Transfemoral implantation of an Edwards-SAPIEN (ES) or Medtronic CoreValve (MCV) aortic valve prosthesis is an alternative to surgical replacement for patients with severe aortic stenosis and a high surgical risk. The study's aim was to compare results obtained with these two devices.
MethodsProspective observational study of transfemoral prosthesis implantation performed at our center.
ResultsOf the 76 patients (age 83±6 years, 63% female, logistic EuroSCORE 18±9) included, 50 were assigned the ES and 26 the MCV device. There was no difference between the groups in age, sex, functional class, valve area, associated conditions, or EuroSCORE. Implantation was successful in 84% of the ES group and 100% of the MCV group (P=.04). There were three cases of tamponade, two aortic dissections and one valve malposition in the ES group. The two groups had similar vascular access complication rates (26% vs. 23%; P=NS), but pacemaker need was greater with the MCV (10% vs. 39%; P=.003). Mortality rates at 30 days were 12% and 20% (P=NS) in the ES and MCV groups, respectively, and at 1 year, 24% and 20% (P=NS), respectively. After a follow-up of 367±266 days in the ES group and 172±159 days in the MCV group, three patients died. Clinical improvement was maintained in other patients and no echocardiographic changes were observed.
ConclusionsIn-hospital mortality, the complication rate and medium-term outcomes were similar with the two devices. The only difference observed was a higher implantation success rate with the MCV, although at the expense of a greater frequency of atrioventricular block.
Keywords
The percutaneous implantation of an aortic valve prosthesis is an alternative to surgery in patients with severe aortic stenosis and at high surgical risk (HSR). Two types of valve have been developed for percutaneous implantation: a balloon-expandable prosthesis (first implantation performed in 20021) and a self-expanding prosthesis (first implantation performed in 20052). In 2008, the results of the initial feasibility and safety studies2,3,4,5,6,7 led to the Edwards-SAPIEN™ (ES) (Edwards Lifesciences LLC, Irvine, California, USA) and Medtronic CoreValve™ System (MCV) (Medtronic CoreValve LLC, Minneapolis, Minnesota, USA) being approved for their use in Europe. By the end of 2009, more than 10000 prostheses had been implanted, approximately two thirds via the transfemoral (TF) approach and one third via the transapical (TA) approach.
After they were launched, several multicenter registries,8,9,10 single-center registries,11,12,13 and some Spanish centers14,15,16,17 provided information on these devices. However, very few centers have incorporated both valves. In fact, a search of the literature revealed just two reported series18,19 with this characteristic. In our hospital, both devices have been implanted via the TF approach; thus, the aim of the present study is to describe the short-term and medium-term results of our TF implantation program, drawing attention to the different aspects of the two devices.
Methods Beginning the ProgramPatient selection commenced in February 2007. In August 2007, the first TF implantation of the Cribier–Edwards prosthesis was performed; this device was superseded by the ES in 2008 and by the ES XT in 2010. In March 2008, the MCV prosthesis program was launched and the first of these devices was implanted in July of the same year.
Patient SelectionCandidates for inclusion in the study were patients with a valve area <0.6cm2/m2, NYHA functional class >2 and at HSR, defined by at least one of the following 4 criteria: logistic EuroSCORE >20%, severe comorbidity (pulmonary, kidney, liver, blood, and cerebrovascular disease, and fragility), age >85 years, or rejected for surgery.
Assessment included transesophageal echocardiogram, coronary angiography, and aortography of the aortic root and iliac bifurcation and, in some patients, computed tomography (CT) of the iliofemoral axis. The minimal luminal diameter of each arterial segment (distal aorta, common iliac, external iliac, and common femoral) on both sides was measured.20 Tortuosity and calcification were classified as mild, moderate, or severe.
Device SelectionThe manufacturer's recommendations were followed for each device (Table 1). During the first year, only the ES valve was available, such that when the iliofemoral axis was unsuitable TA implantation was performed. With the incorporation of the MCV, and after performing the first procedures without applying any specific selection criteria, we decided to use the ES in patients with a suitable femoral artery, the MCV in patients with an annulus of 26–27mm (large for ES, appropriate for MCV) or with femoral arteries of 6–7.5mm (small for ES, acceptable for MCV), and the TA approach in patients with arteries <6mm.
Table 1. Anatomical Criteria for Patient Selection for the Percutaneous Implantation of Aortic Valve Prostheses as Recommended by the Manufacturers
| ES | MCV | |
| Aortic annulus (TEE/CT) | 18–25 | 19–27 |
| Height of the sinus of Valsalva (TEE/CT) | – | >10mm |
| Sinotubular junction (TEE/CT) | – | 30–40 |
| Ascending aorta (TEE/CT) | – | <42 |
| Outflow tract angle-ascending aorta (angiography/CT) | – | <45° |
| Outflow tract diameter (TEE) | >18 | >19 |
| MLD iliofemoral axis (angiography/CT) | >7mm (ES-23) | >6mm |
| >8mm (ES-26) | ||
| Tortuosity (angiography/CT) | ||
| Calcification (angiography/CT) |
Abbreviations: CT, computed tomography; ES, Edwards-SAPIEN prosthesis; MCV, Medtronic CoreValve prosthesis; MLD, minimum luminal diameter; TEE, transesophageal echocardiography.
The size of each device was selected according to the established recommendations (ES-23 for an annulus of 18–21mm, IS-26 for an annulus of 22–25mm, MCV-26 for an annulus of 20–23mm and MCV-29 for an annulus of 24–27mm).
Procedural LogisticsThe procedures were performed in the catheterization laboratory following standard aseptic and antiseptic preparation. In all cases, the team consisted of two interventional cardiologists, three nurses, an anesthesiologist (AC) and an echocardiologist (CA), and when the ES was used a vascular surgeon was present. Antibiotic prophylaxis was administered (cefazolin+gentamicin). At the beginning we used sedation as the anesthetic protocol (remifentanil+propofol); later, this was changed to general anesthesia, but currently we have returned to sedation. Stable blood pressure is essential during implantation, since hypotension inhibits the valve from opening correctly and hypertension favors embolization.
Although the procedure was generally performed under fluoroscopic guidance, conventional 3D transesophageal echocardiography was very useful in measuring the aortic annulus, locating calcium deposits, and assessing post-valvuloplasty opening and regurgitation, positioning during implantation, prosthetic functioning, and the presence of perivalvular leaks, as well as in diagnosing complications (tamponade, aortic dissection).
Instrumentation Non-Therapeutic AccessVenous access for temporary pacemakers via the femoral approach when using ES (electrode catheter <24h) and via the jugular approach when using MCV (electrode catheter 48h). Femoral arterial access using a 6F introducer for pressure monitoring and angiography.
Therapeutic AccessWhen ES was used (except for ES XT), the surgeon exposed the femoral artery and at the end of the procedure sutured the artery and repaired vascular damage. When the MCV or ES XT was implanted, closure was performed with the Prostar XL™ (Abbot Vascular, Chicago, Illinois, USA) device.
ValvuloplastyBalloon valvuloplasty was performed (NuMed Nucleus™, PTV, Numed Inc., Hopkinton, NY, USA) during high-frequency ventricular pacing (PACEL™, St Jude Medical, Minneapolis, Minnesota, USA).
Preparation of the ValveBoth prostheses are supplied in glutaraldehyde and naturally expanded. The ES prosthesis was mounted on a balloon and the MCV was prepared in frozen serum and gradually compressed until being introduced into its delivery sheath.
DevicesThe ES valve consists of a stainless steel stent to which the valve is sewn (bovine pericardium). It requires a 25F and 28F outer diameter introducer except for the ES XT model, which requires a 20F and 22F introducer. The MCV valve is made of porcine pericardium sewn to a self-expanding nitinol stent. It has three portions: the lower portion has high radial force that enables anchoring; the middle region is designed to avoid obstructing the coronary artery outflow tracts; and the upper portion has a larger diameter that helps to orient the prosthesis. It requires an 18F introducer.
ImplantationBoth devices were advanced using the retrograde approach and positioned under fluoroscopic, angiographic, and echocardiographic guidance. In the case of the ES, the reference line was valve calcium. The best projection is usually the left oblique cranial view. Implantation was performed during ventricular pacing at 180–220bpm without the possibility of correction once inflation was started.
The MCV valve was released when the distal extreme of the sheath was 3–4mm below the sinuses of Valsalva in a projection (normally left oblique caudal) in which the three sinuses were seen to be aligned. The device can be repositioned at the beginning of release and can still be reintroduced into its sheath and repositioned again up to release of the distal third.
Post-Implantation ProtocolIn the absence of complications the patients were moved to the cardiovascular critical care unit for 24h (ES) or 48h (MCV) and discharged after 5–6 days under treatment with clopidogrel 75mg and acetylsalicylic acid 100mg (3 months). Patients with complete atrioventricular (AV) block were implanted with a permanent pacemaker.
Follow-upThe patients were followed up at 30 days, 3 months, 6 months and 12 months and every year thereafter. In addition, an echocardiogram was performed at 30 days and at each yearly check-up.
Data CollectionThe data were collected prospectively and introduced in a database for their analysis.
Statistical AnalysisContinuous variables were expressed as mean (standard deviation [SD]) and compared using the Student's t-test. Discrete variables were expressed as percentages and compared using the χ2-test with Fisher's correction if needed. Mortality over time was expressed as Kaplan–Meier curves.
Results Patient SelectionBetween May 2007 and April 2010 159 patients were assessed: 7 were rejected due to having few symptoms, 12 for symptoms of another disease, 6 for nonsevere aortic stenosis, 7 for the lack of HSR criteria, 2 for contraindications (cognitive deterioration, life expectancy <1 year), 2 for predominant aortic regurgitation, 7 for a too large or too small aortic annulus, and 23 for unsuitable femoral arteries; 7 patients did not complete the assessment and 10 patients died during this period. In total, 76 patients were included: 50 were assigned to the ES and 26 to the MCV.
Characteristics of the PatientsTable 2 shows the patient characteristics. The populations were similar except for a smaller iliofemoral diameter in the MCV group.
Table 2. Baseline Clinical Data
| All (n=76) | ES (n=50) | MCV (n=26) | P | |
| Age (year) | 83±6 | 82±6 | 84±5 | .16 |
| Women | 48 (63%) | 34 (68%) | 14 (54%) | .22 |
| Body surface area (m2) | 1.80±0.2 | 1.81±0.2 | 1.77±0.2 | .47 |
| NYHA functional class III–IV | 58 (76%) | 40 (80%) | 18 (69%) | .30 |
| Atrial fibrillation | 19 (25%) | 14 (28%) | 5 (19%) | .40 |
| Ejection fraction (%) | 62±13 | 63±14 | 59±12 | .19 |
| Comorbidity | ||||
| Coronary artery disease | 38 (50%) | 25 (50%) | 13 (50%) | 1 |
| Previous coronary surgery | 6 (8%) | 5 (10%) | 1 (4%) | .66 |
| Previous angioplasty | 25 (33%) | 15 (30%) | 10 (39%) | .46 |
| Ejection fraction <40% | 7(9%) | 5 (10%) | 2 (8%) | 1 |
| Mitral regurgitation ≥moderate | 8 (11%) | 6 (12%) | 2 (8%) | .71 |
| Pulmonary hypertension >60mmHg | 27 (36%) | 15 (30%) | 12 (46%) | .16 |
| Pulmonary disease | 15 (20%) | 11 (22%) | 4 (15%) | .49 |
| Cerebrovascular disease | 6 (8%) | 4 (8%) | 2 (8%) | 1 |
| Peripheral vascular disease | 2 (2%) | 1 (2%) | 1 (4%) | 1 |
| Renal failure | 14(18%) | 10 (20%) | 4 (15%) | 0.76 |
| Age >85 years | 30 (39%) | 16 (32%) | 14 (54%) | .06 |
| Estimated surgical risk | ||||
| EuroSCORE | 17.7±9 | 17.3±7.9 | 18.6±10 | .55 |
| STS score | 6.34±1.8 | 6.31±1.9 | 6.69±2.1 | .73 |
| Rejected for surgery | 52 (68%) | 37 (74%) | 15 (58%) | .15 |
| Valve assessment (TEE) | ||||
| Mean transaortic gradient | 48.6±14.9 | 47.4±14.4 | 51.0±15.9 | .33 |
| Peak transaortic gradient | 79.8±22.8 | 78.8±23.7 | 81.8±21.45 | .60 |
| Valvular area (cm2) | 0.5±0.2 | 0.54±0.16 | 0.57±0.29 | .46 |
| Aortic regurgitation | 45 (59%) | 26 (52%) | 19 (73%) | .08 |
| Annulus diameter (mm) | 21.3±2.4 | 21.6±2.5 | 20.6±2.2 | .09 |
| Ileofemoral axis assessment | ||||
| Minimum luminal diameter (mm), left | 7.4±0.8 | 7.6±0.7 | 6.5±0.6 | .009 |
| Minimum luminal diameter (mm), right | 7.5±0.8 | 7.8±0.6 | 6.5±1.3 | .15 |
| Tortuosity>mild | 47(62%) | 29 (58%) | 18 (69%) | .34 |
| Calcification>mild | 28 (37%) | 16 (32%) | 12 (46%) | .23 |
Abbreviations: ES, Edwards-SAPIEN prosthesis; MCV, Medtronic CoreValve prosthesis; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; TEE, pre-procedural transesophageal echocardiography.
Data express n (%) or mean (standard deviation).
Procedural information is shown in Table 3. It was not possible to implant the ES prosthesis in seven patients: one due to death (tamponade as a consequence of the right ventricle being perforated by the pacemaker); five because the introducer could not be advanced; and one because the valve could not be passed over the valve. Of the 43 patients with an implanted ES, 1 required a second prosthesis because the first device was positioned too high and in another patient the valve was explanted by aortic dissection.
Table 3. Procedural Data
| All (n=76) | ES (n=50) | MCV (n=26) | P | |
| General description of the procedure | ||||
| General anesthesia | 29 (38%) | 22 (44%) | 7 (27%) | .16 |
| Surgical access | 44 (58%) | 44 (88%) | 0 | .0001 |
| Duration (min) | 140±47 | 131±46 | 156±44 | .03 |
| Duration of fluoroscopy (min) | 25±15 | 24±17 | 28±8 | .27 |
| Contrast (mL) | 188±94 | 165±88 | 232±89 | .002 |
| Implantation and valve function (TEE) | ||||
| Valve size | 23: 32 (64%)26: 18 (36%) | 26:7 (27%)29:19 (73%) | ||
| Implantation | 68 (90%) | 42 (84%) | 26 (100%) | .045 |
| Post-procedural gradient (mmHg) | 9.6±5 | 8.8±4 | 10.9±6 | .21 |
| Post-procedural area (cm2) | 1.7±0.4 | 1.6±0.3 | 1.8.0±0.5 | .1 |
| Severe post-procedural aortic regurgitation | 1 (1.3%) | 1 (2%) | 0 | 1 |
| Periprocedural complications (non-exclusive) | ||||
| Tamponade | 3 (3.9%) | 3 (6%) | 0 | .55 |
| Aortic dissection | 2 (2.6%) | 2 (4%) | 0 | .54 |
| Malposition | 1 (1.3%) | 1 (2%) | 0 | 1 |
| Severe arrhythmia | 1 (1.3%) | 1 (2%) | 0 | 1 |
| Stroke | 2 (2.6%) | 2 (4%) | 0 | .54 |
| Vascular complication | 19 (25%) | 13 (26%) | 6 (23%) | .78 |
| Heart surgery | 3 (3.9%) | 3 (6%) | 0 | .55 |
| Vascular surgery | 12 (16%) | 10 (20%) | 2 (8%) | .20 |
| Need for pacemaker | 15 (20%) | 5 (10%) | 10 (38%) | .003 |
| Ventilation >48h | 4 (5%) | 4 (8%) | 0 | .29 |
| Dialysis | 3 (3.9%) | 3 (6%) | 0 | .55 |
| In-hospital mortality | ||||
| Deaths | 12 (16%) | 7 (14%) | 5 (19%) | .74 |
| Cardiac cause | 3 (4%) | 2 (4%) | 1 (4%) | 1 |
| Vascular cause | 4 (5%) | 2 (4%) | 2 (8%) | .63 |
| Pulmonary cause | 2 (3%) | 1 (2%) | 1 (4%) | 1 |
| Sepsis | 1 (1%) | 0 | 1 (4%) | 1 |
| Multiorgan failure | 3 (4%) | 2 (4%) | 0 | .55 |
Abbreviations: ES, Edwards-SAPIEN prosthesis; MCV, Medtronic CoreValve prosthesis; TEE, transesophageal echocardiography.
Data express n (%) or mean (standard deviation).
All the MCV prostheses were successfully implanted. In 1 patient who had a thoracoabdominal aortic stent, the valve remained too high after the distal part was released and so the decision was taken to remove it. Due to the risk of damaging the endoprosthesis, it was decided to release the valve in a safe position and a second valve was implanted in the correct position without adverse events.
Complications TamponadeIn the ES group there were three cases of tamponade; two due to perforation by the pacemaker and one due to aortic dissection. All three patients required pericardial puncture and drainage but only one died because of this.
Aortic DissectionIn addition to the case described, one patient presented a localized dissection 8cm from the valve plane that evolved well with conservative management.
MalpositionOne patient in the ES group required a second valve due to the first valve being positioned too high.
ArrhythmiasAfter ES implantation, one patient developed ventricular fibrillation that was initially refractory, but after some minutes of cardiac massage the patient underwent successful cardioversion.
StrokeTwo patients in the ES group presented neurological abnormalities the first 24h, one of whom had mild sequelae.
Atrioventricular Conduction DisordersIn the ES group, five patients (10%) presented complete AV block during the procedure. There was no case of late AV block.
In the MCV group, 10 patients (38%) needed a pacemaker. The development of AV block was frequently late, progressive and preceded by the development or progression of bundle branch block. The latest AV block we observed occurred 48h after the procedure.
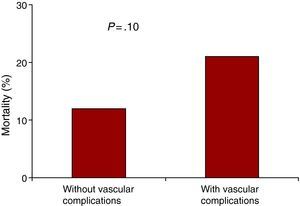
Vascular DamageIn the ES group there were 12 vascular bleeding complications (10 due to femoral or iliac rupture or dissection, 2 due to bleeding at the puncture site), of which 9 required vascular surgery and 3 endovascular treatment. Another patient needed iliofemoral bypass due to ischemia. There were three vascular complications in the MCV group: two due to bleeding after closure with the Prostar™ device, and one due to dissection that affected flow, requiring angioplasty. The vascular complications increased mortality from 12% to 21% although the difference did not reach statistical significance due to the small size of the sample (Figure 1).
Figure 1. 30-Day mortality among the patients with and without vascular complications.
In-Hospital MortalityA total of seven patients died in the ES group: two during the procedure (one tamponade, one post-valvuloplasty cardiogenic shock); one during the first 24h (vascular complications); and four later (three due to multiorgan failure at 7 days, 10 days, and 28 days, and one due to respiratory failure at 65 days). There were three early deaths in the MCV group (two due to vascular complications and one due to post-implantation cardiogenic shock in one patient with very severe left ventricular hypertrophy) and two late deaths: one was due to pneumonia at 6 days (patient with previous lung disease) and one due to urological sepsis at 8 days. No significant differences in mortality were observed between the two series.
Learning CurveTable 4 compares the first and second halves of the ES Group. There was a tendency toward better outcomes in the second half of the group, although only the increase in the implantation rate reached statistical significance.
Table 4. Learning Curve in the Edwards-SAPIEN Series
| Patients 1–25 | Patients 26–50 | P | |
| Baseline characteristics | |||
| Age (years) | 82±6 | 82±6 | .70 |
| Women | 19 (76%) | 15 (60%) | .22 |
| Functional class III/IV | 21 (84%) | 19 (76%) | .48 |
| Peak gradient (mmHg) | 78±23 | 79±30 | .86 |
| Mean gradient (mmHg) | 48±16 | 46±15 | .71 |
| Valvular area (cm2) | 0.58±0.1 | 0.49±0.2 | .06 |
| Coronary disease | 15 (60%) | 10 (40%) | .16 |
| EuroSCORE | 19±7 | 16±8 | .13 |
| Iliofemoral axis diameter | 7.5±0.7 | 7.7±0.6 | .76 |
| Tortuosity ≥moderate | 4 (20%) | 4 (20%) | .33 |
| Calcification ≥moderate | 7 (35%) | 6 (39%) | .14 |
| Procedure | |||
| Implantation | 18 (72%) | 24 (96%) | .049 |
| Post-procedural gradient TEE (mmHg) | 8.9±3 | 8.8±5 | .97 |
| Post-procedural aortic regurgitation ≥moderate (TEE) | 3 (15%) | 2 (10%) | 1 |
| Duration of fluoroscopy (min) | 24±10 | 24±21 | .96 |
| Contrast (mL) | 198±105 | 131±51 | .007 |
| Complications (non-exclusive) | |||
| Tamponade | 3 (12%) | 0 | .24 |
| Aortic dissection | 1 (4%) | 1 (4%) | 1 |
| Heart surgery | 3 (12%) | 0 | .24 |
| Vascular surgery | 7 (28%) | 3 (12%) | .16 |
| Complete atrioventricular block | 2 (8%) | 3 (12%) | 1 |
| Dialysis | 2 (10%) | 1 (4%) | .34 |
| In-hospital mortality | 5 (20%) | 2 (8%) | .67 |
TEE, transesophageal echocardiography.
Data express n (%) or mean (standard deviation).
Both prostheses left small gradients, although the MCV prosthesis left a slightly larger valve area. Mild periprosthetic aortic regurgitation was frequent with both devices.
In the ES group, there were documented alterations in valve function in five patients: two patients with a correctly positioned, well-expanded valve had moderate periprosthetic regurgitation caused by poor valve malposition due to calcium nodules; in two patients the valve was insufficiently expanded, leading to poor valve coaptation with central regurgitation in both patients, one of whom had a 20-mmHg gradient. Finally, in one patient with an apparently well-expanded and correctly positioned ES-23, a 39-mmHg peak gradient with a mean of 22mmHg was documented at the level of the valve. Of the 26 patients with an MCV prosthesis, 2 presented moderate periprosthetic aortic regurgitation.
Medium-Term ResultsThe majority of the patients improved their functional class to class I or II (Table 5). No changes were observed in area or aortic regurgitation except for reduced regurgitation in two patients with ES. Neither were changes observed in the two patients with significant gradients, both of whom remained in a good functional status. The clinical situation of the patient with localized aortic dissection remained satisfactory.
Table 5. Medium-term Course of the Patients Discharged With a Prosthesis
| All (n=58) | ES (n=37) | MCV (n=21) | P | |
| 1-month follow-up | ||||
| Functional class | 1.38±0.6 | 1.45±0.9 | 1.31±0.5 | .20 |
| Functional class III/IV | 2 (4%) | 2 (5%) | 0 | 1 |
| Gradient (mmHg) * | 7.9±3 | 7.1±3 | 10.5±5 | .08 |
| Valve area (cm2) * | 1.7±0.4 | 1.6±0.3 | 2.0±0.5 | .04 |
| Regurgitation ≥moderate * | 1 (2%) | 1 (3%) | 0 | 1 |
| 30-day post-discharge mortality | 0 | 0 | 0 | – |
| Final follow-up | ||||
| Mean follow-up (days) | 281±244 | 343±265 | 172±159 | .003 |
| Functional class | 1.34±0.6 | 1.41±0.8 | 1.17±0.4 | .16 |
| Functional class III/IV | 3 (7%) | 3 (11%) | 0 | .29 |
| Valve area (cm2) * | 1.7±0.4 | 1.7±0.3 | 1.9±0.4 | .01 |
| Regurgitation ≥moderate * | 2 (3.4%) | 2 (5.4%) | 0 | .53 |
| Death (since day 30) | 3 (5%) | 3 (8%) | 0 | .55 |
| Cardiac cause | 1 (2%) | 1 (3%) | 0 | 1 |
| Non-cardiac cause | 2 (3%) | 2 (5%) | 0 | .53 |
Abbreviations: ES, Edwards-SAPIEN prosthesis; MCV, Medtronic CoreValve prosthesis.
Data express n (%) or mean ± standard deviation.
* Gradient, area, and regurgitation were assessed via transthoracic echocardiography.
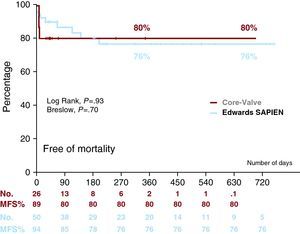
After discharge, three patients in the ES group died: two due to pneumonia at 3 months and 6 months, and one due to aortic dissection (not previously documented by transesophageal echocardiography or post-implantation aortogram) at 4 months. We have no further details of the aortic dissection, as the necropsy was performed in another hospital. Figure 2 shows survival curves with both prostheses.
Figure 2. Kaplan–Meier survival curve for each device. MFS indicates mortality-free survival.
DiscussionThe present series describes our initial experience of the TF implantation of aortic prostheses in which only patients at HSR were included. The baseline characteristics and the results obtained are within ranges similar to other studies5,8,10,11,12,13,14,15,16,17,18,19 although there are wide variations between them.
The novelty of the study lies in the use of two commercial devices which, despite the limited number of patients, allowed us to compare their differences in the same environment and hospital and using the same operators. To date, only two mixed series have been reported. One compiled the joint experience of two hospitals in Toulouse19 that included 21 MCV and 24 ES; and one conducted in Munich18 that included 105 MCV and 4 ES by TF implantation.
The motivation for using two devices in a single center was based on the fact that there is no evidence, at present, to consider one prosthesis as superior to the other regarding periprocedural mortality, functioning or durability, and most of the learning curve is common to both devices.
Increased Number of Patients Treatable by the Transfemoral ApproachThe availability of both devices means that more patients can be treated via the TF approach, not because of differences in the anatomy of the aortic annulus, but because the MCV device can be used in smaller diameter femoral arteries. In the Rouen series,20 only 71% of the patients who needed an ES-23 and 39% of those who needed an E-26 had a suitable femoral diameter for TF implantation. Similarly, in the Canadian ES registry,10 only 50% of the patients could be treated via the TF approach. In the Munich mixed series,18 80% of the patients were treated via the TF approach and in the Toulouse series, 71%.19 In our series, if only the ES valve had been available we would have been able to treat no more than half of the patients who were treated with the MCV valve via the TF approach.
Specific Aspects of the Procedure With Both DevicesThe problem with the ES valve is its size (22–24F) which can lead to vascular problems and implantation failures. In our case, implantation failure decreased from 28% to 4% in the last part of the series when stricter selection criteria were applied. The ES-XT device (18F) markedly reduces difficulties in advancing the device and, potentially, vascular complications.
The other difficulty presented by the ES prosthesis is its correct positioning. This requires good radiological landmarks and correct performance of the pacing–inflation–deflation sequence; transesophageal echocardiography could be of potential use in this regard.
The MCV prosthesis poses fewer access problems and its gradual release enables correct positioning at the beginning of release. In cases of malposition, the implantation of a second valve in the correct position is a possible option with both devices.
ComplicationsAs in other series, vascular complications were frequent with both devices. These involved dissection and vascular rupture (ES), and bleeding in the puncture area due to failed percutaneous closure (MCV and Es XT). It should be pointed out that in the only study we found that provides information on the size of the femoral arteries — the Toulouse series19 — the diameter of the artery in their ES group was 8.9mm vs. 7.8mm in our ES group, and 7.8mm in their MCV group vs. 6.5mm in ours. The strictest patient selection criteria,21,22,23,24 experience in managing the devices, early diagnosis of complications, and their management by percutaneous techniques all contribute to reducing the number of complications and their clinical impact.21,22,23,24
Cardiac complications (perforations, dissections) were severe but infrequent and the majority were not directly related to the implantation procedure.
AV conduction disorders were more frequent with the MCV prosthesis.8,9,13,17 In some cases, the disorder occurred during the procedure, whereas in others this occurred later, with more than one-third of the patients needing a pacemaker. Larger size, low implantation, ventricular hypertrophy, and previous conduction disorders are factors that have led to conduction disorders in other series.25 If the time required for the temporary pacemaker must be extended, patient stay in special units is longer, probe and catheter withdrawal is delayed, and there is an increased likelihood of infection, which was the direct cause of two of the five deaths in patients who had been implanted with an MCV without complications.
With the ES valve, AV block is less frequent11,12,13,26 and there is no tendency toward progression, thus enabling the early withdrawal of the temporary pacemaker lead.
In-Hospital MortalityMortality in our series was somewhat higher than in recently published series,26 but with no difference between the two devices. Of the 12 deaths (1 due to perforation by the pacemaker lead, 5 due to vascular complications, 1 due to respiratory failure, 2 due to infections, 2 due to cardiogenic shock), only 1 was directly related to the valve. Analysis of the causes of mortality suggests that instruments should be handled with extreme caution, intubation avoided (at least in patients with pulmonary disease), probe and catheter time reduced to prevent infections, and vascular complications prevented or treated as early as possible, as a complication is often followed by multiorgan failure leading to the death of the patient.
The operator's degree of experience is an important factor in the success rate, complications, and procedural mortality,12 but so is the level of coordination among the entire multidisciplinary team involved in the assessment, intervention and post-procedural management of these patients.
The procedure still involves appreciable risk, whichever of these devices is used. A consensus document produced by expert representatives of the European Association of Cardio-Thoracic Surgery, the European Association of Percutaneous Cardiovascular Interventions and the European Society of Cardiology27 recommended strict adherence to the initial guidelines for severe symptomatic aortic stenosis and HSR.
ConclusionsThe TF implantation of an aortic valve prosthesis is a procedure that has already been incorporated into clinical practice for patients with severe symptomatic aortic stenosis and at HSR. The use of the two commercial devices can increase the percentage of patients treated using the TF approach. Although there are technical features common to each device, such as their preparation and implantation, they have many other points in common, such as patient selection, instrumentation during the procedure, and the prevention, diagnosis and treatment of complications. Vascular complications are frequent with both devices. In the case of the ES these are due to dissection or femoral rupture caused by the high profile of the device, and in the case of the MCV these are caused by failed percutaneous closure. AV block is more frequent with the MCV prosthesis and may occur later, in contrast to early AV block caused by the ES device. Although the procedural morbidity and mortality rate with each device is acceptable in these types of patients, the risk of severe complications and the lack of data on the durability of these commercial devices suggest that their use should be restricted to high-risk patients. The results of randomized studies currently underway will clarify the efficacy and safety of the various aortic valve replacement techniques (surgical, TA or TF) in the treatment of these patients.
Conflicts of interestDr. E. García is proctor of Edwards Lifesciences.
Acknowledgements
We would like to thank Dr Pepa Pérez-Vizcayno for her invaluable help in processing patient data and the nurses of the Cardiac Catheterization Unit of the San Carlos Hospital for their involvement and enthusiasm in the development of this new technique.
Received 28 January 2010
Accepted 20 July 2010
Corresponding author. Unidad de Hemodinámica y Cardiología Intervencionista, Hospital Clínico San Carlos, C/ Martín Lagos, s/n, Madrid 28040, Spain. rhernandez_antolin@hotmail.com