not reported) | ||||||
| Patients with new lesions | 34 (100) | 6 (100) | .99 | 26 (79) | 23 (85) | NS | 47 (98) | 44 (98) | 1 |
| Total number of lesions | 361 | 42 | NP | NP | NP | NP | NP | NP | NP |
| Number of new lesions/patient | 7.5 [3.0-13.0] | 4.0 [2.0-8.0] | .41 | 4 | 4.5 | NS | 8 [5-13] | 16 [10-24] | .002 |
| Lesion volume (μL), per lesion | 30 [20-50] | 50 [30-70] | .003 | 30.9 [15-65] | 34.8 [20-95] | .3 | NP | NP | NP |
| Total lesion volume (μL), per patient | 305 [130-660] | 180 [75-1115] | .91 | 58.5 ± 52.5 | 68.3 ± 43.8 | NS | 472 [300-1100] | 800 [400-2000] | .02 |
| MRI delay, days | 3 [1-5] | 3 [2-6) | NP | 4 ± 2 | 4 ± 2 | NP | 2 | 2 | NP |
| MRI type | 1.5 T | 1.5 T | NP | NP | 3 T | 3 T | |||
| Lost to post-procedure DW-MRI | 7 (17.1) | 5 (45.5) | NP | 13 (28.3) | 13 (33.3) | NS | 2 (4) | 5 (10) | NP |
| Neurologic and cognitive endpoints | No differences in neurologic evaluation (NIHSS, mRS, Barthel Index). Mild improvement in MoCA at 30 days compared with baseline in the EPD group. No differences in the MMSE assessment | No differences in neurologic evaluation. Better performance on a delayed memory task at discharge and in recovery of normal cognitive function at 30 days in EPD group. No difference in all others cognitive domains. Substantial loss to NIHSS follow-up and to MoCA follow-up (26%) | Higher risk of ataxia in day 2 assessment in control group (non significant in ITT analysis, and significant in per protocol analysis). Cognitive assessments not reported | ||||||
| Worsening NIHSS (pre-discharge) | NP | NP | NP | (3.1) | (15.4) | NS | 8 (16) | 14 (28) | .175 |
| Worsening NIHSS (30 days) | 0 (0.0) | 0 (0.0) | NP | (3.8) | (4.5) | .16 | 6 (12) | 6 (12) | NS |
| Worsening in MoCA (30 days) | NP | NP | NP | (27.3) | (33.3) | NS | NP | NP | NP |
AKI, acute kidney injury; DW-MRI, diffusion-weighted magnetic resonance imaging; EPD, embolic protection device; ITT, intention to treat analysis; LTB, life-threatening bleeding; MRI, magnetic resonance imaging; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; mRS, modified Rankin Scale; NP, not provided; NS, non-significant; NIHSS, National Institutes of Health Stroke Scale; SLV, single lesion volume; TLV, total lesion volume; TAVI, transcatheter aortic valve implantation.
The results of the intention to treat analysis for DEFLECT III and CLEAN TAVI trials. Interquartile ranges for DELFECT III and CLEAN-TAVI trials are presented. were obtained by visual estimation of the plots.
Unless otherwise indicated, the data are expressed as no. (%), mean±standard deviation or median [interquartile range].
The TriGuard (TG) EPD (Keystone Heart Ltd., Herzliya, Israel) is a mechanical system conceived to deflect debris travelling to the brain originating from the aortic valve or ascending aorta during TAVI. The TG device is the only device designed to cover all 3 major arteries in the aortic arch (brachiocephalic trunk, left common carotid artery, left subclavian artery). The device has 5 functional parts: a dual frame nitinol frame, a thin nitinol coated mesh filter with pore size of 250μm (or 130μm for the second generation device), upper and lower stabilizers, and the tail end (distal to the heart) attached to the plunger during the procedure. The coated mesh has chemical and physical properties to reduce the likelihood for thrombus formation. The stabilizers are anchored within the ostium of the innominate artery and in the inner curvature of aortic arch respectively to facilitate the positioning of the filter. The upper stabilizer requires a minimum diameter of 8×11mm into the innominate artery at 15mm of the ostium. The lower stabilizer requires an aortic arch of 35mm or less to anchor properly. Irregularities in the innominate artery anatomy or an innominate ostium diameter less than recommended may result in suboptimal positioning and stability of the device. Special care should be taken with a severely calcified aorta, specifically in the vicinity of innominate ostium. The upper stabilizer angle of the innominate should not deviate from being perpendicular to the arch by more than 45°. The system can be delivered through a 75cm long 9 Fr delivery sheath introduced from the femoral artery (Figure 3D, 3E, 3F and Table 3).49
The DEFLECT III trial is the most relevant study evaluating the TG device. DEFLECT III is a single-arm blinded randomized clinical trial.53 The study enrolled 85 patients (39 controls) across 13 centers to evaluate the feasibility, safety, and efficacy of the TG device in patients undergoing TAVI (Table 2). The trial included patients considered at intermediate surgical risk (Table 4). Technical success (defined as complete 3-vessel cerebral coverage) was achieved in the 88.9% (40 of 45) of patients (Table 3). Time to complete deployment was 13min in the DEFLECT I study, but was not shown in the DEFLECT III trial. In terms of clinical efficacy, there was no between-group difference in the primary safety endpoint (including death, stroke, life-threatening or disabling bleeding, stage 2 or 3 acute kidney injury, or major vascular complications) (Table 5). In the intention-to-treat analysis, the use of the TG device was associated with nonsignificant freedom from of new cerebral DWI lesions (21.2 vs 11.5%), and a nonsignificant reduction in “new neurologic impairment” (a post hoc endpoint defined as worsening in National Institutes of Health Stroke Scale score from baseline with DWI evidence of ischemia). Although the authors noted a reduction in the cerebral TLV in the TG group when TLV was categorized into small, medium and large sizes, the proportion of patients with large ischemic volume was similar between groups (46% in the TG group vs 48% in controls). Although cognitive assessment via the MoCA and the Cogstate Research battery did not show significant between-group differences at discharge and at the 30-day evaluation, on the International Shopping List Test (a measure of episodic memory [delayed recall]), significant differences were observed when patients were evaluated at discharge favoring the interventional arm (65.4% vs 30.4%, P = .022). The main limitation of DEFLECT III was the high rate of loss to follow-up (31% of patients were lost to postinterventional DWI and 26% of patients were lost to the postinterventional cognitive and neurologic assessments).
Claret DeviceThe Claret embolic protection device (CD) (Claret Medical, Inc.; Santa Rosa, California, United States) is the only mechanical system designed to capture and not to deflect debris travelling to the brain. The CD is deployed into the ostia of brachiocephalic trunk and left common carotid artery. The system consists of 2 polyurethane filters with 140μm diameter pores fixed in a flexible nitinol radiopaque frame attached within a 100cm long catheter. The proximal filter is delivered into the brachiocephalic artery (right side) and allows apposition within vessels measuring 9-15mm in diameter. The system enables the delivery of the distal filter (left-sided filter) into the left common carotid artery. The entire system is positioned through the right radial or right brachial artery using a 6 Fr sheath. The CD is deployed before passing the TAVI delivery system into the aortic arch and is withdrawn following removal of the TAVI delivery system (Figure 3G, 3H, 3I, and Table 3).50
There are 2 randomized clinical trials exploring the efficacy of the CD: the Claret Embolic Protection and TAVI trial (CLEAN-TAVI, NCT01833052) with the Medtronic CoreValve system, and the MISTRAL-C trial (Table 2) with the Edwards-SAPIEN 3 system. To date, none of these studies have been published. CLEAN-TAVI is the most relevant trial. The main objective was to evaluate the impact of the use of the CD on the number of cerebral lesions in patients undergoing TAVI. Patients underwent DWI at day 2 and at day 7 post-TAVI, and serial neurological and cognitive assessments were performed. The initial results were encouraging (unpublished data, Dr. A Linke et al., oral presentation, TCT 2014). One hundred patients were enrolled (50 with CD and 50 controls) between April 2013 and June 2014 at the University Hospital of Leipzig (Germany). In the CD group, patients were mainly at intermediate preoperative risk (mean Society of Thoracic Surgeons score of 5.6%), there was a relatively high proportion of women (60%), and a high incidence of prior atrial fibrillation (32%) (Table 4). Device deployment/success was achieved in 96% of participants (Table 3). The causes of unsuccessful device deployment were severe tortuousity of the supra-aortic trunks (left common carotid artery in 1 case and subclavian artery in the other). In addition, there was an accidental dislocation of a correctly deployed filter by a pigtail catheter. Interestingly, National Institutes of Health Stroke Scale evaluation revealed a substantial incidence of neurological deficits at 2 days post-TAVI in both the control and device groups (28 vs 13%, P = 0.8), mainly because an unusually high incidence of ataxia was documented (24% of controls vs 9% in the CD group; relative risk = 1.56 [1.08-2.21], P < .05). However, at 7- and 30-days post-TAVI, the incidence of any neurological symptom was lower, with no between-group differences (day 7: 10 vs 13%; day 30: 12 vs 12%, respectively). In terms of reducing subclinical cerebral injury, the CD failed to reduce the incidence of new DWI cerebral lesions (98% of incidence of new DWI lesions at 2 days post-TAVI). However, in the filter group, there was a 50% reduction in the number of DWI cerebral lesions (day 2: median of 8 lesions in filter group vs median of 16 lesions in control group, P = .023; day 7, median of 5 lesions in filter group vs median of 10 lesions in the control group, P = .012). In addition, there were also a 41% reduction of TLV in the CD group (471 vs 800μL, P = .024) in the magnetic resonance imaging (MRI) performed at day 2, and 53% (220 vs 472μL, P = .013) in the MRI performed within 7 days post-TAVI. These changes were observed largely within cerebral territories that were protected by the filter (day 2 post-TAVI: 246 vs 527μL, P = .002 within cerebral anatomic regions protected by the CD) (Table 5). To date, there are no published data of neurocognitive assessments.54
The MISTRAL-C is a multicenter double-blind randomized trial enrolling 63 patients with symptomatic severe aortic stenosis with a 1:1 randomization to TAVI with or without the use of second generation of Claret Sentinel EPD (Table 2). 56 The use of this device was associated with a trend toward fewer new brain lesions on the 5-day post-TAVI MRI, especially within the cerebral regions corresponding to the areas offered protection by the device (unpublished data, Van Mieghem et al., oral presentation, TCT 2015). However, these results should be interpreted with caution, given that only 57% of the randomized patients underwent MRI.
FUTURE DIRECTIONSThere are several ongoing randomized controlled trials evaluating the efficacy of EPDs during TAVI. The Cerebral Protection in Transcatheter Aortic Valve Replacement trial (SENTINEL, NCT02214277) is using a third generation CD, and the TriGuard Embolic Deflection Device to Reduced Impact of Cerebral Embolic Lesions After Transcatheter Aortic Valve Implantation (REFLECT, NCT02536196) trial is using the TG device. The sample size estimates of these studies are substantially larger than those of prior trials (n = 357 in SENTINEL and n = 285 in REFLECT), and the primary outcome of both trials is the reduction of the ischemic cerebral TLV evaluated by DWI. The results are expected to be presented at the end of 2016 for the SENTINEL trial and during 2017 for REFLECT. These trials will provide pivotal data, collected in a standardized fashion, and adjudicated by expert core laboratories experienced in neuroimaging, regarding the mechanistic efficacy of EPDs during TAVI.
In conclusion, early studies evaluating the role of EPDs during TAVI have demonstrated the feasibility and safety of 3 currently available systems. In general, although EPDs have not been shown to reduce rates of silent cerebral ischemic lesions evaluated by DWI, some subanalyses have documented reductions in total cerebral ischemic volumes. The clinical impact of these findings in terms of stroke/TIA and cognitive decline needs to be confirmed in larger appropriately designed clinical trials. The emergence of TAVI in lower-risk populations seemed not to result in meaningful reductions of clinical CVEs, whilst the potential efficacy of various antithrombotic drugs in terms of stroke/TIA prevention during TAVI are currently under investigation. Until the availability of such data, the current role of EPD during TAVI remains uncertain.
FUNDINGO. Abdul-Jawad Altisent is supported by a research PhD grant from Fundación Alfonso Martín Escudero (Madrid, Spain).
CONFLICTS OF INTERESTJ. Rodés-Cabau has received research grants from Edwards Lifesciences.