There is wide recognition of the importance of healthy eating in cardiovascular health promotion. The purpose of this study was to identify the main dietary patterns among a Spanish population, and to determine their relationship with plasma lipid profiles.
MethodsA cross-sectional analysis was conducted of data from 1290 participants of the Aragon Workers Health Study cohort. Standardized protocols were used to collect clinical and biochemistry data. Diet was assessed through a food frequency questionnaire, quantifying habitual intake over the past 12 months. The main dietary patterns were identified by factor analysis. The association between adherence to dietary patterns and plasma lipid levels was assessed by linear and logistic regression.
ResultsTwo dietary patterns were identified: a Mediterranean dietary pattern, high in vegetables, fruits, fish, white meat, nuts, and olive oil, and a Western dietary pattern, high in red meat, fast food, dairy, and cereals. Compared with the participants in the lowest quintile of adherence to the Western dietary pattern, those in the highest quintile had 4.6mg/dL lower high-density lipoprotein cholesterol levels (P < .001), 8mg/dL lower apolipoprotein A1 levels (P = .005) and a greater risk of having decreased high-density lipoprotein cholesterol (odds ratio = 3.19; 95% confidence interval, 1.36-7.5; P-trend = .03). Participants adhering to the Mediterranean dietary pattern had 3.3mg/dL higher high-density lipoprotein cholesterol levels (P < .001), and a ratio of triglycerides to high-density lipoprotein cholesterol that was 0.43 times lower (P = .043).
ConclusionsAdherence to the Mediterranean dietary pattern is associated with improved lipid profile compared with a Western dietary pattern, which was associated with a lower odds of optimal high-density lipoprotein cholesterol levels in this population.
Keywords
Cardiovascular disease is well recognized as a major public health problem.1 Given the direct influence of unhealthy dietary habits on its development and progression,2 prevention through promoting a healthy way of eating at all population levels is a public health priority.3
The diet-disease relationship can be addressed from different perspectives, from the single nutrient approach to assessment of overall diet quality.4 This latter approach accounts for the likely interactions between dietary components and other lifestyle-related habits and may be better suited to identify behavioral determinants of cardiovascular disease rather than explore nutrient-induced etiological mechanisms. Evidence on how the overall diet quality impacts health is also more easily translated to broader audiences and policymakers, helping to underpin effective public health strategies. In this regard, the traditional Mediterranean dietary pattern (MDP), high in plant-based dietary sources, white meat, fish, and olive oil, and low in red meat and processed food, is well known for its cardioprotective effect5,6 and is recommended worldwide. Moreover, the traditional MDP has also been proposed as a plausible explanation of the Mediterranean paradox, ie, a high prevalence of cardiovascular disease risk factors along with a low incidence of cardiac events,7 and as a priority for primary and secondary cardiovascular disease prevention.8
Although the Mediterranean region has recently experienced a transition toward a more westernized dietary pattern and diet varies significantly between the countries of this area, depending on the agricultural and cultural settings, evidence shows that the MDP is associated with improved plasma lipid profile, including increased high-density lipoprotein cholesterol (HDL-C) concentration and decreased levels of low-density lipoproteins, triglycerides (TG), and total cholesterol.9–11 Furthermore, the effect of the MDP on apolipoprotein A1 (ApoA1) concentration has also been studied.12,13 Some studies have reported an increase of ApoA1 concentrations with Mediterranean diet14 and reductions in ApoA1 catabolic rate.15
In view of these findings, our aim was to identify the current major dietary patterns prevalent in a population of Spanish workers, the Aragon Workers Heath Study cohort, and to investigate their association with plasma lipid profile as an intermediate indicator of future cardiovascular outcomes.
METHODSStudy PopulationDetails of the study design and methodology used have previously been published.16 In brief, the Aragon Workers Health Study is a prospective cohort aimed at investigating the determinants of the development and progression of subclinical atherosclerosis in a middle-aged population. The study population consisted of a random sample of 5690 employees of the General Motors Spain automobile-assembly plant located in Zaragoza (Spain) who were free of cardiovascular disease at baseline.16 Each year, a random one-third of the study participants aged 40 to 55 years are selected for subclinical atherosclerosis imaging, clinical and physical examination, and diet, behavior, and lifestyle assessment. The present cross-sectional study was carried out in a subsample of 1593 participants with complete dietary data at baseline. Of these, 104 participants with extreme values for total energy intake (< 800 or > 4200 Kcal, and < 500 or > 3500 for men and women, respectively),17 and 199 participants with missing data were excluded. The final sample available for analysis consisted of 1290 participants. The study was approved by the central Institutional Review Board of Aragón CEICA (Comité Ético de Investigación de Aragón), and all study participants provided written informed consent.16
Dietary AssessmentHabitual food intakes over the past 12 months were collected through a validated 136-item food-frequency questionnaire, administered by trained dietician.18,19 The frequency of consumption varied from “never or almost never” to “more than 6 times per day”. Individuals’ total energy and nutrient intakes were derived through a standardized nutrient database (ENDB).20 Using this data, factor analysis was used to determine the main dietary patterns prevalent in our population. Furthermore, to validate the results of factor analysis, previously reported diet quality indices (AHEI [Alternate Healthy Eating Index],21 aMED [alternate MD Index],22 MEDAS [MD Adherence Screener],23 and the recently developed MEDLIFE [MEDiterranean LIFEstyle Index]24) were computed. The details of the indices’ development and scoring systems are described elsewhere.21–24
Blood and Urine CollectionAt baseline, participants provided a clinical history, including the occurrence of any clinical events and hospitalizations over the past year, indicating the presence of a personal or family history of early cardiovascular disease, current medication use, and, if diagnosed, hypertension, diabetes, or dyslipidemia. Seated resting blood pressure was measured by using an OMRON M10-IT (OMRON Healthcare Co Ltd; Japan) automatic oscillometric sphygmomanometer. Three measurements were taken and the average of the measurements was used for the analysis. Blood and urine samples were collected at baseline and were processed and stored for further analysis and biobanking. Fasting serum glucose, TG, total cholesterol, and HDL-C concentrations were measured by spectrophotometry (Chemical Analyzer ILAB 650, Instrumentation Laboratory). Low-density lipoprotein cholesterol was calculated using the Friedewald formula. Levels of HDL-C of ≥ 40mg/dL and ≥ 50mg/dL for men and women, respectively, were considered optimal.25 Serum ApoA1, B100, and lipoprotein (a) were measured by kinetic nephelometry (Immunochemistry Analyzer IMMAGE 800, Beckman Coulter), and fasting serum insulin by immunoenzymatic chemiluminiscence (Access Immunoassay System, Beckman Coulter). Whole blood glycated hemoglobin was measured by reverse-phase cationic exchange chromatography and quantification by double wave-length colorimetry quantification (Analyzer ADAMS A1c HA-810, Arkray Factory). The HOMA (Homeostatic Model Assessment) index was used to assess insulin resistance using glucose and insulin data.26
Physical Activity AssessmentLeisure time physical activity was assessed using the Spanish-validated version27 of the Nurses’ Health Study and Health Professionals’ Follow-up study physical activity questionnaires.28,29 Participants were asked about the average weekly time spent on 17 different types of physical activity, which was multiplied by its typical energy expenditure, expressed in metabolic equivalent transfer units,30 and summed over all activities, to estimate the total level of physical activity spent per week.
Assessment of Other VariablesAnthropometric measurements of body weight, height, and waist circumference were performed at baseline following standardized procedures.31 Data were also collected on baseline sociodemographics, education, smoking history, and employment.
Statistical AnalysisThe main dietary patterns were determined by factor analysis by deriving factor loading for predefined food groups using Varimax rotation option. Combinations of eigen values, the scree plot, and interpretability were used to determine the number of factors retained. Each factor had an eigen value > 0.3. Factor scores were computed for each participant for each dietary pattern by summing intakes of food groups weighted by their factor loadings. Based on the score, participants were then divided into quintiles of adherence into the specific dietary pattern. To describe baseline characteristics, categorical variables are presented as count and percentage, and continuous variables as mean (standard deviation). The P-trend was tested using the factor adherence as a continuous term in the regression model. The consistency of the factor analysis-derived patterns was tested by comparing factor loadings with a priori-defined scores, namely the AHEI, the aMED, the MEDAS, and the recently developed MEDLIFE by studying the strength of the association across quintiles of factor score. Linear regression analyses were conducted between plasma lipid concentrations and dietary pattern scores after controlling for the following possible confounders: age, sex, education level, dietary energy, physical activity level, plasma lipid-lowering medication, and body mass index. The odds ratio for decreased HDL-C concentration was assessed through logistic regression (adjusted for the same possible confounders as in linear regression) analysis across quintiles of dietary patterns (the first quintile was set as a reference). STATA 12.0 (StataCorp LP; College Station, Texas, United States) was used for all statistical analyses.
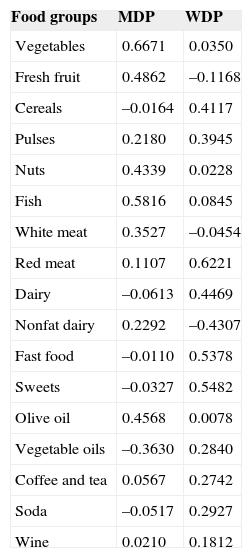
RESULTSFood Consumption PatternFood items from the food frequency questionnaire were classified into 17 main food groups (). Based on the factor loading of the food groups, 2 main dietary patterns were identified (Table 1). The first dietary pattern was characterized by higher intakes of vegetables, fresh fruits, nuts, fish, olive oil and, to a lesser extent, regular consumption of nonfat dairy products and white meat and was named the “Mediterranean dietary pattern” (MDP). The second dietary pattern was characterized by higher intakes of cereals, red meat, full-fat dairy products, fast food, desserts and sweets, and, to a lesser extent, by regular consumption of vegetable oils, soda, coffee, tea and wine/beer and was named the “Western dietary pattern” (WDP). The food group of legume pulses contributed to both patterns and was not considered determinant. These 2 patterns accounted for 22% of the variance of total food intake.
Factor Loading Matrix for Dietary Patterns
| Food groups | MDP | WDP |
|---|---|---|
| Vegetables | 0.6671 | 0.0350 |
| Fresh fruit | 0.4862 | –0.1168 |
| Cereals | –0.0164 | 0.4117 |
| Pulses | 0.2180 | 0.3945 |
| Nuts | 0.4339 | 0.0228 |
| Fish | 0.5816 | 0.0845 |
| White meat | 0.3527 | –0.0454 |
| Red meat | 0.1107 | 0.6221 |
| Dairy | –0.0613 | 0.4469 |
| Nonfat dairy | 0.2292 | –0.4307 |
| Fast food | –0.0110 | 0.5378 |
| Sweets | –0.0327 | 0.5482 |
| Olive oil | 0.4568 | 0.0078 |
| Vegetable oils | –0.3630 | 0.2840 |
| Coffee and tea | 0.0567 | 0.2742 |
| Soda | –0.0517 | 0.2927 |
| Wine | 0.0210 | 0.1812 |
MDP, Mediterranean dietary pattern; WDP, Western dietary pattern.
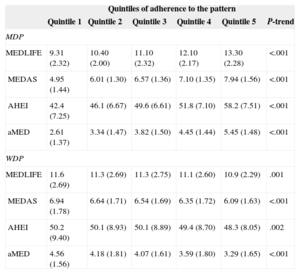
The analysis of the association between the 2 dietary patterns identified in our population and previously reported dietary indices capturing a healthy diet/lifestyle and the Mediterranean diet is shown in Table 2. All indices were positively associated with the MDP, indicating that those participants with higher adherence to the MDP also scored highly in distinct a priori indices. In contrast, all indices were inversely associated with the WDP, suggesting that this pattern is indeed associated with lower quality diets. This association between a priori and a posteriori-derived dietary patterns provides additional reliability to the results.
Association Between a Posteriori-defined Dietary Patterns and a Priori-defined Dietary Indices
| Quintiles of adherence to the pattern | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P-trend | |
| MDP | ||||||
| MEDLIFE | 9.31 (2.32) | 10.40 (2.00) | 11.10 (2.32) | 12.10 (2.17) | 13.30 (2.28) | <.001 |
| MEDAS | 4.95 (1.44) | 6.01 (1.30) | 6.57 (1.36) | 7.10 (1.35) | 7.94 (1.56) | <.001 |
| AHEI | 42.4 (7.25) | 46.1 (6.67) | 49.6 (6.61) | 51.8 (7.10) | 58.2 (7.51) | <.001 |
| aMED | 2.61 (1.37) | 3.34 (1.47) | 3.82 (1.50) | 4.45 (1.44) | 5.45 (1.48) | <.001 |
| WDP | ||||||
| MEDLIFE | 11.6 (2.69) | 11.3 (2.69) | 11.3 (2.75) | 11.1 (2.60) | 10.9 (2.29) | .001 |
| MEDAS | 6.94 (1.78) | 6.64 (1.71) | 6.54 (1.69) | 6.35 (1.72) | 6.09 (1.63) | <.001 |
| AHEI | 50.2 (9.40) | 50.1 (8.93) | 50.1 (8.89) | 49.4 (8.70) | 48.3 (8.05) | .002 |
| aMED | 4.56 (1.56) | 4.18 (1.81) | 4.07 (1.61) | 3.59 (1.80) | 3.29 (1.65) | <.001 |
AHEI, Alternate Healthy Eating Index; aMED, alternate MD Index; MEDAS, MD Adherence Screener; MEDLIFE, MEDiterranean LIFEstyle Index; MDP, Mediterranean dietary pattern; WDP, Western dietary pattern.
The results are expressed as mean (standard deviation).
Baseline characteristics according to quintiles of the 2 major dietary patterns (MDP and WDP) among the 1290 participants are shown in Table 3. As energy intake was not considered during factor analysis, adherence to both factors increased with energy intake due to the wider variety of foods consumed by those with higher caloric intake. On average, those participants in the highest quintile of adherence to the MDP were slightly older (P = .01), were either less likely to currently smoke (P < .001) or were more likely to be a former smokers (P < .001), were more physically active (P < .001), and had higher energy intake (P < .001) compared with those in the lowest quintile. Adherence to none of the patterns was associated with cardiovascular risk factors or biochemistry indicators, either across the quintiles of distribution (P-trend > .05) or when we compared the samples in extreme quintiles (P > .05). In contrast, participants with the highest adherence to the WDP were slightly younger men, belonged to families of 3 or more members, and had a lower education level (P < .05). No differences were found for cardiovascular risk factors except for a lower percentage of medication use (P < .05 for all medication) and a higher prevalence of smoking (P < .001) among those adhering more closely to the WDP.
Baseline Characteristics by Quintiles of Adherence
| Quintiles* of adherence to MDP | |||||||
|---|---|---|---|---|---|---|---|
| Quintile 1*258 (–3.08 to 0.84) | Quintile 2258 (–0.84 to; –0.28) | Quintile 3258 (–0.28 to 0.23) | Quintile 4258 (0.23-0.79) | Quintile 5258 (0.80-3.84) | P-trend | P-value (quintile 1 vs quintile 5) | |
| Demographics | |||||||
| Age, mean (SD), y | 50.8 (3.80) | 51.1 (3.66) | 51.7 (3.6) | 51.4 (3.61) | 51.5 (3.45) | .010 | .021 |
| Number of family members, mean (SD) | 3.20 (1 .06) | 3.23 (1.03) | 3.21 (0.89) | 3.16 (0.93) | 3.19 (0.96) | .669 | .862 |
| Number of children, mean (SD) | 1.50 (0.82) | 1.49 (0.734) | 1.52 (0.755) | 1.51 (0.74) | 1.53 (0.72) | .392 | .648 |
| Gender (female) | 7 (2.71) | 16 (6.20) | 12 (4.65) | 18 (6.98) | 11 (4.3) | .392 | .337 |
| CVD history | 2 (0.78) | 2 (0.79) | 0 (0) | 1 (0.41) | 3 (1.2) | .427 | .637 |
| Education level | |||||||
| ≤ high school | 240 (93.0) | 246 (95.3) | 244 (94.5) | 238 (92.6) | 245 (95) | .952 | .354 |
| > high school | 18 (6.98) | 12 (4.65) | 14 (5.43) | 19 (7.39) | 13 (5.04) | ||
| CVD risk factors | |||||||
| Medication: Dyslipemia | 36 (14.2) | 38 (15.1) | 31 (12.4) | 35 (14.5) | 35 (14.1) | .979 | .970 |
| Medication: Diabetes | 11 (4.40) | 10 (3.98) | 8 (3.19) | 7 (2.89) | 11 (4.5) | .327 | .977 |
| Medication: Hypertension | 60 (23.6) | 54 (21.4) | 47 (18.8) | 51 (21) | 56 (22.5) | .556 | .763 |
| Body mass index, mean (SD), kg/m2 | 27.8 (3.56) | 27.7 (3.62) | 27.7 (3.74) | 28.1 (3.67) | 28.0 (3.33) | .370 | .575 |
| Waist circumference, mean (SD), cm | 98.0 (9.70) | 97.9 (9.47) | 96.9 (10.5) | 97.8 (10) | 97.7 (8.9) | .628 | .687 |
| Systolic blood pressure, mean (SD), mmHg | 125.0 (14.0) | 126.0 (14.7) | 125.1 (13.5) | 125.9 (14.9) | 126 (14.6) | .864 | .379 |
| Diastolic blood pressure, mean (SD), mmHg | 83.5 (9.2) | 83.7 (9.5) | 82.8 (9.2) | 84.3 (10.1) | 83.4 (9.80) | .790 | .664 |
| Smoking status | |||||||
| Smoker | 103 (40.1) | 74 (29.0) | 85 (33.2) | 90 (35.9) | 53 (20.9) | <.001 | <.001 |
| Nonsmoker | 82 (31.9) | 90 (35.3) | 92 (35.9) | 81 (32.3) | 86 (33.9) | .913 | .639 |
| Former smoker | 72 (28.0) | 91 (35.7) | 79 (30.9) | 80 (31.9) | 115 (45.3) | <.001 | <.001 |
| Lifestyle | |||||||
| Energy intake, mean (SD), kcal | 2540 (673) | 2697 (655) | 2797 (573) | 2875 (622) | 3069 (578) | .000 | <.001 |
| Physical activity, mean (SD), METs-h/week | 30.5 (18.3) | 33.3 (19.6) | 31.7 (18.2) | 34.6 (20.3) | 38.2 (22.4) | <.001 | <.001 |
| Sleep, mean (SD), h, business d) | 6.36 (0.92) | 6.25 (0.92) | 6.17 (0.89) | 6.25 (1.07) | 6.27 (1.03) | .619 | .302 |
| Biochemistry | |||||||
| Glucose, mean (SD), mg/dL | 98.7 (15.8) | 101.0 (21.7) | 98.6 (18.3) | 98.7 (17.3) | 99.9 (18.2) | .904 | .458 |
| Insulin, mean (SD), uU/mL | 8.71 (7.01) | 7.68 (5.80) | 7.28 (4.9) | 8.14 (5.6) | 7.75 (5.02) | .230 | .087 |
| Glycated hemoglobin, mean (SD), % | 5.56 (0.47) | 5.56 (0.60) | 5.54 (0.5) | 5.55 (0.5) | 5.57 (0.56) | .836 | .890 |
| Insuline resistance, mean (SD), HOMA | 2.18 (2.00) | 1.95 (1.70) | 1.82 (1.4) | 2.07 (1.7) | 1.96 (1.49) | .342 | .178 |
| C-reactive protein, mean (SD), mg/dL | 0.320 (0.73) | 0.262 (0.20) | 0.322 (0.4) | 0.273 (0.3) | 0.24 (0.29) | .081 | .105 |
| Quintiles* of adherence to WDP | |||||||
|---|---|---|---|---|---|---|---|
| Quintile 1258 (–3.09 to –0.85) | Quintile 2258 (–0.84 to –0.25) | Quintile 3258 (–0.25 to 0.25) | Quintile 4258 (0.25-0.83) | Quintile 5258 (0.84-4.41) | P-trend | P-value (quintile 1 vs quintile 5) | |
| Demographics | |||||||
| Age, mean (SD), y | 51.6 (3.60) | 51.3 (3.85) | 51.7 (3.44) | 51.0 (3.57) | 50.8 (3.69) | .003 | .012 |
| Number of family members, mean (SD) | 3.00 (0.98) | 3.20 (0.97) | 3.17 (0.99) | 3.29 (0.97) | 3.33 (0.92) | <.001 | <.001 |
| Number of children, mean (SD) | 1.38 (0.76) | 1.53 (0.74) | 1.51 (0.79) | 1.55 (0.76) | 1.59 (0.69) | <.001 | .001 |
| Sex (female) | 30 (11.60) | 17 (6.59) | 14 (5.43) | 2 (0.77) | 1 (0.39) | <.001 | <.001 |
| CVD history | 4 (1.61) | 0 (0.00) | 1 (0.40) | 2 (0.81) | 1 (0.39) | .310 | .168 |
| Education level | |||||||
| ≤ high school | 236 (91.5) | 237 (92.2) | 245 (94.9) | 244 (94.5) | 251 (97.3) | .002 | .004 |
| > high school | 22 (8.50) | 20 (7.78) | 13 (5.04) | 14 (5.43) | 7 (2.70) | ||
| CVD risk factors | |||||||
| Medication: dyslipemia | 50 (20.40) | 31 (12.50) | 42 (16.90) | 31 (12.60) | 21 (8.24) | <.001 | <.001 |
| Medication: diabetes | 17 (7.00) | 8 (3.24) | 8 (3.21) | 8 (3.25) | 6 (2.36) | .026 | .014 |
| Medication: hypertension | 71 (28.9) | 49 (19.7) | 67 (26.7) | 47 (19.0) | 34 (13.3) | <.001 | <.001 |
| Body mass index, mean (SD), kg/m2 | 28.1 (3.71) | 27.8 (3.69) | 27.8 (3.60) | 27.9 (3.64) | 27.5 (3.27) | .123 | .054 |
| Waist circumference, mean (SD), cm | 97.6 (10.80) | 97.3 (10.40) | 97.9 (9.36) | 97.8 (9.52) | 97.7 (8.50) | .637 | .983 |
| Systolic blood pressure, mean (SD), mmHg | 126 (14.9) | 125 (14.2) | 125 (14.6) | 126.8 (14.1) | 125 (14.0) | .626 | .742 |
| Diastolic blood pressure, mean (SD), mmHg | 83.4 (9.80) | 82.8 (9.30) | 83.8 (9.90) | 84.3 (9.80) | 83.5 (8.96) | .281 | .874 |
| Smoking status | |||||||
| Smoker | 54 (20.9) | 77 (30.2) | 73 (29.1) | 83 (32.9) | 118 (45.9) | <.001 | <.001 |
| Nonsmoker | 107 (41.5) | 86 (33.7) | 82 (32.7) | 93 (36.9) | 63 (24.5) | <.001 | <.001 |
| Former smoker | 97 (37.60) | 92 (36.19) | 96 (38.20) | 76 (30.20) | 76 (29.60) | .014 | .054 |
| Lifestyle | |||||||
| Energy intake, mean (SD), kcal | 2093 (455) | 2518 (413) | 2769 (448) | 3085 (410) | 3514 (408) | <.001 | <.001 |
| Physical activity, mean (SD), METs-h/week | 31.4 (19.3) | 35.2 (20.9) | 33.6 (20.7) | 34.7 (20.5) | 33.3 (18.2) | .194 | .266 |
| Sleep, mean (SD), h, business d | 6.25 (0.91) | 6.21 (1.01) | 6.31 (0.92) | 6.26 (1.10) | 6.27 (0.91) | .808 | .847 |
| Biochemistry | |||||||
| Glucose, mean (SD), mg/dL | 99.9 (18.0) | 98.5 (14.5) | 99.8 (17.1) | 99.2 (19.9) | 99.1 (21.3) | .915 | .657 |
| Insulin, mean (SD), uU/mL | 7.49 (6.03) | 7.6 (4.48) | 7.88 (5.90) | 8.32 (6.28) | 7.87 (5.90) | .919 | .902 |
| Glycated hemoglobin, mean (SD), % | 5.56 (0.57) | 5.53 (0.46) | 5.56 (0.52) | 5.56 (0.59) | 5.55 (0.55) | .721 | .828 |
| Insuline resistance, mean (SD), HOMA | 2.00 (1.75) | 1.89 (1.26) | 2.00 (1.70) | 2.13 (1.89) | 1.98 (1.77) | .863 | .909 |
| C-reactive protein, mean (SD), mg/dL | 0.290 (0.39) | 0.337 (0.35) | 0.272 (0.35) | 0.276 (0.31) | 0.250 (0.21) | .131 | .235 |
CVD, cardiovascular disease; HOMA, Homeostatic Model Assessment; MDP, Mediterranean dietary pattern; WDP, Western dietary pattern.
Data are expressed as No. (%) or median (standard deviation).
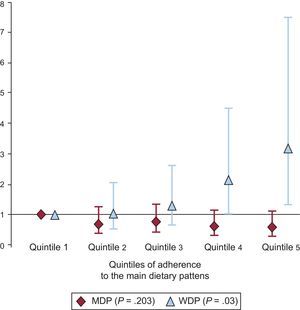
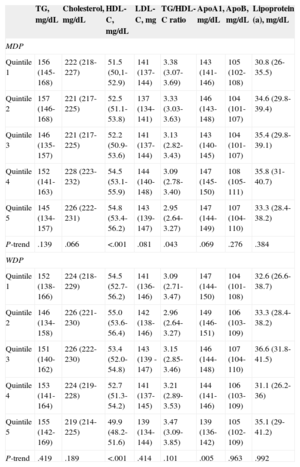
Plasma lipid concentrations across quintiles of adherence to the MDP and WDP are shown in Table 4. Fully adjusted linear regression models were used for comparison. On average, participants adhering more closely to the MDP had higher HDL-C (quintile 1 = 51.5 mg/dL; quintile 5 = 54.8mg/dL; P < .001) and a lower TG/HDL-C ratio (quintile 1 = 3.38; quintile 5 = 2.95; P = .043). In contrast, participants who scored high on the WDP had significantly (P < .001) lower HDL-C (quintile 1 = 54.5mg/dL; quintile 5 = 49.9mg/dL), and serum ApoA1 (quintile 1 = 147mg/dL; quintile 5 = 139mg/dL; P = .005). Figure shows the risk of having HDL-C lower than 40mg/dL for men and lower than 50mg/dL for women across quintiles of adherence to the WDP and MDP. The risk of having lower HDL-C increased with greater adherence to the WDP (quintile 5 vs quintile 1, odds ratio = 3.19; 95% confidence interval, 1.36-7.50; P-trend = .03). Regarding the MDP, the trend across quintiles was not significant (quintile 5 vs quintile 1, odds ratio = 0.603; 95% confidence interval, 0.329-1.100; P-trend = .203).
Plasma Lipids by Quintiles of Adherence to Main Dietary Patterns
| TG, mg/dL | Cholesterol, mg/dL | HDL-C, mg/dL | LDL-C, mg | TG/HDL-C ratio | ApoA1, mg/dL | ApoB, mg/dL | Lipoprotein (a), mg/dL | |
|---|---|---|---|---|---|---|---|---|
| MDP | ||||||||
| Quintile 1 | 156 (145-168) | 222 (218-227) | 51.5 (50,1-52.9) | 141 (137-144) | 3.38 (3.07-3.69) | 143 (141-146) | 105 (102-108) | 30.8 (26-35.5) |
| Quintile 2 | 157 (146-168) | 221 (217-225) | 52.5 (51.1-53.8) | 137 (134-141) | 3.33 (3.03-3.63) | 146 (143-148) | 104 (101-107) | 34.6 (29.8-39.4) |
| Quintile 3 | 146 (135-157) | 221 (217-225) | 52.2 (50.9-53.6) | 141 (137-144) | 3.13 (2.82-3.43) | 143 (140-145) | 104 (101-107) | 35.4 (29.8-39.1) |
| Quintile 4 | 152 (141-163) | 228 (223-232) | 54.5 (53.1-55.9) | 144 (140-148) | 3.09 (2.78-3.40) | 147 (145-150) | 108 (105-111) | 35.8 (31-40.7) |
| Quintile 5 | 145 (134-157) | 226 (222-231) | 54.8 (53.4-56.2) | 143 (139-147) | 2.95 (2.64-3.27) | 147 (144-149) | 107 (104-110) | 33.3 (28.4-38.2) |
| P-trend | .139 | .066 | <.001 | .081 | .043 | .069 | .276 | .384 |
| WDP | ||||||||
| Quintile 1 | 152 (138-166) | 224 (218-229) | 54.5 (52.7-56.2) | 141 (136-146) | 3.09 (2.71-3.47) | 147 (144-150) | 104 (101-108) | 32.6 (26.6-38.7) |
| Quintile 2 | 146 (134-158) | 226 (221-230) | 55.0 (53.6-56.4) | 142 (138-146) | 2.96 (2.64-3.27) | 149 (146-151) | 106 (103-109) | 33.3 (28.4-38.2) |
| Quintile 3 | 151 (140-162) | 226 (222-230) | 53.4 (52.0-54.8) | 143 (139 -147) | 3.15 (2.85-3.46) | 146 (144-148) | 107 (104-110) | 36.6 (31.8-41.5) |
| Quintile 4 | 153 (141-164) | 224 (219-228) | 52.7 (51.3-54.2) | 141 (137-145) | 3.21 (2.89-3.53) | 144 (141-146) | 106 (103-109) | 31.1 (26.2-36) |
| Quintile 5 | 155 (142-169) | 219 (214-225) | 49.9 (48.2-51.6) | 139 (134-144) | 3.47 (3.09-3.85) | 139 (136-142) | 105 (102-109) | 35.1 (29-41.2) |
| P-trend | .419 | .189 | <.001 | .414 | .101 | .005 | .963 | .992 |
Apo, apolipoprotein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MDP, Mediterranean dietary pattern; TG, triglycerides; WDP, Western dietary pattern.
Adjusted means (95% confidence interval) for age, sex, education level, energy intake, physical activity level, plasma lipid-lowering medication, and body mass index.
In this study, we investigated the relationship between overall diet quality expressed by adherence to a posteriori-derived dietary patterns and plasma lipid profile as an intermediate indicator of cardiovascular risk. Previous studies have suggested that adherence to a healthy diet is linked to improved health-related behaviors.32 Analysis of the dietary patterns of a Dutch population revealed that individuals who followed a pattern high in dairy products, meat, and processed food were more likely to be less educated, less physically active, and heavy smokers.33 In contrast, those with a higher intake of vegetables and vegetable oils, pasta, rice, fish, white meat, and wine were likely to have a higher education level and to be more physically active.33
There is scientific evidence that choosing a healthier dietary pattern depends on socioeconomic status.34 This is true in Spain, where a recent study among university graduates found that the Mediterranean diet, to some extent, was expensive.35 In our study, individuals with a lower education and income might face economic barriers to the MDP, and are thus prone to the WDP or a similar high-fat pattern, giving preference to a less expensive and less healthy diet, whereas a more highly educated population with a higher income might be more able to afford certain healthier food items, which on average are considered expensive. Additionally, more highly educated participants might have better nutrition knowledge and greater awareness of the effects of exposure to a low-quality diet on risk of disease progression; therefore they would develop the healthier dietary pattern. As for lifestyle habits, in our study only physical activity was positively associated with increased adherence to the MDP and smoking with a lower or higher adherence to MDP or WDP, respectively. Current smokers tended to be less health-conscious and to have a lower degree of self-awareness of their health-related behaviors, including diet, than those who either never smoked or gave up smoking.36 Smoking causes an increased turnover of micronutrients,37 thus putting smokers who follow the WDP at greater risk of chronic disease compared with nonsmokers, not only due to the unhealthy dietary intake characterized by the WDP, but also due to malnutrition.38
The results of our study on HDL-C are in agreement with both observational studies and clinical trials.9,10,39 Overall, a higher intake of refined and processed food, which characterizes the WDP, is associated with a lower HDL-C level, while closer adherence to the MDP will more likely result in increased HDL-C. One plausible underlying mechanism explaining the association between the WDP and lower HDL-C could be greater consumption of refined carbohydrates among those who follow the WDP. Refined carbohydrates tend to increase visceral adiposity, decrease insulin sensitivity, and stimulate hepatic de novo lipogenesis, which result in reduced HDL-C levels.40 In contrast, the favorable effect of the MDP on HDL-C could be due to higher consumption of olive oil among the MDP population. Olive oil has been related to higher levels of trienoic prostaglandins, resulting in amelioration of plasma lipid profile, such as an increase in serum HDL-C.41
Some observational studies have shown an independent beneficial effect of physical activity on lipid profile.42 The role played by physical activity in altering the association between diet and lipid profile was to some extent observed in our study. Controlling for physical activity in the model slightly weakened the association between the MDP and TG/HDL-C ratio, and ApoA1 among those who followed the MDP, indicating that physical activity could indeed be partly associated with improvement of some components of the lipid profile.
Some authors have claimed that the effect of adherence to the healthy MDP on lipid profile is due to its favorable effect on obesity.43 Although greater compliance with the MDP was seen to be associated with a lower obesity risk, adjustment for body mass index as a proxy of obesity, according to our results, only slightly altered the association between diet and lipid profile, indicating that the improvements in plasma lipids observed were more likely to be due to adherence to the dietary pattern rather than to a change in body mass index.
Although not directly observed in our study, diet influences serum TG level, which has been reported in several studies.44 In our study, we observed an inverse association between the MDP and the TG/HDL-C ratio, known as a surrogate index directly related to coronary heart risk, especially myocardial infarction.45 Omega-3 polyunsaturated fatty acids, which are abundant in the MDP, are known to reduce TG synthesis, which will result in a lower TG/HDL-C ratio,46 therefore adherence to the MDP, even with nonincreasing HDL-C concentration, will likely result in a lower risk for the development of coronary heart disease.
Even though a direct association between adherence to the MDP and ApoA1 was observed in our study, as well as in several other studies,11,14,47 the evidence regarding this relationship is still inconsistent. Taking into account the importance of apolipoproteins in cardiovascular disease prediction,48 future studies are required to confirm our results.
Over the years, epidemiological studies have demonstrated an inverse association between HDL-C levels and cardiovascular risk,49 such as in the Framingham Heart Study, where an increase of 5mg/dL in HDL-C concentrations was associated with a 21% lower cardiovascular risk.50 However, some recent studies have challenged this evidence, showing that increases in HDL-C are not necessary related to a lower risk of myocardial infarction and that its protective effect still needs to be further investigated.51
A posteriori-derived dietary patterns could only partially explain the variance in total food intake in this population, and therefore the influence of other minor dietary patterns attenuating the observed associations cannot be ruled out. In addition, the results of studies based on factor analyses largely depend on subjective decisions taken by the researchers regarding the grouping of food items into food categories. In our study, consumption of food items such as legume pulses, cereals, and wine was associated with a high-fat, high-carbohydrate diet (WDP). These items were originally considered as essential parts of the traditional MDP, and could indicate an ongoing dietary transition from the traditional MDP to the current Mediterranean-based pattern followed in Spain. It is known that the same dietary pattern significantly differs between distinct populations, suggesting, for instance, that the MDP studied in 2 different countries might also differ.52
Strengths and LimitationsAn advantage of our study is the validation of an a posteriori-defined dietary pattern through comparison with a priori defined indices of diet quality. Based on our results, it can be concluded that factor analysis-defined dietary patterns are valid tools to assess the relationship between overall diet quality and biomarkers of diseases. In relation to the associations found, and due to the cross-sectional design of our study, a causal relationship cannot be established. In addition, this sample may not be representative of the general population, because these participants were mostly men and active workers and therefore healthy or at least without disabling diseases. As in most diet-related studies, reporting biases due to social desirability of overreporting healthier food items and underreporting less favorable foods also cannot be ruled out.
CONCLUSIONsHigher adherence to the MDP was associated with improved plasma lipid profile while adherence to a WDP decreased the odds of optimal HDL-C levels in this cohort of Spanish workers.
FUNDINGThis study was financially supported by the FIS (Fondo de Investigaciones Sanitarias) of the ISCIII (Instituto de Salud Carlos III) of Spain, project PI11/00403.
CONFLICTS OF INTERESTNone declared.
We thank the participants and the personal of the Aragon Health Workers Study cohort for their collaboration.