To describe the epidemiology and treatment of a large contemporary cohort of patients with heart failure (HF).
MethodsObservational, retrospective, population-based study using the BIG-PAC database, which includes people aged ≥ 18 years seeking care for HF between 2017 and 2019. The main variables were the prevalence/annual incidence rate, comorbidities, clinical variables, and medication administered.
ResultsWe identified 19 762 patients with HF from a total of 1 189 003 persons seeking medical attention from 2017 to 2019 (2019: mean age, 78.3 years; 53.0% men). Distribution by type of left ventricular ejection fraction (LVEF) was as follows: 51.7% reduced, 40.2% preserved, and 8.1% mid-range. In 2019, the prevalence was 1.89% (95%CI, 1.70-2.08), with an incidence rate of 2.78 new cases per 1000 persons/y. No statistically significant differences were observed in prevalence and/or incidence from 2017 to 2019. Among patients with HF with reduced ejection fraction (HFrEF), 64% received beta-blockers, 80.5% angiotensin-converting enzyme inhibitor/angiotensin receptor blockers or sacubitril-valsartan, and 29.8% an aldosterone antagonist. In addition, from the diagnosis (baseline) to 24 months of follow-up, there was discreet treatment optimization, which was notable in the first 3 to 6 months.
ConclusionsEpidemiological data on HF remained stable during the study period, with a lower prevalence than that reported in non–population-based studies. There is wide room for improvement in the optimization of medical treatment of HFrEF.
Keywords
Heart failure (HF) is a complex clinical syndrome resulting from alterations to cardiac structure or function. Symptom progression in HF results in frequent hospitalizations, poor quality of life for patients, and a high mortality rate.1–3
HF can be classified according to left ventricular ejection fraction (LVEF) into three basic phenotypes: a) HF with reduced LVEF (HFrEF, ≤ 40%); b) HF with preserved LVEF (HFpEF, ≥ 50%); and c) HF with mid-range LVEF (HFmrEF, 41-49%).3,4 HFrEF accounts for between 40% and 50% of HF patients.2,4,5 Classification of HF patients according to LVEF is important because LVEF phenotype is linked to differences in etiology, comorbidities, and especially responses to treatment.1–3
Several studies have shown that the prognosis of patients with HFrEF and—to a lesser degree—those with HFmrEF can be improved by neurohormonal modulation with angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB) in combination with beta-blockers and aldosterone antagonists (AA); moreover, prognosis is also improved by treatments that potentiate the natriuretic peptide system and other endogenous vasodilatory systems.6,7
HF prevalence in developed countries is around 2% overall and exceeds 10% in people older than 70 years.8 In Spain, overall prevalence has been estimated at >5%, perhaps reflecting a lack of national population-based studies that would provide more precise estimates of the impact of this disease.5,9 Furthermore, current knowledge of the medical treatment received by patients is derived from registries compiled by specialist HF units and may not accurately represent the population at large.10 To allow appropriate planning of present and future interventions, there is therefore an urgent need for up-to-date population data that accurately describe HF prevalence and the use of prognosis-modifying treatments. In the present study, we report the epidemiology (prevalence and incidence), baseline characteristics, and treatment of the HF population, especially HFrEF patients, in standard clinical practice in Spain during the period 2017-2019. The study is called PATHWAYS-HF to reflect the goal to provide information about the current state of HF and treatment trajectories in these patients.
METHODSStudy design and populationWe performed a retrospective observational analysis of electronic medical records (EMR) retrieved from the BIG-PAC administrative database, a source of secondary data. BIG-PAC currently holds information from 1 853 412 individuals and is owned by Atrys Health. The primary data are the EMRs from 7 health care zones in 7 Spanish autonomous communities and include data from primary health centers and referral hospitals within the Spanish national health system. Before export to BIG-PAC, data are rigorously anonymized at the health center or hospital of origin. The data are thus dissociated before import to BIG-PAC, and it is not possible to identify the territory, health care provider, treating physician, or patient or access any other information that would permit individual identification. This procedure ensures adherence to current law governing the protection of personal data (Ley General de Protección de Datos de Carácter Personal). Because the encryption algorithm is applied to EMRs at the source hospitals and health centers, it is opaque and irreversible for Atrys Health. The company therefore has no means of accessing the primary data source. BIG-PAC is registered with the European Medicines Agency.
A demographic comparison conducted in 2019 confirmed that the BIG-PAC data are representative of the whole Spanish population; the results of that study are summarized in the . For the present study, we included epidemiological and medication data for all patients requiring care during 2017, 2018, and 2019. Patients newly diagnosed with HFrEF in the first year of the study period were analyzed in more detail to assess medication changes over a 2-year period.
Inclusion and exclusion criteriaThe following inclusion criteria were established. Patients had to be a) aged ≥ 18 years; b) registered on the database at least 12 months before the initiation of the study period; c) included in the prescription program (with verified records of daily doses, interdose intervals, and treatment duration for each treatment and at least 2 prescriptions during the follow-up period), and d) traceable through certified regular follow-up (at least 2 recorded consultations in the electronic records). Patients were excluded if they a) had moved or were located outside the included health care areas; b) were permanent elderly care home residents; c) had a severe mental illness or end-stage disease or were on dialysis, or d) lacked LVEF data (missing echocardiogram).
Heart failure diagnosisHF patient records were obtained according to the International Disease Classification, Ninth Revision, Clinical Modification (ICD-9-CM, code 428). HF was diagnosed according to clinical criteria (the presence of clinical symptoms or signs as assessed by the treating physician) or the detection of a structural or functional abnormality on echocardiography.
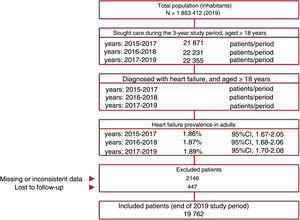
Point prevalence and incidence ratePrevalence was determined as the number of diagnosed HF patients relative to the total population seeking medical attention (by year, age, and sex) and was calculated for 3 consecutive years (figure 1). The incidence rate was calculated as the number of patients with a new HF diagnosis/1000 persons/y within the population seeking medical attention (for 2017, 2018, and 2019). Results were not standardized because the age and sex distribution of the study population was similar to that of the general Spanish population ().
Demographic variables and comorbiditiesSociodemographic variables and comorbidities were obtained for 2019. The sodiodemographic variables were age (continuous) and sex. The assessed comorbidities (ICD-9-CM) were a history of hypertension, dyslipidemia, diabetes, obesity, cerebrovascular disease (ischemic stroke or transient ischemic attack), peripheral artery disease, anemia, kidney failure (glomerular filtration rate < 60mL/min/1.73 m2 according to the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] method), glomerular filtration rate <30mL/min/1.73 m2, chronic obstructive pulmonary disease, cancer, and atrial fibrillation. The proportion of patients dying in the study period was calculated for cardiovascular or noncardiovascular causes. The Charlson comorbidity index was used as a general comorbidity variable.11
MedicationInformation on medication was obtained from dispensary records and was classified according to the Anatomical Therapeutic Chemical Classification System (ATC).12 The following therapeutic groups and active ingredients were selected: ivabradine (C01EB17), diuretics (C03, excluding AAs), AAs (C03DA), beta-blockers (C07), ACEI (C09A-C09B), ARB (C09C-C09D, excluding sacubitril-valsartan), sacubitril-valsartan (C09DX04), and sodium-glucose cotransporter 2 inhibitors. The choice of medication for each patient was made by the treating physician according to clinical criteria.
Clinical variablesEMRs were examined to obtain the following variables for each treated patient: a) LVEF phenotype (HFrEF, ≤ 40%; HFmrEF, 41-49%; and HFpEF, ≥ 50%); b) HF etiology (ischemic or nonischemic heart disease); and c) New York Heart Association (NYHA) functional class (I-IV). For the analysis, we selected the first available values after inclusion. For the analysis of changes over time, we considered the values closest to the time period in question.
Statistical analysisThe database was interrogated with SQL script commands. The data were carefully reviewed through exploratory studies and during their preparation for analysis, including thorough scrutiny of distribution and frequency and screening for possible recording and code errors. We performed a descriptive univariate analysis. Qualitative data are presented as absolute or relative frequencies, quantitative data are presented as mean±standard deviation, and 95% confidence intervals (95%CI) were calculated for the whole study population. In the bivariate analysis, differences between independent groups were assessed by the chi-square test and ANOVA. Differences between paired groups (for time comparisons) were analyzed by the McNemar test; statistical significance was set at P <.05. Data were analyzed with SPSSWIN version 23.
RESULTSBaseline characteristics (2019)Of the 1 853 412 people included in the BIG-PAC database in 2019, we excluded the population younger than 18 years. The database thus included 1 189 003 adults who sought medical attention between 2017 and 2019.
This population included 22 355 patients with a diagnosis of HF. Of these patients, 2593 were excluded due to incomplete data (mostly a lack of LVEF data) or loss to follow-up. The final study population therefore included 19 762 patients. The selection of the study population is illustrated in the flow diagram in figure 1.
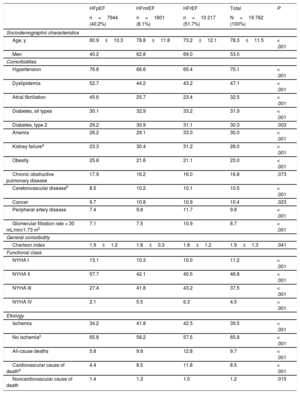
The mean age of the HF population was 78.3±11.5 years; 53% were men. Within this population, 70.1% had hypertension, 47.1% dyslipidemia, and 32.5% atrial fibrillation. The mean Charlson index was 1.9 points. The LVEF phenotype distribution was as follows: a) HFrEF, 51.7%; b) HFpEF, 40.2%, and HFmrEF, 8.1%. The most frequent NYHA class was class II (46.8%).
HFrEF patients tended to be younger (mean age 73.2±12.1 years, 69.0% men), and 65.4% of them had hypertension, 43.2% dyslipidemia, and 31.1% type 2 diabetes mellitus. The mean Charlson index among HFrEF patients was 1.8 points. Mortality was higher than in the general HF population (12,8% among HFrEF patients vs 9.7% for HF overall; P <.001). Baseline population characteristics are shown in table 1.
Population baseline characteristics stratified by LVEF phenotype (2019)
| HFpEF | HFmrEF | HFrEF | Total | P | |
|---|---|---|---|---|---|
| n=7944 (40.2%) | n=1601 (8.1%) | n=10 217 (51.7%) | N=19 762 (100%) | ||
| Sociodemographic characteristics | |||||
| Age, y | 80.9±10.3 | 78.8±11.8 | 73.2±12.1 | 78.3±11.5 | < .001 |
| Men | 40.2 | 62.8 | 69.0 | 53.0 | |
| Comorbidities | |||||
| Hypertension | 76.8 | 66.6 | 65.4 | 70.1 | < .001 |
| Dyslipidemia | 52.7 | 44.2 | 43.2 | 47.1 | < .001 |
| Atrial fibrillation | 45.6 | 25.7 | 23.4 | 32.5 | < .001 |
| Diabetes, all types | 30.1 | 32.9 | 33.2 | 31.9 | < .001 |
| Diabetes, type 2 | 29.2 | 30.9 | 31.1 | 30.3 | .003 |
| Anemia | 26.2 | 29.1 | 33.0 | 30.0 | < .001 |
| Kidney failurea | 23.3 | 30.4 | 31.2 | 28.0 | < .001 |
| Obesity | 25.8 | 21.6 | 21.1 | 23.0 | < .001 |
| Chronic obstructive pulmonary disease | 17.9 | 16.2 | 16.0 | 16.8 | .073 |
| Cerebrovascular diseaseb | 8.5 | 10.2 | 10.1 | 10.5 | < .001 |
| Cancer | 9.7 | 10.8 | 10.9 | 10.4 | .023 |
| Peripheral artery disease | 7.4 | 9.8 | 11.7 | 9.8 | < .001 |
| Glomerular filtration rate < 30 mL/min/1.73 m2 | 7.1 | 7.5 | 10.9 | 8.7 | < .001 |
| General comorbidity | |||||
| Charlson index | 1.9±1.2 | 1.8±0.3 | 1.8±1.2 | 1.9±1.3 | .041 |
| Functional class | |||||
| NYHA I | 13.1 | 10.3 | 10.0 | 11.2 | < .001 |
| NYHA II | 57.7 | 42.1 | 40.5 | 46.8 | < .001 |
| NYHA III | 27.4 | 41.8 | 43.2 | 37.5 | < .001 |
| NYHA IV | 2.1 | 5.5 | 6.3 | 4.5 | < .001 |
| Etiology | |||||
| Ischemia | 34.2 | 41.8 | 42.5 | 39.5 | < .001 |
| No ischemiac | 65.8 | 58.2 | 57.5 | 65.8 | < .001 |
| All-cause deaths | 5.8 | 9.8 | 12.8 | 9.7 | < .001 |
| Cardiovascular cause of deathd | 4.4 | 8.5 | 11.8 | 8.5 | < .001 |
| Noncardiovascular cause of death | 1.4 | 1.3 | 1.0 | 1.2 | .015 |
HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Data are expressed as No. (%) or mean±standard deviation.
Kidney failure is defined as glomerular filtration rate < 60mL/min/1.73 m2 according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) method.
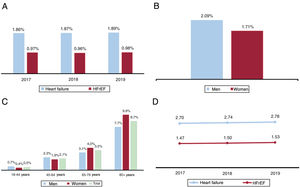
HF prevalence values were as follows: a) 2015-2017, 1.86% (95%CI, 1.67%-2.05%); b) 2016-2018, 1.87% (95%CI, 1.68%-2.06%); and c) 2017-2019, 1.89% (95%CI, 1.70%-2.08%). The small differences in these prevalence values were not statistically significant (P=.574).
Prevalence values for total HF and HFrEF during the period 2017-2019 are shown in figure 2A. Stratification of HF prevalence in 2019 by sex and by sex and age range is shown in figure 2B,C. In 2019, HF prevalence was close to 2%, with HFrEF accounting for 51% of cases and prevalence higher in men. Prevalence was very low in people younger than 45 years but approached 9% in those older than 80 years.
In the final year of the 2017-2019 study period, the incidence rate for HF was 2.78/1000 persons/y, and the rate for HFrEF was 1.53/1000 persons/y. The incidence rate increased slightly over the 3 years of this study period, but this change was not statistically significant (P=.213) (figure 2D).
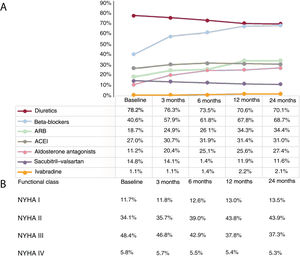
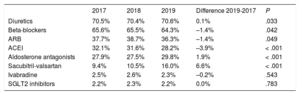
Medical treatment of HF (2017-2019)For the analysis of medication prescribed to HF patients, we focused on HFrEF because of the availability of evidence-based treatment guidelines and drugs able to modify the prognosis of this condition. During 2019, 64.3% of HFrEF patients were treated with beta-blockers, 80.5% with ACEI/ ARBs or sacubitril-valsartan, and 29.8% with AAs (table 2). Treatment optimization was assessed by analyzing the treatment trajectories of the 1550 patients diagnosed with HFrEF in 2017 over a 24-month period (figure 3A). Only 40.6% of patients were treated with beta-blockers initially, but treatment increased more than for any other drug class during the follow-up period, to 68.7% of patients at 24 months (P <.001). For ACEIs and ARBs, prescription increased from 45.7% of patients initially to 65.4% after 24 months (P <.001). Only 11.2% of patients were treated with AAs initially, increasing to just 27.4% at 24 months (P <.001). The only treatment showing a decline was sacubitril-valsartan, from 14.8% of patients initially to 11.6% at the end of the follow-up period (P=.012).
Medication prescribed to HFrEF patients, 2017-2019
| 2017 | 2018 | 2019 | Difference 2019-2017 | P | |
|---|---|---|---|---|---|
| Diuretics | 70.5% | 70.4% | 70.6% | 0.1% | .033 |
| Beta-blockers | 65.6% | 65.5% | 64.3% | –1.4% | .042 |
| ARB | 37.7% | 38.7% | 36.3% | –1.4% | .049 |
| ACEI | 32.1% | 31.6% | 28.2% | –3.9% | < .001 |
| Aldosterone antagonists | 27.9% | 27.5% | 29.8% | 1.9% | < .001 |
| Sacubitril-valsartan | 9.4% | 10.5% | 16.0% | 6.6% | < .001 |
| Ivabradine | 2.5% | 2.6% | 2.3% | –0.2% | .543 |
| SGLT2 inhibitors | 2.2% | 2.3% | 2.2% | 0.0% | .783 |
ACEI, angiotensin-converting enzyme inhibitor;ARB, angiotensin II receptor blocker; HFrEF, heart failure with reduced ejection fraction; SGLT2, sodium-glucose cotransporter 2.
Temporal changes in medication administered (by therapeutic group) and functional class in patients diagnosed with HFrEF in 2017 (N=1550). Data are expressed as percentages. All comparisons (baseline vs 24 months) showed statistically significant differences; P < .01. ACEI, angiotensin-converting enzyme inhibitor;ARB, angiotensin II receptor blocker; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association.
The analysis revealed that treatment optimization tended to take place within the first 12 months, with little further change in the second year. On diagnosis in 2017, 82.5% of HFrEF patients were in NYHA functional class II or III, and this figure remained stable at 81.2% after 24 months (P=.142). NYHA functional class III included 48.4% patients initially, decreasing to 37.3% at 24 months (P <.001). The reduction mostly occurred during the first 12 months and was concentrated between months 6 and 12 (in which period there was a 5.1% reduction). NYHA functional class II moved in the opposite direction, increasing from 34.1% initially to 43.9% 2 years later (P <.002). Regarding the outermost functional classes, 5.8% of HFrEF patients were in NYHA class IV on diagnosis, decreasing by 0.5% to 5.3% at 24 months (P <.001). Just 13.5% of patients were in NYHA class I 2 years after diagnosis (figure 3B).
DISCUSSIONTo the best of our knowledge, this study is the largest population-based study of HF performed in Spain to date. The cohort is large, contemporary, and representative of the Spanish population, thus providing a good estimate of the real-world situation. The main study findings are that HF prevalence in Spain in 2019 was 1.89% of the population older than 18 years, with an incidence of 2.78/1000 persons/y, and that both figures were stable over the preceding 3 years. The results also clearly reflect the association of HF with aging, with the prevalence reaching 9% in the population older than 80 years. The most frequent HF type was HFrEF (51.7%). In contrast with the conclusions of other national and international studies, the present results show that there is wide room for improvement in the treatment optimization of HFrEF patients in accordance with current guidelines.1,3
During the period analyzed, the epidemiological data remained stable, showing a slight upward trend that did not reach statistical significance. The incidence rate remained steady at around 2.8 cases/1000 persons/y. The few previous epidemiological studies of HF in Spain have reported widely differing prevalence values depending on the geographical area and study population examined. For example, the PRICE study reported an HF prevalence of 6.8% in a randomized sample of patients aged ≥ 45 years from 15 hospitals and 55 health care centers in 9 autonomous communities.13 The EPISERVE study reported prevalence values of 2% in primary care, 17% in cardiology, and 12% in internal medicine.14 In a small sample (n=391) in Asturias, Cortina et al. reported a prevalence of 4.9%.15 These values contrast with the considerably lower prevalence detected in population-based studies. For example, Farre et al. reported prevalence values in Catalonia of 1.2% for the population older than 15 years and 2.7% among people older than 44 years.16 These findings are in line with those reported by Carmona et al. in the Community of Madrid.17 The prevalence values reported in non–population-based studies in Spain are thus much higher than the true values; in contrast, other population-based studies have published prevalence values similar to those reported here.1–4,18,19
In the present study, 51.7% of HF patients had HFrEF and 40.2% had HFpEF. These figures are somewhat higher than those published in previous reports.4,5,10 However, the rates for the most prominent comorbidities in our study (hypertension, dyslipidemia, atrial fibrillation, diabetes, and kidney failure) are comparable to those reported in other studies.4,15,16 Conde et al.5 reported that HF-associated comorbidities are a frequent cause of hospital readmissions, and the same study also showed that hospitalized HF patients tend to be elderly and have multiple comorbidities that hinder diagnosis and treatment, interfere with patient progress, and are associated with a worse prognosis. The published literature reveals some variability in comorbidity rates in HF2,5,13,15,16,20; however, the reported comorbidity rate is high in all studies, and the proportion of HF patients with HFrEF is consistently reported at between 40% and 60%.1,3,7
The analysis of medical treatment for HFrEF in the most recent study year (2019) reveals that 35.7% of patients were not prescribed beta-blockers, 19.5% were not prescribed ACEI/ARBs or sacubitril-valsartan, and 70.2% were not prescribed AAs. Current European Society of Cardiology treatment guidelines for HFrEF recommend an ACEI or ARB, a beta-blocker, and an AA (indication class I, level of evidence A).3 The high nonprescription rates found here thus demonstrate that treatment optimization should be prioritized as an essential step toward reducing symptoms, hospitalization, and mortality and increasing functional capacity and quality of life.1–4,9 Growing evidence for the benefits of sacubitril-valsartan recently led to this treatment being recommended as a replacement for ACEI/ARB therapy.21 However, examination of published studies shows that this recommendation is not being followed in clinical practice.22–25 Our data differ from those for the 2834 Spanish patients included in the European Heart Failure registry, which reveal the use of ACEI/ARBs, beta-blockers, and AAs by 92%, 93.3%, and 74.5% of patients, respectively.10 However, while these are real-world data, they reflect the specific experience of HF units; large population-based cohorts not linked to specialized HF units have produced data very similar to ours. For example, results from the recent American CHAMP-HF registry show that, in the absence of contraindications, 27% of HFrEF patients were not prescribed ACEI/ARBs or sacubitril-valsartan, 33% were not prescribed beta-blockers, and 67% were not prescribed AAs.26 There is thus clear room for improvement in treatment optimization for HFrEF.
In our sample of 1550 patients diagnosed with HFrEF in 2017, treatment optimization was concentrated in the first 3 to 6 months, and treatments tended to not to change in the 12–24-month period. In our view, this treatment trajectory indicates an important element of therapeutic inertia and therefore suggests that the treatment of these patients could be improved. However, the slowdown in treatment optimization may in part be due to specific factors or patient characteristics.10 It will be important to establish training programs and simple clinical procedures to improve the alignment of clinical practice with guideline recommendations,1,2 together with strategies to improve treatment adherence27 through coordination between different specialties, especially during transition from one area of care to another.
The cause of HF was ischemic (coronary) in 40% of the patients in the study population. Published rates of ischemic HF vary, but the proportion of ischemic HF in our study is below the > 60% reported in some reviewed series.28 This discrepancy could be due to 2 factors. a) In many patients, the presence of 2 or more overlapping causal factors makes it difficult to link HF to a single, specific etiology. For example, coronary atherosclerosis is frequently detected but is not always sufficiently severe to explain a patient's HF. Likewise, in other patients, hypertension can be considered both a risk factor for ischemic heart disease and a direct, intrinsic cause of HF. b) The retrospective nature of our study may have resulted in an under-recording of ischemic etiology. Nevertheless, our results are in line with other reported findings.4,25 Our analysis is at risk of the usual limitations associated with retrospective database reviews. These include disease under-reporting, possible variability among health care professionals and patients, the potential for classification bias in an observational study, and imprecise diagnosis coding. Another potential limitation is missing data, either due to nonsystematic determination of prognostic biomarkers (such as natriuretic peptides) or of variables such as LVEF or NYHA class. There is also a risk that the proportion of patients in treatment may be underestimated due to missing information related to dose changes, treatment adherence, medication prescribed by private health care providers, or paper prescriptions. It would also have been interesting to have information about how the different drug treatments were distributed according to the 3 LVEF phenotypes associated with HF. However, this was not considered as a study objective because HFrEF is the only phenotype whose prognosis can be modified with available medication. Finally, because our analysis did not consider the cause of death of patients who died at home, death due to a cardiovascular cause may have been underestimated.
CONCLUSIONSDuring the period analyzed, the epidemiological data for HF remained stable, with a prevalence close to 2%. This figure is lower than that reported in some non–population-based studies but is in line with the reported prevalence in comparable countries. A high proportion of HF patients have multiple comorbidities, and there is wide room for improvement in the optimization of medical treatment of HFrEF in line with scientific society recommendations.
FUNDINGThis study was funded by AstraZeneca, Spain.
AUTHOR CONTRIBUTIONSAll authors contributed to the study conception and design. A. Sicras-Navarro collected data and performed the statistical analysis. All authors interpreted data, wrote and revised the text, and approved the submitted manuscript.
CONFLICTS OF INTERESTA. Sicras-Mainar and A. Sicras-Navarro were independent consultants in relation to the preparation of this manuscript. B. Palacios and L. Varela are on the staff of AstraZeneca.
- -
HF is a complex clinical syndrome originating in altered cardiac structure or function.
- -
Estimates of HF prevalence in Spain show considerable variability and include likely overestimates.
- -
Current knowledge about medication prescribed to HF patients mostly comes from studies conducted in specialist cardiac units.
- -
The prevalence of HF in Spain is close to 2% in the adult population (≥ 18 years). HF is a health problem associated with aging.
- -
The most frequent HF phenotype is HFrEF (51.7%).
- -
The optimization of medical treatment for HFrEF leaves a lot to be desired and there is wide room for improvement.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.09.033