Cumulative data indicate that chronic heart failure (HF) is a major and growing public health problem worldwide. Even though the incidence of HF is stable, the prevalence is set to rise because of the aging population and the increase in the prevalence of HF risk factors (eg, diabetes and hypertension). This will cause further increases in health care costs. Consequently, finding cures for HF represents a global unmet medical need of the utmost importance. Despite significant advances in the treatment of HF with reduced ejection fraction (HFrEF) during the past decades, there is broad consensus that new treatments are needed because the residual risk in this disorder remains unacceptably high. In addition, no progress has been made in reducing mortality and morbidity in patients with HF with preserved ejection fraction (HFpEF) in recent years.
Preserving function and the ability to perform activities of daily living are not only powerful markers of longevity but also appear to play a more important role in moderating cardiovascular and all-cause mortality.1 Thus, monitoring and preserving functional capacity in HF patients might be a primary focus for clinicians, given that it negatively influences wellbeing, social adaptation, quality of life, and prognosis. In this context, low levels of muscle strength and accelerated loss of muscle mass (ie, sarcopenia) are also prevalent among patients with the development of HFpEF, especially in those aged> 70 years.2 Therefore, to advance HF therapy in terms of improving clinical outcomes, muscle strength and mass, functional capacity, and quality of life, it seems necessary to implement novel strategies based on emerging aspects.
One of the most important symptoms of HF patients that negatively influences their quality of life and prognosis is exercise intolerance and deep fatigue. In contrast to the traditional vision that considers a malfunction of the heart as the leading cause of reduced exercise capacity of HF patients, in the last 20 years, there has been mounting evidence of the critical role of skeletal myopathy. Additionally, associations have been described between skeletal muscle-related alterations and the clinical severity and outcomes of HF patients. This editorial describes the potential pathophysiological involvement and clinical implications in HF of a group of molecules, known as myokines, which are secreted in skeletal muscle, and pays special attention to those aspects related to their direct myocardial impact.
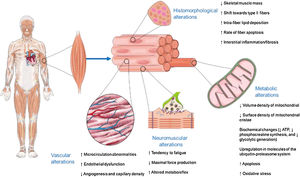
CONVENTIONAL VIEW OF SKELETAL MYOPATHY IN HF: THE MUSCLE AS A VICTIMAlterations in skeletal muscle morphology, metabolism, and function have been described in HF patients and may be critically involved in reduced exercise capacity in these patients (figure 1).3 Conventionally, the origin of these alterations has been related to decreased muscle blood flow, partly due to decreased cardiac output, and partly to vasoconstriction of the small muscular arteries secondary to the neuro-humoral-mediated systemic vasoconstriction that accompanies HF.3 Chronic systemic inflammation is emerging as a cardiovascular disease risk factor. Indeed, skeletal muscle inflammation and subsequent fibrosis, as a component of the systemic inflammation and reparative response that characterizes HF, have been also implicated in the initiation and perpetuation of skeletal muscle damage.4 On the other hand, HF is generally associated with systemically enhanced metabolic catabolism and reduced anabolism. For example, an increase in blood levels of catabolic hormones (eg, cortisol) and a decrease in anabolic hormones (eg, testosterone) have been shown to be closely associated with muscle atrophy, myopathy severity, and the poor prognosis in HF patients.5
EMERGING VIEW OF SKELETAL MYOPATHY IN HF: THE MUSCLE AS A CULPRITThe concept of muscle as a secretory organ, developed in recent decades, partially answers the issue of how the crosstalk between skeletal muscle and distant tissues occurs.6 The beneficial effects of exercise transcend the simple improved skeletal muscle functionality: systemic responses to exercise (particularly during muscle contraction) have been observed in distal organs such as the heart, kidney, brain, adipose tissue, and liver. Increasing data have also accumulated regarding the synthesis, kinetics of release and biological roles of muscular cytokines, now called myokines. A myokine is a molecule that is produced by skeletal muscle cells mostly in response to muscle contraction and subsequently released into the extracellular space and the circulation to exert autocrine and paracrine effects on skeletal muscle cells, and endocrine effects in other cells, tissues or organs, respectively.6 Since the identification of interleukin-6 as the prototypical myokine, interest in the concept and the known muscle secretome, defined here as the “myokinome”, has steadily grown, with an exponential rise in the number of studies describing myokines in recent years. In fact, a secretome analysis of human muscle cells recently identified over several hundred nonredundant secreted molecules, of which more than 300 were classified as potential myokines.7 However, myokine discovery is challenging, and molecules are often prematurely labeled myokines before appropriate validation or an in-depth analysis of the proposed effects of the molecule once it is released from muscle.
Acting in an autocrine-paracrine manner, most of myokines are involved in myogenesis and growth (both in terms of myoblast proliferation and fiber hypertrophy), protection against cell death processes such as apoptosis and autophagy, metabolic autoregulation (eg, related to both oxidative and nonoxidative generation of adenosine triphosphate, as well as density and composition of mitochondria), and general function (eg, exercise capacity and tolerance) of skeletal muscle cells.8,9 Most of these effects are mediated via intracellular signaling pathways, including the Janus 1 and 2 kinases/3 and 5 signal transducer and activator of transcription proteins/nuclear factor kappa B, phosphoinositide 3 (PI3) kinase, and mitogen-activated protein kinase pathways.8,9
In addition, myokines have a large spectrum of endocrine actions resulting mainly in the regulation of white adipose tissue (eg, decreased inflammation, and increased insulin sensitivity, thermogenesis, browning, and lipid peroxidation) and in facilitating a systemic anti-inflammatory environment (namely, mediated by interleukin-6, which acts directly on inflammatory cells or through adrenal production of cortisol and subsequent regulation of immune-inflammatory cell trafficking).10
The concept developed in recent decades that the contracting muscle may act by releasing myokines, some of which are found in the bloodstream to an extent dependent on the type and intensity of the exercise exerted, implies that the regulation of the growth, metabolism, and mechanical functions of the muscle during and after training may also be derived (and is furthermore guaranteed) by the effects of these molecules.11 In this conceptual framework, it has been proposed that myokine alterations may contribute to the development of HF-related skeletal myopathy and other metabolic comorbidities accompanying HF, thereby influencing the clinical course of this syndrome.12
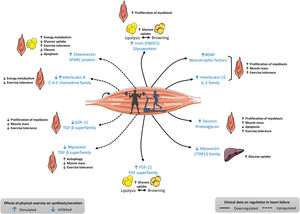
ALTERED REGULATION OF MYOKINES IN HF: CLINICAL AND EXPERIMENTAL DATAIn addition to data obtained in experimental models of HF, the regulation of some myokines has also been shown to be altered in patients with HF as assessed by measuring their blood concentrations and/or tissue expression (figure 2).6,11,12 For instance, plasma irisin concentrations were shown to be lower in cachectic women with either HFrEF or HFpEF than in their counterpart noncachectic HF women.13 Another study performed in patients with HFrEF showed that expression of the gene encoding irisin in muscle biopsies was positively related to aerobic performance (as assessed using oxygen consumption and ventilatory efficiency).14 In contrast, it has been reported that serum myostatin levels were higher in HF patients than in controls.15 In addition, Ishida et al.16 demonstrated that myostatin protein was significantly upregulated in the muscle of female patients with advanced HFrEF, but not in that of male patients with advanced HFrEF, and female patients displayed lower body mass index than male patients.
Characteristics of some myokines analyzed in patients with heart failure and whose regulation is altered in blood and/or its tissue expression. BDNF, brain-derived neurotrophic factor; CTRP15, myonectin; FGF-21, fibroblast growth factor; GDF-11, growth differentiation factor 11; IL-2, interleukin-2; TGF-β, transforming growth factor beta. Adapted with permission from Fiuza-Luces et al.,6 Di Raimondo et al.,11 and Berezin et al.12.
The analysis of the data presented in figure 2 shows that most of the myokines downregulated in HF are those that physiologically exert beneficial effects in terms of exercise tolerance or systemic metabolism. In contrast, among the myokines upregulated in HF, some are detrimental and some are beneficial in terms of their reported effects on skeletal muscle tolerance and systemic metabolism under physiological conditions. In addition, the response of skeletal muscle secreting specific myokines may vary depending on both the modality and the dose of physical exercise.
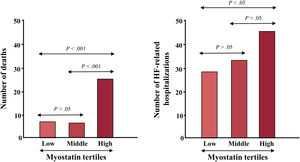
The analysis of the data shown in table 1 indicates that myokines may exert pro- or antiremodeling effects in the myocardium, therefore opening up the possibility of their potential contribution to the severity and clinical progression of HF. For example, it has been reported that plasma irisin was positively correlated with left ventricular ejection fraction and negatively correlated with left ventricular dimensions in women with HF.13 In addition, it has been shown that increased serum myostatin levels were associated with the severity of HF (as assessed by the New York Heart Association class and the plasma level of N-terminal pro-B-type natriuretic peptide).15 In addition, patients in the high tertile myostatin group had a lower survival rate and larger number of HF hospitalization than those in the low tertile group (figure 3), and Cox regression analysis showed that serum myostatin was an independent predictor of mortality.15
Myocardial effects of some myokines altered in patients with heart failure
| Myokine | Effects on the cardiomyocyte | Effects on the interstitium | Effects on the coronarymirocirculationtion | Global myocardial pro- or antiremodeling effects |
|---|---|---|---|---|
| Irisin | Inhibits cardiomyocyte hypertrophy and apoptosis | Unknown | Protects endothelial function | Antiremodeling |
| BDNF | Unknown | Unknown | Stimulates angiogenesis | Antiremodeling |
| Interleukin-15 | Inhibits cardiomyocyte apoptosis | Stimulates inflammation | Activates endothelium | Inconclusive orproremodeling |
| Decorin | Inhibits cardiomyocyte hypertrophy | Stimulates fibrosis | Unknown | Proremodeling |
| Myonectin | Inhibits cardiomyocyte apoptosis | Inhibits inflammation | Protects endothelial function | Antiremodeling |
| Myostatin | Stimulates cardiomyocyte autophagy and apoptosis | Stimulates fibrosis | Unknown | Proremodeling |
| FGF-21 | Inhibits cardiomyocyte hypertrophy and apoptosis | Unknown | Unknown | Antiremodeling |
| GDF-11 | Inhibits cardiomyocyte hypertrophy and apoptosis | Unknown | Unknown | Antiremodeling |
| Interleukin-8 | Unknown | Stimulates inflammation | Activates endothelium | Proremodeling |
| Osteonectin | Unknown | Stimulates inflammation and fibrosis | Unknown | Proremodeling |
Association of serum myostatin tertiles with number of nonsurvivors and heart failure-related hospitalizations. The mean [± standard deviation] length of follow-up was 51.40 [± 15.01] months. HF, heart failure. Adapted with permission from Chen et al.15.
Most of the evidence regarding physical exercise in HF is derived from studies implementing exercise training programs that are considered safe and highly recommended in stable patients on optimal medical therapy.17 Although meta-analyses of these studies have demonstrated significant but modest improvements in exercise tolerance and quality of life, they have failed to prove significant beneficial effects on all-cause and HF-specific mortality and hospitalization.14 Strikingly, the prescription of physical exercise in HF patients is not based on objective criteria for the individualization of its modality (either aerobic and/or resistance) or its dose (ie, frequency, intensity, duration, mode, and progression).17
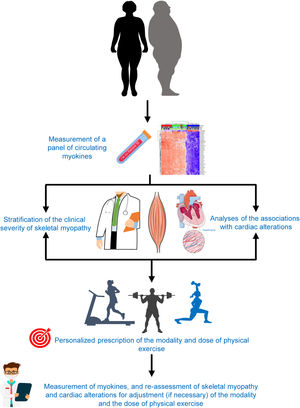
Myokines, at least those currently measurable in blood, offer the possibility of acting as biomarkers useful to personalize the prescription of physical exercise in HF patients (figure 4). Indeed, having a panel of measurements of the most representative myokines would help to individualize the modality and dose of physical exercise in each HF patient with the triple aim of increasing exercise tolerance, improving lipid and glucose metabolism, and protecting the myocardium from remodeling.
As a hypothetical example of this approach and based on the data presented in figure 2 and table 1 for a HF patient with low brain-derived neurotropic factor and high myostatin, either a cardiovascular or more specifically muscle strengthening exercise program (either acute or regular) would be indicated to both stimulate brain-derived neurotropic factor and inhibit myostatin and thus achieve greater exercise tolerance and myocardial protection.
CONCLUSIONS AND PERSPECTIVESTo date, it has been suggested that myokines may be useful biomarkers for monitoring exercise prescription for people with, for example, cancer, diabetes, or neurodegenerative diseases.7 The data discussed here also suggest that their assessment may provide new insights into the pathophysiology of HF-related skeletal myopathy and serve as biomarkers for the stratification of this condition in HF patients. In addition, they may be useful to personalize the modality and dose of physical exercise to be prescribed in HF patients.
Therefore, it is not surprising that the pharmacology of myokines is an active field. However, there are several challenges and limitations currently facing the therapeutic development of myokines or myokine secretagogues and/or analogs. First, myokines—by their very definition—are proteins or peptides, and therefore significant hurdles facing the use of biologics as therapies must be overcome. Protein biopharmaceuticals have become widely available after the rapid development of recombinant DNA technology over the past few decades. The most important problems to be overcome in developing proteins for therapeutic administration include their physicochemical instability (in particular, aggregation), limited solubility, proteolytic instability, short plasma half-life, immunogenicity, and toxicity. Nevertheless, it is important to mention that there are currently several ongoing trials testing molecules derived from myostatin (see ClinicalTrials.gov), hypothesizing that myostatin inhibition may potentially offer a therapeutic option to counteract skeletal muscle wasting and, in turn, increase exercise tolerance in clinical conditions other than HF. This opportunity could be offered to HF patients in the future, but more focused research is needed.
Therefore, the authors of this editorial strongly believe that further research on myokines and a better understanding of their synthesis and secretion, functions, mechanisms of action and downstream pathways in experimental and clinical HF is likely to lead to the identification of novel physical exercise-based therapeutic approaches for HF patients, namely for those with HFpEF who usually have metabolic disorders and for which exercise programs are a cornerstone in their holistic management.18 Importantly, establishing molecular links between exercise and improved HF management can only strengthen the scientific objective of precision medicine-based management of HF, as well as the public message that myokine-guided physical exercise results in a better quality of life and probably an improved prognosis of HF patients, and contributes to a better sustainability of the health system in terms of cost-effectiveness.
FUNDINGThe present article received no funding.
CONFLICTS OF INTERESTNone of the authors declare a conflict of interest with respect to this article.




![Association of serum myostatin tertiles with number of nonsurvivors and heart failure-related hospitalizations. The mean [± standard deviation] length of follow-up was 51.40 [± 15.01] months. HF, heart failure. Adapted with permission from Chen et al.15. Association of serum myostatin tertiles with number of nonsurvivors and heart failure-related hospitalizations. The mean [± standard deviation] length of follow-up was 51.40 [± 15.01] months. HF, heart failure. Adapted with permission from Chen et al.15.](https://static.elsevier.es/multimedia/18855857/0000007400000012/v4_202203130557/S1885585721001791/v4_202203130557/en/main.assets/thumbnail/gr3.jpeg?xkr=eyJpdiI6IjFuZ3cwTXBtdnNOdFdwRU5vZCtBWEE9PSIsInZhbHVlIjoiTURCUzNiRXMveDdxRDNHdlVDSVRnTGJhYmhESWR0TzZXNDBKVHhHdzdQdz0iLCJtYWMiOiJkMjIyZjY4ZTk5NWU1M2Q5YzQ5MjliYzJiNWI3NGQ3ZGNkYzk2ZjQwMzY4NTEwYzczYTA0ZGNmODVhY2JlNDAwIiwidGFnIjoiIn0=)
