Degenerative aortic stenosis (DAS) is the most frequent valvular heart disease. It remains unclear how to identify asymptomatic DAS patients with normal left ventricular ejection fraction who have a high probability of event occurrence and would thus benefit from early intervention. Here, we describe a protocol for exercise hemodynamics in true asymptomatic patients with moderate or severe DAS and assess the prognostic value of the data obtained in this population.
MethodsThis study involved a prospective single-centre registry of consecutive asymptomatic patients with moderate or severe DAS. Patients underwent cardiopulmonary exercise testing to confirm symptom absence during exercise and then right heart catheterization (RHC) at rest and during exercise. Events were defined as death, surgical aortic valve replacement, or transcatheter aortic valve implantation according to clinical guidelines.
ResultsThirty-three patients underwent baseline and exercise RHC. The mean aortic valve area was 1.08 cm2 and the aortic gradient was 39mmHg. The mean pulmonary artery pressure was 21mmHg with a pulmonary artery occlusion pressure of 14mmHg and cardiac output of 5.6 L/min. The mean pulmonary artery pressure at peak exercise was 34mmHg. After a mean follow-up of 27 months, 8 patients experienced an event (24%). There were no differences in baseline variables, aortic valve area, or cardiopulmonary exercise testing parameters between the event and event-free groups. Patients with an event did not have higher pulmonary or filling pressures after peak exercise but had lower pulmonary artery oxygen saturation on effort (median, 48% vs 57%, P=.03).
ConclusionsExercise RHC is feasible and safe in this population. Peak pulmonary artery oxygen saturation might identify patients with increased risk of serious adverse events.
Keywords
Degenerative aortic stenosis (DAS), already the most frequent valvular heart disease, is showing ever increasing prevalence in western societies due to population aging.1 In symptomatic patients, the only effective treatments are surgical aortic valve replacement or percutaneous transcatheter aortic valve implantation.1 Despite its frequency, it is unclear how to identify asymptomatic patients with normal left ventricular ejection fraction but a high probability of event occurrence. Serial testing and follow-up of asymptomatic patients with moderate-to-severe aortic stenosis is the most usual approach, but some parameters, such as severe pulmonary hypertension, the hemodynamic progression rate of the stenosis, and elevated plasma levels of natriuretic peptides,1 suggest an adverse prognosis and might tip the balance in favor of early elective intervention.
Although exercise-induced symptoms are the main reason for intervention in most patients, noninvasive resting parameters are used to indicate early surgery in asymptomatic patients. Exercise noninvasive hemodynamic results have been linked to poor outcomes in several cardiac conditions and in asymptomatic aortic stenosis.2 However, the evidence regarding their true value is controversial because pulmonary pressures during exercise depend not only on pulmonary vascular resistance and left ventricular end-diastolic pressure, but also on cardiac output, which is not usually measured during exercise echocardiography and whose reliability during exercise is unclear. High exercise pulmonary pressures have been found on stress echocardiography even in healthy young individuals3 and show well-documented variability when compared with invasive hemodynamics.4
Invasive hemodynamics have been proven to provide accurate prognostic information in a wide range of cardiac conditions, especially in heart failure, but information on exercise invasive hemodynamics is lacking and exercise protocols for right heart catheterization (RHC) have not been standardized. In this study, our objective was to describe the protocol for exercise hemodynamics in true asymptomatic patients with moderate or severe aortic stenosis and to assess the prognostic value of the data obtained in this population.
METHODSStudy populationThis study involved a prospective single-centre registry of consecutive asymptomatic patients with moderate or severe valvular DAS detected by echocardiography. Patients were enrolled from May 2015 to April 2018. The study was approved by the local ethics committee before patient inclusion. Absence of cardiovascular symptoms was confirmed at the enrollment visit and echocardiography was repeated to confirm the presence of DAS. We excluded patients with reduced left ventricular systolic function (< 50%), other severe valvular diseases, or other cardiac conditions that contraindicated treadmill testing. Only patients younger than 85 years of age able to provide informed consent and to perform exercise by walking on the treadmill were included.
By protocol, patients underwent cardiopulmonary exercise testing (CPET) to confirm the absence of symptoms during exercise or blood pressure falls or other CPET parameters of poor prognosis according to the investigator's criteria. Within 1 month after the CPET, patients underwent RHC at rest and during exercise. All patients had a clinical follow-up every 6 months in a dedicated clinic. Events were defined as the occurrence of death, surgical aortic valve replacement, or transcatheter aortic valve implantation according to clinical guidelines, or the development of symptoms related to aortic stenosis with an intervention planned.
Evaluation of aortic stenosis severityEchocardiography assessment was performed according to clinical guidelines.5 Continuous-wave Doppler was used to measure transaortic velocities. Peak and mean transaortic pressure gradients were calculated using the simplified Bernoulli equation. Aortic valve area was calculated using the continuity equation. The aortic stenosis was considered severe if the valve area was ≤ 1cm.2
Cardiopulmonary exercise testingAll patients underwent CPET on a Mortara Xscribe device (Mortara Instrument, Inc., Milwaukee, United States) and Full Vision treadmill (Full Vision, Kansas, United States). Data were processed with Blue Cherry version 1.2.2.2 software from Geratherm Respiratory (Geratherm Respiratory GmbH, Bad Kissingen, Germany). The exercise protocol (Naughton, modified Bruce, Bruce, or ramp) was selected on an individual basis according to the patient's mobility and comorbidities and at the investigator's discretion. Patients were encouraged to exercise until exhaustion. Blood pressure was carefully monitored at the end of each stage using a calibrated sphygmomanometer, and heart rate and continuous 12-lead electrocardiogram monitoring were also recorded. An experienced cardiologist closely monitored all patients during the test. The test was promptly stopped if symptoms or any other complications developed. The peak oxygen consumption (peak oxygen uptake [VO2]), percentage of the estimated VO2, respiratory exchange ratio, VE/VCO2 slope, VE/VO2, VE/VCO2, presence of exercise oscillatory ventilation, baseline and peak end-tidal CO2 pressure, and respiratory reserve were analyzed.
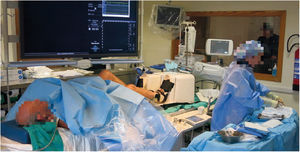
Right heart catheterization at rest and during exerciseRHC was performed with a 6-French Swan-Ganz catheter through a cephalic vein under fluoroscopy guidance. Measurements were taken at rest and after performance of controlled supine exercise with a Cardio Step device (Ergospect GmbH, Innsbruck, Austria; Figure 1 and ) until exhaustion or symptom occurrence, with patients trying to reach the same workload as in the CPET (Watts vs peak VO2) according to the available evidence.6 The exercise protocol comprised incremental step resistance every 30seconds and patients were encouraged to follow a 60 steps per minute pace. Blood pressure and electrocardiography were monitored according to usual practice during the test. After measurement of baseline pressures, pulmonary artery saturation, and cardiac output, patients started supine exercise with the Swan-Ganz catheter floating in the pulmonary artery (). Pulmonary pressures at peak effort and pulmonary artery oxygen saturation (PaO2s) were recorded. Immediately after the exercise was stopped, the catheter was moved to obtain the pulmonary artery occlusion pressure and rapidly pulled back to the right ventricle and right atrium. Baseline cardiac output was calculated using the indirect Fick method because our aim was to compare PaO2s at rest and after exercise. Thermodilution was not considered adequate to assess peak cardiac output because the current guidelines recommend at least 3 measurements,7 which takes too long to be reliable for assessing peak cardiac output.
Statistical analysisAll data were prospectively collected in an anonymous database. To detect events, only patients with at least 1 year of follow-up after inclusion were analyzed. Results are expressed as mean±standard deviation, median with interquartile range, or percentage, as appropriate. Statistical differences between groups were assessed using a Mann-Whitney U test and chi-square test as appropriate. Values of P <.05 were considered significant. All statistical analyses were performed with SPSS version 20.0 (SPSS Inc., Chicago, Illinois, United States).
RESULTSFrom May 2015 to April 2018, 43 patients meeting the inclusion criteria underwent CPET. Of these, 5 (11.6%) developed symptoms during exercise and were referred for surgical aortic valve replacement or transcatheter aortic valve implantation. The other 38 patients underwent CPET without showing symptoms and were scheduled to undergo baseline and exercise RHC. Four of them did not have the test because of problems with the vascular access (ie, if access was not possible through the cephalic vein, because no other access site was allowed by protocol) and 1 patient only had a baseline RHC (the exercise test was canceled due to the patients’ very high blood pressure). Of the 33 patients, 1 developed transient atrial fibrillation during the procedure, which was the only complication of this study.
Baseline data are shown in Table 1. The mean age was 74 years, mean aortic valve area 1.08cm2, and mean aortic gradient 39mmHg. Patients performed well on CPET with a mean peak VO2 of 18.7mL/kg/m2, which resulted in a mean of 90% of the theoretical peak VO2 adjusted by age and sex (normal> 80%).
Baseline characteristics of the entire cohort
| Age, y | 73.84±8.30 | End-diastolic LV volume indexed, mL/m2 | 62.90±18.01 |
| Sex, % male | 91 | LA area, cm2 | 22.10±6.50 |
| Hypertension, % | 73 | RA area, cm2 | 17.00±4.72 |
| Diabetes mellitus, % | 15 | TAPSE, mm | 24.02±3.16 |
| Dyslipidemia, % | 60 | Peak VO2, mL/min/m2 | 18.70±3.68 |
| Active smoker, % | 6 | %maxVO2 | 90.27±13.62 |
| Atrial fibrillation, % | 3 | RER | 0.99±0.08 |
| Coronary artery disease, % | 9 | VE/VCO2 slope | 33.32±4.87 |
| Stroke, % | 6 | Basal PETCO2, mmHg | 31.84±4.22 |
| Aortic valve area, cm2 | 1.08±0.28 | Peak PETCO2, mmHg | 37.32±3.49 |
| Aortic valve area indexed, cm2/m2 | 0.58±0.15 | Grade III AR, % | 21 |
| Aortic valve mean gradient, mmHg | 39.02±12.78 | Grade III MR, % | 3 |
| Severe aortic stenosis, % | 48.5 | LVEF, % | 65.30±6.54 |
| End-diastolic LV volume, mL | 120.39±42.66 |
AR, aortic regurgitation; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; PETCO2, end-tidal carbon dioxide; RA, right atrium; RER, respiratory exchange ratio; TAPSE, tricuspid annular plane systolic excursion; VO2, peak oxygen consumption.
Results are expressed as mean±standard deviation and percentages.
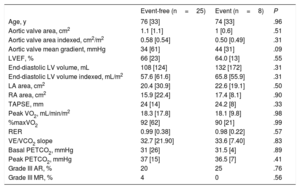
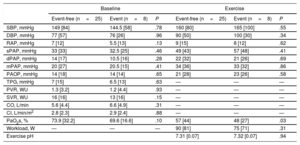
Baseline and exercise invasive hemodynamic data are shown in Table 2. The mean pulmonary artery pressure was 21mmHg with a mean pulmonary artery occlusion pressure of 14mmHg and mean cardiac output of 5.6 L/min. The mean peak workload was 83 Watts and the mean pulmonary artery pressure at peak exercise was 34mmHg. The mean baseline PaO2s was 74%, which decreased to a mean of 54% during peak exercise.
Baseline and peak exercise right heart catheterization data
| Baseline | Exercise | |
|---|---|---|
| Heart rate, bpm | 69.06±9.98 | 102.69±16.84 |
| SBP, mmHg | 146.66±18.11 | 171.36±18.25 |
| DBP, mmHg | 76.18±11.88 | 94.85±10.42 |
| RAP, mmHg | 7.57±3.65 | 9.45±4.48 |
| sPAP, mmHg | 33.61±8.31 | 52.75±13.12 |
| dPAP, mmHg | 13.60±4.98 | 21.81±8.01 |
| mPAP, mmHg | 21.48±6.14 | 34.63±9.22 |
| PAOP, mmHg | 14.45±4.84 | 22.21±8.42 |
| TPG, mmHg | 6.96±3.60 | — |
| PVR, WU | 1.70±1.13 | — |
| SVR, WU | 16.56±4.82 | — |
| CO, L/min | 5.59±1.32 | — |
| CI, L/min/m2 | 2.91±0.62 | — |
| PaO2s, % | 73.94±5.96 | 54.71±12.55 |
| Workload, W | — | 83.07±25.16 |
CI, cardiac index; CO, cardiac output; DBP, diastolic blood pressure; dPAP, diastolic pulmonary artery pressure; mPAP, mean pulmonary artery pressure; PaO2s, pulmonary artery oxygen saturation; PAOP, pulmonary artery occlusion pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure; SVR, systemic vascular resistance; TPG, transpulmonary gradient.
Results are expressed as mean±standard deviation.
After a mean follow-up of 27.6±7.8 months, 8 patients had an event (24%): 2 patients died (1 from sudden cardiac death; the other developed cardiogenic shock days after being hospitalized with pancreatitis) and 6 patients underwent aortic valve replacement or transcatheter aortic valve implantation due to the development of symptoms or left ventricular dysfunction.
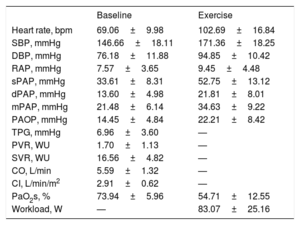
Comparisons between the event-free and event groups are shown in Table 3 and Table 4. There were no statistically significant differences between the 2 groups in baseline variables, aortic valve area, and CPET parameters (Table 3), although patients who had an event tended to have higher mean aortic valve gradients. There were also no differences in baseline invasive hemodynamics (Table 4). Patients with an event did not have higher pulmonary pressures or right or left filling pressures after peak exercise but did have significantly lower PaO2s on effort (median, 48% vs 57%; P=.03) without a difference in workload (median, 75W vs 90W; P=.31). This difference in peak PaO2s was not found after stratification for severe or moderate DAS according to echocardiographic classification (median, 53% vs 55%; P=.49). Exercise PaO2s performed reasonably well in the ROC curve analysis (Figure 2), with an area under the curve of 0.76. A peak PaO2s under 51% showed a sensitivity and specificity of 75%, whereas a peak PaO2s under 54% showed a sensitivity of 87.5% and specificity of 67%. The median peak exercise pH indicated metabolic acidosis without differences between the event and event-free groups, confirming that the patients exceeded the anaerobic threshold.
Baseline characteristics of the event and event-free groups
| Event-free (n=25) | Event (n=8) | P | |
|---|---|---|---|
| Age, y | 76 [33] | 74 [33] | .96 |
| Aortic valve area, cm2 | 1.1 [1.1] | 1 [0.6] | .51 |
| Aortic valve area indexed, cm2/m2 | 0.58 [0.54] | 0.50 [0.49] | .31 |
| Aortic valve mean gradient, mmHg | 34 [61] | 44 [31] | .09 |
| LVEF, % | 66 [23] | 64.0 [13] | .55 |
| End-diastolic LV volume, mL | 108 [124] | 132 [172] | .31 |
| End-diastolic LV volume indexed, mL/m2 | 57.6 [61.6] | 65.8 [55.9] | .31 |
| LA area, cm2 | 20.4 [30.9] | 22.6 [19.1] | .50 |
| RA area, cm2 | 15.9 [22.4] | 17.4 [8.1] | .90 |
| TAPSE, mm | 24 [14] | 24.2 [8] | .33 |
| Peak VO2, mL/min/m2 | 18.3 [17.8] | 18.1 [9.8] | .98 |
| %maxVO2 | 92 [62] | 90 [21] | .99 |
| RER | 0.99 [0.38] | 0.98 [0.22] | .57 |
| VE/VCO2 slope | 32.7 [21.90] | 33.6 [7.40] | .83 |
| Basal PETCO2, mmHg | 31 [26] | 31.5 [4] | .89 |
| Peak PETCO2, mmHg | 37 [15] | 36.5 [7] | .41 |
| Grade III AR, % | 20 | 25 | .76 |
| Grade III MR, % | 4 | 0 | .56 |
AR, aortic regurgitation; LA, left area; LV, left ventricle; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; PETCO2, end-tidal carbon dioxide; RA, right area; RER, respiratory exchange ratio; TAPSE, tricuspid annular plane systolic excursion; VO2, oxygen consumption.
Unless otherwise indicated, results are expressed as median [range].
Baseline and exercise right heart catheterization data in the event and event-free groups
| Baseline | Exercise | |||||
|---|---|---|---|---|---|---|
| Event-free (n=25) | Event (n=8) | P | Event-free (n=25) | Event (n=8) | P | |
| SBP, mmHg | 149 [84] | 144.5 [58] | .78 | 160 [80] | 165 [100] | .55 |
| DBP, mmHg | 77 [57] | 76 [26] | .96 | 90 [50] | 100 [30] | .34 |
| RAP, mmHg | 7 [12] | 5.5 [13] | .13 | 9 [15] | 8 [12] | .62 |
| sPAP, mmHg | 33 [33] | 32.5 [25] | .46 | 49 [43] | 57 [48] | .41 |
| dPAP, mmHg | 14 [17] | 10.5 [16] | .28 | 22 [32] | 21 [26] | .69 |
| mPAP, mmHg | 20 [27] | 20.5 [15] | .41 | 34 [36] | 33 [32] | .86 |
| PAOP, mmHg | 14 [18] | 14 [14] | .65 | 21 [28] | 23 [26] | .58 |
| TPG, mmHg | 7 [15] | 6.5 [13] | .63 | — | — | — |
| PVR, WU | 1.3 [3.2] | 1.2 [4.4] | .93 | — | — | — |
| SVR, WU | 16 [16] | 13 [16] | .15 | — | — | — |
| CO, L/min | 5.6 [4.4] | 6.6 [4.9] | .31 | — | — | — |
| CI, L/min/m2 | 2.8 [2.3] | 2.9 [2.4] | .88 | — | — | — |
| PaO2s, % | 73.9 [32.2] | 69.6 [16.6] | .10 | 57 [44] | 48 [27] | .03 |
| Workload, W | — | — | — | 90 [81] | 75 [71] | .31 |
| Exercise pH | 7.31 [0.07] | 7.32 [0.07] | .94 | |||
CI, cardiac index; CO, cardiac output; DBP, diastolic blood pressure; dPAP, diastolic pulmonary artery pressure; mPAP, mean pulmonary artery pressure; PaO2s, pulmonary artery oxygen saturation; PAOP, pulmonary artery occlusion pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure; SVR, systemic vascular resistance; TPG, transpulmonary gradient.
Results are expressed as median [range].
One of the holy grails common to all medical specialties is the ability to predict clinical deterioration in patients with chronic conditions, particularly when this information could prompt a significant change in management, such as surgery. Exercise testing in patients with chronic cardiovascular conditions seems to be the most physiological way to evaluate clinical status, with the clinical management usually altered by the detection of symptoms during exercise, not at rest, because this situation represents a later stage of disease progression. In DAS, only conventional exercise stress testing is recommended to unmask symptoms or detect blood pressure falls in selected individuals and is not part of the routine follow-up in patients with otherwise asymptomatic DAS.1,8 Due to their well-recognized prognostic value, invasive hemodynamics provide a direct and accurate assessment of cardiac output, pulmonary pressures, and filling pressures and are widely used in critical care and in heart failure management to guide treatment and clinical decision-making in both stable and unstable patients.9 Exercise invasive hemodynamics are considered a highly attractive and physiological way to assess patients’ status and prognosis, but a lack of standardization and evidence about their clinical significance leave them relegated to a research tool in different clinical scenarios such as scleroderma-related pulmonary hypertension and heart failure.10,11
In this report, we show that exercise RHC in true asymptomatic (with a normal exercise capacity in CPET) patients with moderate or severe aortic stenosis is feasible and safe and provides useful information that correlates with poor outcomes. A lower peak exercise PaO2s in our cohort was significantly associated with the occurrence of major events (death and need for intervention) after a mean follow-up of about 2 years. Resting PaO2s is a crucial biological parameter when assessing the hemodynamic status of heart failure and critically ill patients. PaO2s is influenced by cardiac output and peripheral oxygen extraction, 2 of the main determinants of exercise tolerance.12 In our cohort, there were no differences in resting PaO2s between the event and event-free groups. The difference was only found in peak PaO2s, suggesting poorer cardiac performance of the event group because there were no differences in workload or other baseline parameters. Although the peak PaO2s after treadmill exercise has classically been linked to poorer functional class,12 this is the first time that this parameter has been associated with poorer outcomes in any cardiac condition, including DAS.
Although the event group showed slightly higher exercise pulmonary artery pressures during peak exercise, the difference was not significant. It is likely that cardiac output was not as greatly increased by exercise in the event group, which is feasible because PaO2s is critical for calculating output with the Fick method. We can speculate that lower exercise cardiac output in the event group led to a less than expected increase in pulmonary pressures. Cardiac output can increase up to 5 to 8 times at peak exercise,3 but limits to the ability of pulmonary vasodilatation to accommodate this amount of volume overload increase pulmonary pressures. This explains why, in healthy athletes, even “severe” pulmonary hypertension has been found with noninvasive hemodynamic assessment.3 Although severe exercise pulmonary hypertension has been linked to poor outcomes in different conditions, an association that is probably correct in many cases, this result should be taken with caution because lower pulmonary pressures could be related to even worse outcomes if cardiac output or cardiac performance is poor. In addition, there is a weak relationship between noninvasive and invasive assessment of pulmonary pressures,4 indicating that careful consideration is required of the noninvasive calculation of pulmonary pressures.
We consider our work and its results primarily as a proof of concept, a demonstration that the idea behind it—the identification of new prognostic factors in DAS using exercise hemodynamics—is possible, feasible, and physiologically reasonable. Additionally, we believe that our findings might have a potential practical application. Accordingly, given that this study was conducted in selected patients and probably in a simpler manner than required, further research is warranted.
LimitationsA small number of patients was enrolled in this study, which otherwise is similar to other experiences in the literature.11 This protocol is very demanding and includes an invasive procedure, and some patients were reluctant to participate, despite the approval of our ethics committee. Ideally, research such as that presented here would be performed with the inclusion of an oxygen consumption device, such as an iCPET.13 This device would allow us to directly compare exercise cardiac output with the direct Fick method. Unfortunately, due to technical reasons, we were unable to use the CPET device in the catheterization laboratory. Although thermodilution at peak exercise was used in other experiences in the literature,11 we did not consider this approach to be the best option, for the reasons already given. Although the workload achieved was relatively low (but higher than that of previous experiences11), it correlates well with the peak VO2 achieved in the CPET, which was normal (> 80%) for age and sex. In addition, compared with cycloergometer exercise, treadmill exercise achieves a higher workload and degree of tachycardia,14 due to leg fatigue, which may be even more evident with our supine stepper.
CONCLUSIONSExercise RHC is feasible and safe in true asymptomatic patients with moderate-to-severe and severe aortic stenosis. Peak PaO2s might identify patients with an increased midterm risk of serious adverse events and may be useful in selected scenarios.
FUNDINGThis work has been funded with a grant from the Instituto de Salud Carlos III (PFIS).
Conflicts of interestNone declared.
- –
Some parameters, including severe pulmonary hypertension, the hemodynamic progression rate of the stenosis, and elevated plasma levels of natriuretic peptides, suggest adverse prognosis and might tip the balance in favor of early elective intervention, but strong evidence is lacking.
- –
Exercise right heart catheterization in asymptomatic patients with moderate or severe degenerative aortic stenosis is feasible and safe and provides useful information that is correlated with poor outcomes.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.03.005