Optimal medical therapy decreases mortality and heart failure (HF) hospitalizations in HF patients with reduced left ventricular ejection fraction. Women have been underrepresented in clinical trials and not specifically evaluated. This study aimed to compare the safety and effectiveness of drug titration in women vs men.
MethodsThis post hoc gender study of the ETIFIC multicenter randomized trial included hospitalized patients with new-onset HF with reduced ejection fraction and New York Heart Association II-III and no contraindications to beta-blockers. A structured 4-month titration process was implemented in HF clinics. The primary endpoint was the mean relative dose (% of target dose) of beta-blockers achieved by women vs men. Secondary endpoints included the mean relative doses of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and mineralocorticoid receptor antagonists, adverse events, and other clinical outcomes at 6 months.
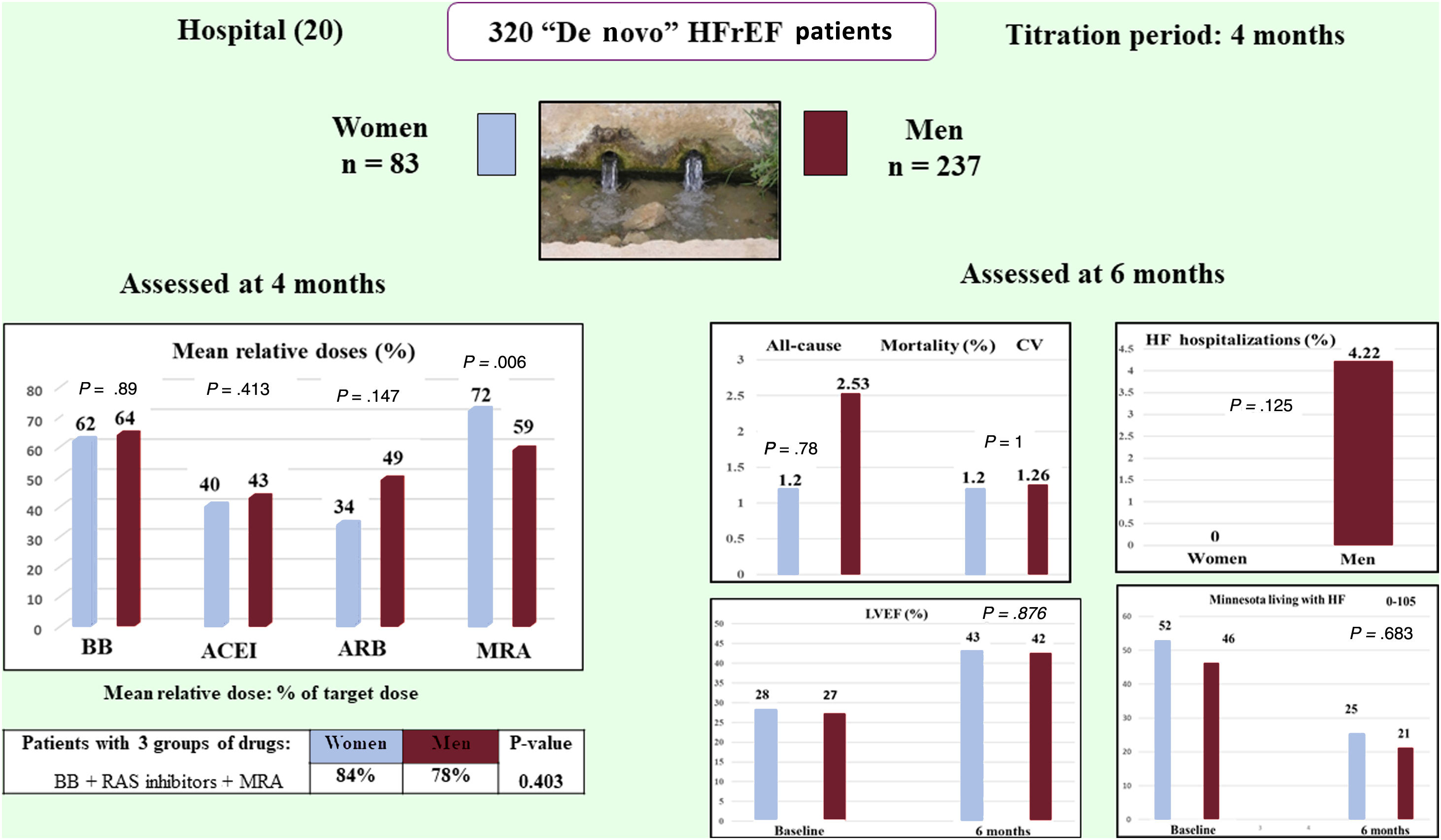
ResultsA total of 320 patients were included, 83 (25.93%) women and 237 (74.06%) men (76 vs 213 analyzed). The mean±standard deviation of the relative doses achieved by women vs men were as follows: beta-blockers 62.08%±30.72% vs 64.4%±32.77%, with a difference of−2.32% (95%CI,−10.58-5.94), P = .580; and mineralocorticoid receptor antagonists 79.85%±27.72% vs 67.29%±31.43%, P =.003. No other differences in drug dosage were found. Multivariate analysis showed nonsignificant differences. CV mortality was 1 (1.20%) vs 3 (1.26%), P=1, and HF hospitalizations 0 (0.00%) vs 10 (4.22%), P=.125.
ConclusionsIn a post hoc analysis from the HF-titration ETIFIC trial, we found nonsignificant gender differences in drug dosage, cardiovascular mortality, and HF hospitalizations.
Trial registry number: NCT02546856.
Keywords
Heart failure (HF) has a high prevalence, mortality, hospital admissions, and social and health system impacts. To improve prognosis and reduce mortality and HF hospitalizations, clinical practice guidelines recommend administration of beta-blockers (BB), renin angiotensin system inhibitors, mineralocorticoid receptor antagonists (MRA) and education and follow-up programs with multidisciplinary teams of specialized nurses and cardiologists in HF patients with reduced ejection fraction (HFrEF). Careful drug titration is recommended.1–3 However, dose optimization is deficient in clinical practice. Women have been underrepresented in most original HFrEF trials. Their clinical characteristics, prescription, achieved dose and adverse events associated with titration have not been specifically analyzed. Few trials have evaluated the effects on mortality and hospitalization based on sex4–10 (see ).
There is limited evidence on the differences between women and men from meta-analyses, systematic reviews, and observational studies.4,11–23 It is recommended that sex and gender analysis be carried out in studies to deepen knowledge of possible differences, avoid harm due to inappropriate generalization of results, and increase the applicability of treatments in women.4,12,16 To our knowledge, no results of the HF drug titration process in women vs men have been published within the framework of a clinical trial with a structured titration protocol and follow-up. The limited available evidence raises the need to deepen study of this topic.
ETIFIC was a multicenter randomized trial, which demonstrated noninferiority in the safety and effectiveness of drug titration by HF-nurses vs HF-cardiologists in patients with de novo HFrEF.24,25 This post hoc analysis aimed to compare gender differences in drug titration, selection process, characteristics, prescription, achieved dose, adverse events and clinical results in women vs men.
METHODSStudy design and participantsETIFIC was a randomized controlled open label trial carried out in 20 Spanish hospitals with HF units (2015-2018) to compare the safety and effectiveness of HF drug titration by HF-nurses vs HF-cardiologists. Its design and results have been previously published.24,25
Patients with de novo HFrEF and New York Heart Association (NYHA) II-III were included after hospitalization in a cardiology ward. Exclusion criteria were planned surgery, contraindication to BB or already receiving target or maximum tolerated dose, home or terminal care, or inability for self-care.
An active supervision system for recruitment, with centralized randomization, 4-month titration period, and a 6-month follow-up period after inclusion were established. A safety and clinical adjudication committee, blinded to the group assignment, monitored the safety of the research activity and evaluated all adverse events. Written informed consent forms were signed. The study was approved by the Clinical Research Ethics Committee of the Basque Country and complied with the Declaration of Helsinki.
ETIFIC confirmed the noninferiority safety and effectiveness of drug titration by HF-nurses vs drug titration by HF-cardiologists.
Study protocolThe previously published study protocol24 was based on clinical practice guidelines.1,2 The titrating professional was the HF-nurse vs the HF-cardiologist. In both cases, drug prescription was the responsibility of the cardiologist. HF-nurse tasks also included clinical assessment, education on self-care, psychosocial support, and care coordination. All HF-nurses and half of the HF-cardiologists were women.
The main objective of this post hoc substudy was to compare the safety and effectiveness of drug titration in women vs men from the ETIFIC study and to assess the possible factors associated with any differences.
Primary endpointTo compare the achieved BB mean relative dose (% relative to target dose) after 4 months of titration in women vs men. The % of target dose was defined according to ESC HF guidelines.24
Secondary endpointsTo compare the following between women and men: a) patient selection process and baseline characteristics; b) mean relative doses of angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), and MRA at 4 months; c) percentage of patients with 100% and ≥ 50% of the target dose; d) mean relative doses and number of visits according to type and gender of the professional; e) percentage of adverse events associated with titration; f) variables influencing target dose achievement; g) rates of cardiovascular mortality and readmissions at 6 months; and h) changes in left ventricular ejection fraction (LVEF), NYHA class, 6-minute walk distance, N-terminal pro b-type natriuretic peptide (NT-proBNP) levels and quality of life scores throughout the study. Variables are shown in the design article.2 Definitions of sex and gender are provided in the .
Statistical analysisThe analysis was performed on an intention-to-treat basis. Both the Student t-test (or nonparametric Wilcoxon test if continuous data were not normally distributed) and the chi-square test (or Fisher exact test) were used to compare the baseline sociodemographic and clinical characteristics of patients in the 2 groups (women vs men). The effect attributable to the intervention was estimated by comparing the differences in the relative dose of BB, ACEI, ARB and MRA achieved between the groups, assessed at 4 months after the start of titration, and the 95% confidence interval was calculated. We performed a multivariate analysis for the primary and secondary endpoints as predefined in the original study design.24 The model was adjusted by the variables established as relevant related factors with a possible effect on dosing, based on a review of the literature, shown in . All analyses were performed considering the 2 target populations (women and men). All variables with a P value<.20 were included as explanatory variables in the multivariate model, with the relative dose as the response variable. The multivariate analysis was conducted using ANCOVA within the framework of a linear mixed regression analysis. To take into account the difference between baseline and end of the titration period at 4 months, mixed linear regression models with fixed effects and random effects (specific effect of each participant and center and the effect of time expressed as visit 1 (baseline) and visit 2 (at 4 months) were used. The models were adjusted for women, men, and the total number of patients. These models took into account the longitudinal structure of the 2 repeated measurements, as well as the hierarchical structure of the data. All the statistical analyses were performed using R (version 4.0.4): R Foundation (Statistical Computing, Vienna, Austria). Statistical significance was set at P<.05.
RESULTSPatient populationA total of 824 patients with de novo HFrEF, 221 women and 603 men, were evaluated, and 320 patients were included, 83 women and 237 men. Finally, 289 patients (76 women and 213 men) were analyzed at 4 months, and 274 (74 women, 200 men) were analyzed at 6 months. The selection process and causes for exclusion are shown in figure 1, and in .
Patient characteristics were generally well-balanced between the 2 groups, without significant differences in LVEF, ischemic heart disease, or NT-proBNP (table 1). However, women were older (4 years), had a higher proportion of systolic blood pressure (SBP) ≤ 100mmHg, lower hemoglobin level, lower 6-minute walk distance, and worse quality of life. In contrast, men had a higher proportion of smokers, alcohol abuse, atrial fibrillation/flutter, and worse scores on the Lawton Instrumental Activities of Daily Living Scale and age-adjusted Charlson index.
Baseline patient characteristics
| Variables (at hospital discharge) | Women n=83 | Men n=237 | P |
|---|---|---|---|
| Demographics | 83 (25.93) | 237 (74.06) | <.001 |
| Age, y | 64.83±12.27 | 60.04±11.95 | .002 |
| Education level, ≤ 10 y | 31 (37.25) | 77 (32.63) | .434 |
| Patients ≥ 70 y | 30 (36.14) | 53 (22.36) | .0137 |
| Memory impairment screening ≤ 4 | 4 (14.29) | 8 (17.02) | .755 |
| Lawton test: inability to administer medication | 10 (38.46) | 23 (46.94) | .482 |
| Cardiovascular risk factors | |||
| Hypertension | 41 (49.4) | 125 (52.74) | .600 |
| Smoker | 14 (16.87) | 83 (35.02) | .002 |
| Alcohol consumption>2 units/d | 7 (8.43) | 87 (36.71) | .001 |
| Diabetes | 19 (22.89) | 76 (32.07) | .115 |
| Heart disease | |||
| Ischemic heart disease | 18 (21.69) | 70 (29.54) | .168 |
| Atrial fibrillation/flutter | 14 (18.42) | 78 (34.98) | .007 |
| NYHA | |||
| II | 64 (77.11) | 203 (85.65) | .071 |
| III | 19 (22.89) | 34 (14.35) | .071 |
| Left ventricular ejection fraction, % | 28.02±7.05 | 27.59±6.9 | .6232 |
| Comorbidities | |||
| Peripheral arterial disease | 2 (2.41) | 20 (8.44) | .062 |
| Stroke | 6 (3.66) | 10 (6.41) | .259 |
| Chronic respiratory disease | 9 (10.84) | 32 (13.5) | .533 |
| Charlson index, adjusted by age | 5.11±1.65 | 4.69±2.03 | .048 |
| Vital signs | |||
| SBP mmHg | 112.95±18.65 | 116.39±18.48 | .147 |
| SPB ≤ 100 mmHg | 24 (28.92) | 44 (18.64) | .049 |
| Heart rate beats/min | 72.41±14.4 | 72.75±13.84 | .851 |
| Laboratory tests | |||
| NT-proBNP pg/mL | 75; 1901 [1042; 4642] | 207; 1590 [860; 3196] | .231 |
| BNP pg/mL | 5; 358 [126;404] | 24; 352.5 [193.8; 835.2] | .544 |
| Creatinine mg/dL | 0.91±0.39 | 1.14±0.52 | .001 |
| eGFR mL/min/1.73 m2 | 72.21±22.75 | 75.55±21.71 | .234 |
| eGFR<60 mL/min/1.73 m2 | 16 (19.28) | 49 (20.68) | .735 |
| Potassium>5 mEq/L | 7 (8.43) | 29 (12.24) | .345 |
| Hemoglobin g/dL | 13.52±1.99 | 14.3±2.03 | .0025 |
| Anemia | 22 (26.51) | 60 (25.32) | .831 |
| 6-minute walk test, meters | 318.29±96.82 | 383.28±102.85 | .001 |
| Meters ≤ 300 | 38 (46.34) | 37 (16.23) | .001 |
| European HF Self-care Behavior Scale (12-60) | 37.6±11.98 | 35.78±11.68 | .229 |
| Question 10 Irregular medication intake (score 3-5) | 18 (21.95) | 26 (11.06) | .014 |
| Quality of life | |||
| Minnesota Living with HF Questionnaire (0-105) | 52.76±21.14 | 46.76±22.83 | .038 |
| Physical dimension (0-40) | 25.68±10.32 | 20.77±11.11 | .001 |
| Emotional dimension (0-25) | 11.49±7.35 | 9.49±6.77 | .025 |
| EQ-5 D index | 0.66±0.24 | 0.76±0.23 | .001 |
| Daily living tasks, score 2-3 | 43 (51.80) | 75 (31.64) | .040 |
| Anxiety and depression score 2-3 | 52 (62.65) | 109 (45.99) | .002 |
| VAS EQ-5D (0-100) | 53.89±17.73 | 58.94±20.21 | .047 |
| Drugs | |||
| BB | 78 (93.98) | 232 (97.89) | .078 |
| ACEI | 70 (84.34) | 196 (82.7) | .732 |
| ARB | 9 (10.84) | 23 (9.7) | .766 |
| MRA | 63 (75.9) | 186 (78.48) | .627 |
| Psychotropic drugs | 32 (38.55) | 43 (18.14) | .001 |
| Antidepressants | 17 (20.48) | 21 (8.86) | .005 |
| Anxiolytics | 19 (22.89) | 24 (10.13) | .003 |
| Hypnotics | 6 (7.23) | 4 (1.69) | .013 |
| Neuroleptics | 3 (3.61) | 2 (0.84) | .080 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta-blockers; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; EQ-5D, EuroQol-5 Dimension; HF, heart failure; MRA, mineralocorticoid receptor blocker; NT-proBNP, N-terminal probrain natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; VAS, Visual analogue scale.
The data are expressed as No. (%), mean±standard deviation, or No.; median [interquartile range].
Baseline prescription of HF guideline-recommended drugs did not differ significantly. Women more frequently took psychotropic drugs ( shows other baseline characteristics).
Primary endpointBB dosageThere were no significant differences between women and men in the mean relative doses of BB at 4 months or in the percentage of patients with 100% and ≥ 50% of target dose (table 2). Equally, there were also no differences between women and men in each group of titrating professional (table 3), or in the number of visits for women vs men (table 4).
Dosage: baseline to 4 months (titration period)
| Womenn=76 | Menn=213 | Diff. (95%CI) | P* | |
|---|---|---|---|---|
| BB | ||||
| At baseline | 76 | 213 | ||
| Relative dose % | 34.54±17.95 | 34.90±19.89 | −0.36 (−5.46 to 4.75) | .890 |
| At 4 mo | 76 | 213 | ||
| Relative dose % | 62.08 (30.72) | 64.4 (32.77) | −2.32 (−10.58 to 5.94) | .580 |
| Patients with 100% target dose | 24 (31.57) | 83 (38.96) | −7.38 (−19.7 to 4.94) | .252 |
| Patients with ≥ 50% target dose | 54 (71.05) | 149 (69.95) | 1.09 (−10.8 to 13.01) | .857 |
| ACEI | ||||
| At baseline | 63 | 176 | ||
| Relative dose % | 40.58±27.61 | 43.93±27.91 | −3.35 (−11.40 to 4.70) | .413 |
| At 4 mo | 57 | 173 | ||
| Relative dose % | 57.67±48.81 | 66.21±62.05 | −8.54 (−18.42 to 1.35) | .089 |
| Patients with 100% target dose | 19 (33.33) | 64 (36.99) | −3.66 (−17.85 to 10.53) | .617 |
| Patients with ≥ 50% target dose | 37 (64.91) | 138 (79.76) | −14.85 (−28.61 to−1.09) | .023 |
| ARB | ||||
| At baseline | 8 | 16 | ||
| Relative dose % | 30.70±19.55 | 35.39±17.55 | −4.69 (−13.03 to 22.40) | .577 |
| At 4 mo | 13 | 23 | ||
| Relative dose % | 34.11±24.46 | 49.62±35.62 | −15.51 (−36.80 to 5.78) | .147 |
| Patients with 100% target dose | 1 (7.69) | 6 (26.08) | −18.39 (−41.45 to 4.66) | .180 |
| Patients with ≥ 50% target dose | 3 (23.07) | 12 (52.17) | −29.09 (−59.77 to 1.58) | .089 |
| MRA | ||||
| At baseline | 67 | 185 | ||
| Relative dose % | 72.01±36.55 | 59.10±31.50 | 12.91 (3.66 to 22.16) | .006 |
| At 4 mo | 67 | 185 | ||
| Eplerenone | 30/67 (44.77) | 131/185 (70.81) | −26.03 (−40.64 to -11.43) | <.001 |
| Relative dose % | 79.85±27.72 | 67.29±31.43 | 12.55 (4.46 to 20.65) | .003 |
| Patients with 100% target dose | 42 (62.68) | 81(43.78) | 18.90 (4.28 to 33.53) | .012 |
| Patients with ≥ 50% target dose | 65 (97.01) | 161 (87.02) | 9.9 (2.64 to 17.33) | .039 |
95%CI, 95% confidence interval; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta-blocker; Diff., difference; MRA, mineralocorticoid receptor blocker.
The data are expressed as No. (%) or mean±standard deviation.
* P value of the interaction between treatment and each subgroup.
Mean relative dose by intervention group. Baseline to 4 months (titration period)
| DrugNo.Titrating professionalRelative dose (%) at 4 moMean±SD | Womenn=76 | Menn=213 | Diff. (95%CI) | P* |
|---|---|---|---|---|
| BB | ||||
| No. | 76 | 213 | ||
| HF-nurse group (all women) | 40 | 104 | ||
| Relative dose, % | 68.44±30.7 | 72.48±31.7 | −4.03 (−15.54 to 7.46) | .486 |
| HF-cardiologist group | 36 | 109 | ||
| Relative dose, % | 55.03±29.5 | 56.71±32 | −1.67 (−13.25 to 9.91) | .774 |
| Female cardiologist | 18 | 47 | ||
| Relative dose, % | 65.28±33.09 | 62.37±33.34 | 2.91 (−15.83 to 21.65) | .754 |
| Male cardiologist | 18 | 62 | ||
| Relative dose, % | 44.79±17.53 | 52.42±30.52 | −7.63 (−20.69 to 5.43) | .244 |
| ACEI | ||||
| No. | 57 | 173 | ||
| HF-nurse group | 30 | 85 | ||
| Relative dose, % | 68.75±32.3 | 73.2±28.7 | −4.45 (−17.90 to 8.93) | .504 |
| HF-cardiologist group | 27 | 88 | ||
| Relative dose, % | 45.37±30.6 | 59.43±29.7 | −14.06 (−27.57 to−0.55) | .042 |
| Female cardiologist | 14 | 39 | ||
| Relative dose, % | 48.21±32.84 | 61.35±29.20 | −13.14 (−33.82 to 7.56) | .201 |
| Male cardiologist | 13 | 49 | ||
| Relative dose, % | 42.31±30.17 | 57.91±30.27 | −15.6 (34.74 to 3.54) | .104 |
| ARB | ||||
| No. | 13 | 23 | ||
| HF-nurse group | 7 | 12 | ||
| Relative dose, % | 36.85±30.8 | 48.93±35.5 | −12.08 (−45.26 to 21.09) | .448 |
| HF-cardiologist group | 6 | 11 | ||
| Relative dose, % | 30.92±22.8 | 50.38±37.5 | −19.46 (−50.78 to 11.85) | .205 |
| Female cardiologist | 2 | 2 | ||
| Relative dose, % | 22.75±14.50 | 43.75±44.19 | −21 (−299.93 to 257.92) | .622 |
| Male cardiologist | 4 | 9 | ||
| Relative dose, % | 35±27.1 | 51.85±38.76 | −16.85 (−59.69 to 25.99) | .393 |
| MRA | ||||
| No. | 67 | 185 | ||
| HF-nurse group | 34 | 91 | ||
| Relative dose, % | 83.82±26.7 | 66.21±32.8 | 17.61 (6.19 to 29.04) | .003 |
| HF-cardiologist group | 33 | 94 | ||
| Relative dose, % | 75.76±28.3 | 68.35±30.5 | 7.41 (−4.28 to19.1) | .210 |
| Female cardiologist | 17 | 38 | ||
| Relative dose, % | 70.59±30.92 | 63.82±39.50 | 6.77 (−12.10 to 25.65) | .471 |
| Male cardiologist | 16 | 56 | ||
| Relative dose, % | 81.25±25.00 | 71.43±34.04 | 9.82 (−5.13 to 24.77) | .189 |
95%CI, 95% confidence interval; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta-blocker; Diff., difference; HF, heart failure; MRA, mineralocorticoid receptor blocker.
Unless otherwise indicated, the results are expressed as No. or mean±standard deviation (SD).
* P value of the interaction between treatment and each subgroup.
Visits according to titrating professional
| Visits/professional(n women/n men) | Womenn=76 | Menn=213 | Diff. (95%CI) | P |
|---|---|---|---|---|
| HF-nurse and HF-cardiologist, n *75/*211 | 4.57±2.97 | 4.63±2.91 | −0.05 (−0.84 to 0.73) | .895 |
| HF-nurse (all women), n *39/*103 | 6.28±2.95 | 6.50±2.80 | −0.21 (−1.30 to 0.88) | .698 |
| HF-cardiologist, n 36/*108 | 2.72±1.56 | 2.84±1.60 | −0.12 (−0.72 to 0.48) | .692 |
| Male cardiologist, n 18/47 | 3.22±1.77 | 3.43±1.65 | −0.20 (−1.17 to 0.77) | .670 |
| Female cardiologist, n 18/*61 | 2.22±1.17 | 2.30±1.33 | −0.07 (−0.73 to 0.59) | .823 |
| Patients with ≤ 2 visits according to the titrating professional | ||||
| HF-nurse and HF cardiologist | *23/75 (30.67) | *62/211 (29.38) | 1.28 (−11.73 to 14.30) | .950 |
| HF-nurse | *3/39 (7.69) | *4/103 (3.88) | 3.81 (−7.12 to 14.73) | .616 |
| HF-cardiologist | 20/36 (55.55) | *58/108 (53.70) | 1.85 (−18.76 to 22.46) | .999 |
| Male cardiologist | 8/18 (44.44) | 18/47 (38.30) | 6.15 (−24.53 to 36.82) | .865 |
| Female cardiologist | 12/18 (66.67) | *40/61 (65.57) | 1.09 (−24.83 to 27.01) | .999 |
Diff., difference; HF, heart failure.
Unless otherwise indicated, the data are expressed as No. (%) or mean±standard deviation.
However, women achieved significantly higher BB doses when titration was performed by a female HF-cardiologist compared with a male HF-cardiologists (P=.037) and higher BB doses, when comparing HF-nurses vs HF-cardiologists, and this result was almost significant (P=.057). This was associated with a higher number of visits ().
Secondary endpointsACEI/ARB dosageThere were no significant differences between women and men in the relative ACEI and ARB doses achieved at 4 months (table 2). However, relative doses of ACEI were significantly lower in women vs men (P=.042) when titration was performed by HF-cardiologists but not by HF-nurses (table 3).
Women achieved significantly higher ACEI doses on comparison of titration by HF-nurses vs H-cardiologists, P=.007 ().
The percentage of patients receiving ≥ 50% of the target dose of ACEI was significantly higher in men, P=.0226 (table 2).
MRA dosageThe relative MRA doses, the percentage of patients with 100% and ≥ 50% of the target dose at 4 months, were significantly higher in women vs men (table 2). Women achieved higher MRA doses vs men when titration was performed by HF-nurses, P=.003 (table 3).
Variables potentially associated with higher drug doses at the end of the up-titration periodSignificant differences were found between women and women. Women showed slightly better self-care, while men showed lower NYHA class and a lower proportion of patients with body mass index ≤ 19.
Moreover, the type and gender of the titrating professional, associated with their respective number of visits, influenced the achievement of higher doses, both among men and among women.
Although women had significantly lower creatinine at baseline and 4 months, estimated glomerular filtration rates (eGFR) showed no significant differences. No differences were found in other clinical variables or prescription ().
Multivariate analysisA multivariate analysis disaggregated by sex was carried out, using mixed linear regression models with fixed effects and following the recommendations for Sex and Gender Equity in Research SAGER guidelines.12 Factors related to the relative dose of BB, ACEI, MRA achieved by women, men and the total number of patients are shown in table 5.
Multivariate linear mixed regression models
| Estimate | 95%CI | P | |
|---|---|---|---|
| Beta-blockers, all patients, n=289 | |||
| Intercept | −53.02 | (−146.97 to 40.92) | .269 |
| Female sex | 2.01 | (−10.16 to 14.19) | .746 |
| Time (baseline vs 4 mo) | 29.29 | (−29.71 to 88.28) | .331 |
| Time (baseline vs 4mo), female sex* | −1.87 | (−9.55 to 5.81) | .634 |
| BB relative dose at baseline | 0.83 | (0.73 to 0.93) | <.001 |
| Baseline heart rate, bpm | 0.29 | (0.16 to 0.41) | <.001 |
| Visits with the titrating professional | 1.23 | (0.42 to 2.04) | .003 |
| HF-nurse vs HF-cardiologist | 4.63 | (0.61 to 8.65) | .024 |
| Beta-blockers, women, n=76 | |||
| Intercept | −59.23 | (−163.61 to 45.15) | .268 |
| BB relative dose at baseline | 0.83 | (0.64 to 1.02) | <.001 |
| Baseline heart rate, bpm | 0.35 | (0.12 to 0.58) | .004 |
| Visits with the titrating professional | 2.4 | (1.15 to 3.66) | <.001 |
| Time (baseline vs 4 mo) | 27.42 | (−37.29 to 92.12) | .408 |
| Beta-blockers, men, n=213 | |||
| Intercept | −45.76 | (−151.84 to 60.32) | .398 |
| BB relative dose at baseline | 0.82 | (0.71 to 0.92) | <.001 |
| Baseline heart rate, bpm | 0.29 | (0.14 to 0.44) | <.001 |
| HF-nurse vs HF-cardiologist | 7.78 | (3.72 to 11.85) | <.001 |
| Atrial fibrillation | −5.44 | (−9.99 to−0.88) | .02 |
| Time (baseline vs 4 mo) | 29.13 | (−37.51 to 95.76) | .392 |
| ACEI, all patients, n=239 | |||
| Intercept | −31.77 | (−119.52 to 55.98) | .478 |
| Sex: female | 4.09 | (−8.5 to 16.68) | .525 |
| Time (baseline vs 4 months) | 22.83 | (−31.98 to 77.65) | .415 |
| Time (baseline vs 4 months) *Sex: female | −4.26 | (−12.34 to 3.81) | .301 |
| ACEI relative dose at baseline | 0.7 | (0.64 to 0.77) | <.001 |
| SBP (baseline, mmHg) | 0.26 | (0.16 to 0.36) | <.001 |
| eGFR<60 (no vs yes) | −6.56 | (−11.31 to−1.81) | .007 |
| HF-nurse vs HF-cardiologist | 7.1 | (3.52 to 10.68) | <.001 |
| Age, y | −0.18 | (−0.33 to−0.02) | .025 |
| ACEI, women, n=63 | |||
| Intercept | −46.71 | (−130.67 to 37.26) | .278 |
| ACEI relative dose at baseline | 0.72 | (0.58 to 0.86) | <.001 |
| SBP (baseline, mmHg) | 0.31 | (0.1 to 0.52) | .004 |
| HF-nurse vs HF-cardiologist | 11.14 | (3.69 to 18.58) | .004 |
| Diabetes mellitus | −12.08 | (−22.15 to−2.02) | .02 |
| Time (baseline vs 4 mo) | 18.37 | (−32.59 to 69.32) | .481 |
| ACEI, men, n=176 | |||
| Intercept | −27.17 | (−105.39 to 51.04) | .999 |
| ACEI relative dose at baseline | 0.71 | (0.64 to 0.78) | <.001 |
| SBP (baseline, mmHg) | 0.25 | (0.14 to 0.36) | <.001 |
| eGFR<60 (no vs yes) | −5.65 | (−10.95 to−0.34) | .038 |
| HF nurse vs HF cardiologist | 5.72 | (1.65 to 9.79) | .006 |
| Age, y | −0.23 | (−0.41 to−0.05) | .012 |
| Time (baseline vs 4 mo) | 22.83 | (−25.6 to 71.25) | .999 |
| MRA, all patients, n=252 | |||
| Intercept | 14.71 | (−29.88 to 59.3) | .999 |
| Sex: female | 3.03 | (−10.17 to 16.24) | .653 |
| Time (baseline vs 4 mo) | 8.51 | (−19.5 to 36.52) | .999 |
| Time (baseline vs 4 mo) *Sex: female | −0.68 | (−8.97 to 7.61) | .873 |
| MRA relative dose at baseline | 0.75 | (0.68 to 0.83) | <.001 |
| eGFR<60 (no vs yes) | −6.28 | (−11.15 to−1.41) | .012 |
| K (≥ 5.5 mEq/L vs<5.5 mEq/L) | −14.09 | (−27.47 to−0.7) | .04 |
| Combination of 3 drugs, baseline | −8.65 | (−14.64 to−2.66) | .005 |
| MRA, women, n=67 | |||
| Intercept | 14.5 | (−75.86 to 104.86) | .754 |
| MRA relative dose at baseline | 0.69 | (0.59 to 0.79) | <.001 |
| Time (baseline vs 4 mo) | 7.84 | (−49.15 to 64.82) | .788 |
| MRA, men, n=185 | |||
| Intercept | 31.28 | (−148.71 to 211.27) | .999 |
| MRA relative dose at baseline | 0.77 | (0.68 to 0.85) | <.001 |
| K (≥ 5.5 mEq/L vs<5.5 mEq/L) | −22.88 | (−38.21 to−7.55) | .004 |
| Combination of 3 drugs, baseline | −9.09 | (−16.47 to−1.71) | .016 |
| NYHA at baseline | −8.18 | (−14.15 to−2.22) | .008 |
| Time (baseline vs 4 mo) | 8.43 | (−105.05 to 121.9) | .999 |
95%CI, 95% confidence interval; ACEI, angiotensin-converting enzyme inhibitors; BB, beta-blockers; eGFR, estimated glomerular filtration; HF, heart failure; K, potassium; n, number of patients; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; SBP, systolic blood pressure.
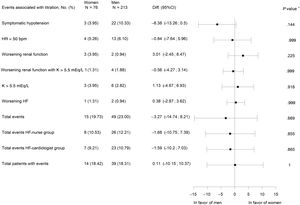
There were no significant differences in terms of the occurrence of overall or individual adverse events between groups (figure 2).
Adverse events associated with titration. 95%CI, 95% confidence interval; bpm, beats per minute; Diff., difference; HF, heart failure; HR, heart ratio; K, potassium; worsening renal function, creatinine>50% baseline, creatinine>3mg/dL, estimated glomerular filtration rate<25mL/min/1.73 m2; N total number of patients, No. (%), number of cases. * P value for difference between treatment groups.
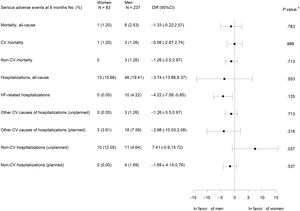
There were no statistically significant differences between women and men in all-cause and cardiovascular mortality or cardiovascular hospitalizations. No HF hospitalizations were observed in women, but this difference was not statistically significant. However, unplanned noncardiovascular hospitalizations were significantly more frequent in women (figure 3).
Clinical outcomes at 6 monthsThere were significant improvements in all clinical outcomes at 6 months, but without significant differences in the change from baseline to 6 months between the 2 groups, except in NYHA class, with men having better functional class at 6 months (table 6).
Outcomes at 6 months
| Variables | Womenn=74 | Menn=200 | Difference of change from baselineto 6 monthsbetween groups(95%CI) | P | ||
|---|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | |||
| LVEF % | 28.23±7.17 | 43.09±11.29 | 27.26±6.94 | 42.58±12.32 | −0.46 (−3.80 to 2.87) | .786 |
| LVEF<35% | 57 (77.03) | 15(20.27) | 161 (80.50) | 48 (24.00) | 0.26 (−13.21 to 13.73) | .999 |
| LVEF>40% | 0 | 44 (59.46) | 0 | 96 (48.00) | 11.46 (−2.62 to 25.54) | .121 |
| NT-proBNP, pg/mL n 68/176 | 1654 [952-3850] | 611 [195-1017] | 1476 [789-2954] | 526 [152-1277] | −182 (−650 to 92) | .158 |
| BNP, pg/ml, n 3/19 | 358 [200-381] | 160 [155-166] | 333 [188-762] | 162 [71- 490] | 33.5 (−967 to 992) | .999 |
| NYHA classa | ||||||
| I | 0 | 18 (24.32) | 0 | 84 (41.00) | −16.68 (−30.53 to−4.82) | .011 |
| II | 57 (77.03) | 53 (71.62) | 171 (85.50) | 110 (55.00) | −25.09 (−34.22 to 15.97) | <.001 |
| III | 16 (21.62) | 2 (2.70) | 29(14.50) | 6 (3.00) | 7.42 (−3.47 to 18.30) | .163 |
| 6-minute-walk test, meters | 315.9±94.59 | 359.59±99.86 | 382.25±97.41 | 433.20±117.64 | −7.25 (−30.29 to 15.80) | .535 |
| Minnesota scoreb | 52.81±21.48 | 26.07±19.60 | 46.5±22.73 | 21.11±20.01 | −1.34 (−7.83 to 5.14) | .683 |
| Physical dimension | 25.42±10.28 | 10.94±9.08 | 20.8±11.32 | 7.4±8.66 | −1.08 (−14.48 to 2.40). | .540 |
| Emotional dimension | 11.60±7.30 | 6.81±6.08 | 9.46±6.54 | 5.21±5.91) | −0.60 (−2.47 to 1.27). | .524 |
| Euroqol-5 dimension index | 0.65±0.24 | 0.74±0.24 | 0.77±0.21 | 0.82±0.21 | 0.04 (−0.02 to 0.11) | .210 |
| Visual analogue scale | 53.52±18.50 | 66.19±20.28 | 58.78±19.85 | 71.65±18.93 | −0.19 (−6.32 to 5.93) | .950 |
95%CI, 95% confidence interval; BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal probrain natriuretic peptide; NYHA, New York Heart Association.
Unless otherwise indicated, the data are expressed as No. (%), mean±standard deviation, or No.; median [interquartile range].
In this post hoc study of HF drug titration in the ETIFIC trial, nonsignificant gender differences were found in BB/ACEI/MRA dosage in the multivariate analysis, cardiovascular mortality, HF hospitalizations, and other clinical outcomes at 6 months (figure 4).
Post hoc analysis of the HF-titration ETIFIC trial: There were no significant gender differences regarding dosage of HF medications in the multivariate analysis, cardiovascular mortality or HF hospitalizations. ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; BB, beta-blockers; CV, cardiovascular; HF, heart failure; HFrEF, heart failure patients with reduced ejection fraction; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor blocker; RAS, renin-angiotensin system.
The possible barriers to women's participation were analyzed.4,12 The proportion of women in ETIFIC (25.93%) could be considered underenrolment, given the established reference (< 32%) in a systematic review of 317 HFrEF trials.4 However, participation was higher than that in trials of BB (23%), ACEI (21%), ARB (26%) and MRA (21%) (see : BB,1-20; ACEI 21-23; ARB 24,25 MRA 26-28), but lower than that in observational optimization studies (30%)5–10 (see ). This may have been influenced by recruitment in cardiology wards (lower proportion of elderly patients or with comorbidity). The lower proportion of women in cardiology services vs other services is also reflected in the literature20–26 (see ).
Some baseline differences between women vs men were observed in ETIFIC (table 1). No clinical trial or observational optimization study has evaluated the baseline characteristics of women.5–10 (see ). These were analyzed by 2 meta-analyses17,18 and 6 studies with other objectives.15,19–23 The HFrEF women generally had similar characteristics to those in ETIFIC (table 1): they were 1 to 4 years older,15,17,18,20–22 had a lower proportion of smokers,15,18,21 lower alcohol consumption,15 less frequently had atrial fibrillation,15,18,20,21 a lower 6-minute walk distance,22 worse quality of life,22,23 a higher proportion of NYHA III-IV,17,18,20,21 and minimal differences in LVEF (0.5-2%) vs men.15,17–19,21
Drug prescription in ETIFIC showed no significant differences, except for greater prescription of psychotropic drugs in women. A lower prescription of BB15,18 and ACEI in women vs men has been reported in the literature.15,20
Primary endpointBB relative doseNo gender differences were found in the mean relative doses of BB reached by all patients at 4 months (table 2) or in the multivariate analysis (table 5). Factors associated with dose were the relative dose of BB and heart rate at baseline for both women and men, and atrial fibrillation only for men (table 5), probably associated with a higher prevalence (table 1).
Both women and men achieved higher BB doses when they were titrated by HF-nurses vs HF-cardiologists. All HF-nurses were female. Female patients achieved higher BB doses when titrated by female cardiologists vs male cardiologists. In both cases, this was associated with a higher number of visits. The multivariate analysis also reflected the influence of these organizational issues (table 5, and ). The association of the achieved dose with the titrating professional, and the number of visits was previously demonstrated in ETIFIC patients.25 The possible influence of the professional's gender on the doses achieved by women has also been mentioned previously.16
No original BB trial has evaluated optimal doses for women or analyzed their prescription and the mean relative doses achieved (see ). These doses in women in ETIFIC, 62%, were lower (−15%), as with men, than the mean doses achieved by all patients in BB trials (more selected patients), although they were in the high dose range of observational optimization studies (33-63%)5–10 (see ).
We found limited and heterogeneous information on the BB doses achieved by women vs men in other types of studies. As in ETIFIC, some studies found no dose differences, namely a meta-analysis,17 a trial on exercise (see ), and 3 observational studies,9,10,21 while others reported lower target doses in women, namely, a HF program trial (see ) and 2 observational studies5 (see ).
Secondary endpointsNo gender differences were found in the relative doses of ACEI/ARB (table 2). Equally, there were no significant differences between women and men during titration in SBP or eGFR (), in symptomatic hypotension events (figure 2), or the mean relative dose of ACEI (table 2). However, there was a lower proportion of women with ≥ 50% the target dose of ACEI (table 2).
The multivariate analysis showed that the relative dose of ACEI at baseline and SBP were associated with the achieved doses of ACEI in both women and men (table 5). The association of achieved ACEI dose with the titrating professional, and the number of visits previously demonstrated in ETIFIC25 was also confirmed for women, as shown in table 3 and table 5 (see also ).
Women achieved significantly higher relative MRA doses at 4 months, associated with higher use of spironolactone and lower use of eplerenone (table 2). No gender differences were found in the multivariate analysis including all patients (table 5). However, there were no differences in the potassium level at baseline and 4 months () or in adverse events associated with potassium levels (figure 2), but potassium ≥ 5 mEq/L was associated with a lower dose only in men in the multivariate analysis (table 5).
No original ACEI, ARB and MRA trial has reported the prescription and mean relative dose achieved disaggregated by gender. Both women and men achieved lower ACEI/ARB doses in ETIFIC than those achieved by all patients in trials. This difference could be explained by the selected population, a higher SBP and a lower prescription of MRA and BB in these trials compared with ETIFIC (see ).
We found limited and heterogeneous information from some observational studies, 2 of them showing lower ACEI and ARB doses achieved by women6,10 but no other.21 In contrast, higher MRA doses have been reported in women.7
The drug prescription rate in the ETIFIC trial was high for both women and men, without significant differences. In addition, the joint prescription of 3 groups of drugs (BB, renin angiotensin system inhibitors and MRA) was 84% in women vs 78% in men, far higher than in trials and observational studies5–10 (see ).
Both women and men showed good self-care and adherence, although women showed slightly better results. Previous studies of adherence in HF have reported contradictory results in relation to gender (see ).
There were no differences between women and men in adverse events associated with titration (figure 2). Clinical trials and observational optimization studies did not report adverse events disagregated by sex5–10 (see references ).
Differences in pharmacodynamics and pharmacokinetics have been described in women that could lead to higher blood levels at the same drug dose, lower tolerance of higher doses or beneficial effects with lower doses. Moreover, differences in renal function could lead to greater adverse events, suggesting that optimal doses in women should be lower.13,16,21 However, the ETIFIC trial showed no significant differences in women vs men in dosage and adverse events.
Other secondary endpointsNo gender differences were found in the mortality rate or cardiovascular admissions. Both were lower than in the literature, probably because the patients in this study had novo HF, received therapeutic optimization, close follow-up by the HF program, and showed good self-care and adherence.1,3
Although it did not reach statistical significance, probably due to the small number of events, it could be clinically relevant that there were no HF admissions in women, considering that there were no significant differences in cardiovascular mortality either (figure 3).
Three meta-analyses have shown the effectiveness of BB, ACEI and MRA in reducing mortality and admissions in women.17,18,27 The BB and MRA meta-analysis17,18 and the European Long-Term registry28 observed fewer serious adverse events in women.
A substudy of BIOSTAT21 observed a 30% reduction of death or hospitalization with 50% of the recommended doses of ACEI, ARB and 60% of BB, without finding a greater reduction with higher doses, although the authors reported that the optimal doses for women are unknown. No titration protocol, adverse events, number of visits or reasons for not reaching the target dose were described. Moreover, the BB and MRA prescription and BB doses achieved were significantly lower than in the ETIFIC trial.
A clear improvement in LVEF (14.86% vs 15.32%), NT-proBNP, NYHA, 6-minute walk test and quality of life, with no significant differences between women and men, was shown, except in NYHA class, where men had a better functional class at 6 months than women (table 6).
LVEF recovery has been associated with being female, nonischemic etiology, atrial fibrillation, adherence to BB and shorter duration of HF19 (see ).
Similar improvements in women vs men have also been described in quality of life and 6-minute walk test, associated with the use of BB, ACEI, ARB, and a close follow-up in clinical studies.22
A paradox previously reported in the literature15,20,29 was also observed in women who, despite being older, more symptomatic and having worse quality of life, had lower mortality and HF hospitalization. According to previous publications, this may be due to possible late diagnoses, less access to health care, socioeconomic and educational factors, and physicians’ misperception of women's symptoms and consequent undertreatment.
LimitationsThe ETIFIC clinical trial was not designed with the aim of evaluating gender differences in drug titration. Therefore, the sample in this post hoc analysis was not calculated for this purpose.
CONCLUSIONSIn a post hoc analysis of the HF-titration ETIFIC trial, multivariate analysis identified nonsignificant gender differences in the dosage of HF medications at 4 months after discharge. There were also nonsignificant differences in cardiovascular mortality, HF hospitalizations, and other clinical outcomes at 6 months.
To our knowledge, ETIFIC is the first study to show that a controlled environment such as a randomized trial in HF clinics, with a structured protocol, close follow-up and patient education allows women to tolerate a prescription and dosage without significant differences vs men, without a higher mortality rate or cardiovascular admissions and a clear improvement in clinical outcomes. Higher dosage was associated with HF-nurse involvement, female gender of the titrating professional, and the number of visits.
Sex and gender analysis should be carried out in clinical trials to gain greater in-depth knowledge of the possible differences and increase the applicability of treatments in women.
- -
Women have been underrepresented in HFrEF trials. No original BB, ACEI, ARB, MRA trial has prospectively evaluated optimal doses by sex on a continuous scale, or specifically evaluated the characteristics, prescription, achieved doses and adverse events in women. Few trials have evaluated their effects on mortality and hospitalizations.
- -
Some studies (meta-analyses, systematic reviews, observational studies) have described differences between women and men in characteristics, drug prescription, dosage, and effects, but this evidence is limited by their design.
- -
To our knowledge, ETIFIC is the first study to show that a controlled environment such as a randomized trial in a HF unit, with a structured protocol, close follow-up and patient education allows women to tolerate prescription and dosage without significant differences vs men, without a higher mortality rate or cardiovascular admissions and a clear improvement in LVEF, NT-proBNP, 6-minute-walk test, and quality of life.
- -
Higher dosage was associated with HF-nurse involvement, female gender of the titrating professional, and the number of visits.
This work was supported by grants from Carlos III Health Research Institute (FIS PI14/01208) in coordination with the European Regional Development Fund, and the Government of the Basque Country (Exp. 2014111143). Neither funding source was an industry sponsor, and both are public institutions. ETIFIC researchers were independent from funders in the study design, collection, analysis, and interpretation of the data, drafting of the report and in the decision to submit the article for publication.
AUTHORS’ CONTRIBUTIONSAll authors had full access to all the data (including statistical reports and tables) in the study and take responsibility for data integrity and data analysis. All authors participated in the study design, writing and review of this manuscript and approval of the final version. P. Latorre-García, E. Arana-Arri, S. Pérez-Fernández were responsible for its methodological accuracy and statistical analysis. J. Oyanguren is the guarantor and attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
CONFLICTS OF INTERESTNone declared.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.11.002