The influence of hemoglobin kinetics on outcomes in heart failure has been incompletely established.
MethodsHemoglobin was determined at the first visit and at 6 months. Anemia was defined according to World Health Organization criteria (hemoglobin < 13g/dL for men and hemoglobin < 12g/dL for women). Patients were classified relative to their hemoglobin values as nonanemic (both measurements normal), transiently anemic (anemic at the first visit but not at 6 months), newly anemic (nonanemic initially but anemic at 6 months), or permanently anemic (anemic in both measurements).
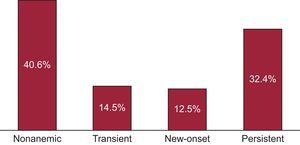
ResultsA total of 1173 consecutive patients (71.9% men, mean age 66.8±12.2 years) were included in the study. In all, 476 patients (40.6%) were considered nonanemic, 170 (14.5%) had transient anemia, 147 (12.5%) developed new-onset anemia, and 380 (32.4%) were persistently anemic. During a follow-up of 3.7±2.8 years after the 6-month visit, 494 patients died. On comprehensive multivariable analyses, anemia (P < .001) and the type of anemia (P < .001) remained as independent predictors of all-cause mortality. Compared with patients without anemia, patients with persistent anemia (hazard ratio [HR] = 1.62; 95% confidence interval [95%CI], 1.30-2.03; P < .001) and new-onset anemia (HR = 1.39; 95%CI, 1.04-1.87, P = .03) had higher mortality, and even transient anemia showed a similar trend, although without reaching statistical significance (HR = 1.31; 95%CI, 0.97-1.77, P = .075).
ConclusionsAnemia, especially persistent and of new-onset, and to a lesser degree, transient anemia, is deleterious in heart failure.
Keywords
Despite outstanding therapeutic advances over the past 2 decades, heart failure (HF) is first among cardiovascular diseases in terms of mortality and the presence of coexisting comorbidities. Anemia is a frequent comorbidity among HF patients and ranges from 5% to 55% in this patient group, depending on the cutpoint used to define anemic status,1,2 and is associated with poor outcomes.3–5 In a systematic review of the literature, Groenveld et al6 showed that the presence of anemia carries a 2-fold higher risk of all-cause mortality with a linear relationship between lower values of hemoglobin and mortality. Moreover, patients with anemia have worse exercise tolerance and greater functional impairment,7,8 an effect that seems to be similar in both patients with preserved ejection fraction and reduced ejection fraction.9
The etiology of anemia in HF is multifactorial, and many contributing factors have been proposed,10–12 including reduced intestinal absorption, increased inflammatory cytokines, hemodilution, renal impairment, decreased erythropoietin production, and loss of transferrin via proteinuria. Treatment of anemia with iron supplementation or erythropoietin has failed to improve mortality in randomized controlled trials,13–15 yet benefits have been reported in functional class and reduction in hospitalization for worsening HF.16,17 Indeed, little is known about hemoglobin kinetics in HF, and, at present, whether changes in anemic status influence outcomes during long-term follow-up is incompletely understood.2,18,19 Accordingly, the objective of our study was to examine whether changes in anemic status over 6 months affect long-term survival in a cohort of HF outpatients followed-up in a structured HF clinic.
METHODSStudy PopulationAll consecutive ambulatory patients referred to a structured HF clinic of a university hospital from 1 August 2001 to 31 December 2012, regardless of etiology, were included in an outpatient setting. The criteria for clinical practice referral to the HF unit have been reported elsewhere.20,21 Briefly, the criteria were HF with at least 1 hospitalization and/or reduced left ventricular ejection fraction (LVEF) < 40%. Most patients were referred from cardiology and internal medicine departments, and a few were from the emergency room/short-stay unit or other hospital departments. Less than 10% of patients were admitted to the HF unit for asymptomatic reduced LVEF after acute myocardial infarction. Two patients were excluded due to valvular surgical repair between baseline and 6 months.
All patients were seen regularly during follow-up visits at the HF clinic according to their clinical needs. Follow-up visits included a minimum of 1 visit from a nurse every 3 months and 1 visit from a physician (cardiologist, internist, or family physician) every 6 months, as well as optional visits from specialists in geriatrics, psychiatry, and rehabilitation.20,21 During the baseline visit, patients provided written consent for analytical samples and the use of their clinical data for research purposes.
The study was performed in compliance with the law protecting personal data in accordance with the international guidelines on clinical investigation of the World Medical Association's Declaration of Helsinki.
Death AssessmentThe main outcome was death from all causes. The number and causes of death during follow-up were obtained from clinical records at the HF unit, other hospital departments, other hospital records, or by contacting the patient's relatives. Data were verified using the databases of the Catalan and Spanish health systems. Five patients were lost during follow-up and were adequately censored in the survival analysis.
Anemia DefinitionHemoglobin was determined at first visit and at 6 months. Anemia was defined according to World Health Organization (WHO) criteria (hemoglobin < 13g/dL for men and < 12g/dL for women). Patients were classified relative to their hemoglobin values as nonanemic (both measurements normal), transiently anemic (anemic at the first visit but not at 6 months), newly anemic (nonanemic initially but anemic at 6 months), or persistently anemic (anemic in both measurements). Resolution of anemia was defined as normalization of hemoglobin levels (≥ 13g/dL for men and ≥ 12g/dL for women).
Statistical AnalysisCategorical variables are expressed as frequencies and percentages. Continuous variables are expressed as mean±standard deviation or as median [interquartile range] for cases with skewed distribution. Normal distribution was assessed with normal Q-Q plots. Statistical differences between groups were assessed using the chi-square test for categorical variables, Student t test for continuous variables with normal distribution, or the Mann-Whitney U test for nonnormal distributions. Univariate Cox proportional hazards regression analysis was performed using all-cause mortality as the dependent variable and the anemia prespecified subgroup as the independent variable. Proportional assumptions needed to use Cox proportional hazard regression models were tested for all variables. To fulfil the assumption of linearity, the logarithmic function of the covariable HF duration was used. A multivariable Cox proportional hazards model was also created with the same dependent and independent variables and different covariables were included in the model due to their significance in the univariate analysis or because they were considered clinically significant: age, sex, New York Heart Association (NYHA) functional class, duration of HF, etiology of HF, LVEF, diabetes mellitus, hypertension, atrial fibrillation, renal failure, chronic obstructive pulmonary disease, peripheral vasculopathy, heart rate, systolic blood pressure, and treatments (backward stepwise). Statistical analyses were performed using SPSS 15 (SPSS Inc.; Chicago, Ilinois, United States). A 2-sided P < .05 was considered statistically significant.
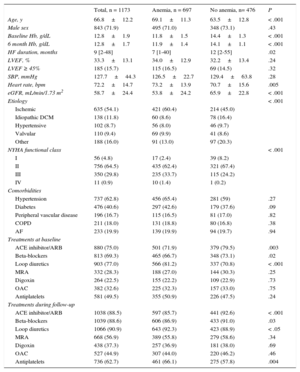
RESULTSFrom August 2001 to December 2012, this study included 1173 consecutive patients. Table 1 provides the demographic, clinical, and biochemical data at enrollment, as well as treatment during follow-up. Mean age was 66.8±12.2 years, and patients were predominantly male (71.9%). The main etiology of HF was ischemic heart disease (54.1%), and most patients were in NYHA functional classes II (64.5%) and III (29.8%) at inclusion. Most patients had depressed systolic function (mean LVEF 33.3%±13.1%) while LVEF ≥ 45% was present in 15.6% of the patients. Most of our patients were treated with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, and loop diuretics at baseline, and this figure increased during follow-up (Table 1). Mean hemoglobin level at the first visit was 13.1±1.9g/dL for men and 12.1±1.6g/dL for women.
Demographic and Clinical Characteristics
| Total, n = 1173 | Anemia, n = 697 | No anemia, n= 476 | P | |
|---|---|---|---|---|
| Age, y | 66.8±12.2 | 69.1±11.3 | 63.5±12.8 | < .001 |
| Male sex | 843 (71.9) | 495 (71.0) | 348 (73.1) | .43 |
| Baseline Hb, g/dL | 12.8±1.9 | 11.8±1.5 | 14.4±1.3 | < .001 |
| 6 month Hb, g/dL | 12.8±1.7 | 11.9±1.4 | 14.1±1.1 | < .001 |
| HF duration, months | 9 [2-48] | 7 [1-40] | 12 [2-55] | .02 |
| LVEF, % | 33.3±13.1 | 34.0±12.9 | 32.2±13.4 | .24 |
| LVEF ≥ 45% | 185 (15.7) | 115 (16.5) | 69 (14.5) | .32 |
| SBP, mmHg | 127.7±44.3 | 126.5±22.7 | 129.4±63.8 | .28 |
| Heart rate, bpm | 72.2±14.7 | 73.2±13.9 | 70.7±15.6 | .005 |
| eGFR, mL/min/1.73 m2 | 58.7±24.4 | 53.8±24.2 | 65.9±22.8 | < .001 |
| Etiology | < .001 | |||
| Ischemic | 635 (54.1) | 421 (60.4) | 214 (45.0) | |
| Idiopathic DCM | 138 (11.8) | 60 (8.6) | 78 (16.4) | |
| Hypertensive | 102 (8.7) | 56 (8.0) | 46 (9.7) | |
| Valvular | 110 (9.4) | 69 (9.9) | 41 (8.6) | |
| Other | 188 (16.0) | 91 (13.0) | 97 (20.3) | |
| NYHA functional class | < .001 | |||
| I | 56 (4.8) | 17 (2.4) | 39 (8.2) | |
| II | 756 (64.5) | 435 (62.4) | 321 (67.4) | |
| III | 350 (29.8) | 235 (33.7) | 115 (24.2) | |
| IV | 11 (0.9) | 10 (1.4) | 1 (0.2) | |
| Comorbidities | ||||
| Hypertension | 737 (62.8) | 456 (65.4) | 281 (59) | .27 |
| Diabetes | 476 (40.6) | 297 (42.6) | 179 (37.6) | .09 |
| Peripheral vascular disease | 196 (16.7) | 115 (16.5) | 81 (17.0) | .82 |
| COPD | 211 (18.0) | 131 (18.8) | 80 (16.8) | .38 |
| AF | 233 (19.9) | 139 (19.9) | 94 (19.7) | .94 |
| Treatments at baseline | ||||
| ACE inhibitor/ARB | 880 (75.0) | 501 (71.9) | 379 (79.5) | .003 |
| Beta-blockers | 813 (69.3) | 465 (66.7) | 348 (73.1) | .02 |
| Loop diuretics | 903 (77.0) | 566 (81.2) | 337 (70.8) | < .001 |
| MRA | 332 (28.3) | 188 (27.0) | 144 (30.3) | .25 |
| Digoxin | 264 (22.5) | 155 (22.2) | 109 (22.9) | .73 |
| OAC | 382 (32.6) | 225 (32.3) | 157 (33.0) | .75 |
| Antiplatelets | 581 (49.5) | 355 (50.9) | 226 (47.5) | .24 |
| Treatments during follow-up | ||||
| ACE inhibitor/ARB | 1038 (88.5) | 597 (85.7) | 441 (92.6) | < .001 |
| Beta-blockers | 1039 (88.6) | 606 (86.9) | 433 (91.0) | .03 |
| Loop diuretics | 1066 (90.9) | 643 (92.3) | 423 (88.9) | < .05 |
| MRA | 668 (56.9) | 389 (55.8) | 279 (58.6) | .34 |
| Digoxin | 438 (37.3) | 257 (36.9) | 181 (38.0) | .69 |
| OAC | 527 (44.9) | 307 (44.0) | 220 (46.2) | .46 |
| Antiplatelets | 736 (62.7) | 461 (66.1) | 275 (57.8) | .004 |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation/flutter; ARB, angiotensin receptor blockers; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralcorticoid receptor antagonist; NYHA, New York Heart Association; OAC, oral anticoagulants; SBP, systolic blood pressure.
Data are expressed as no. (%), mean±standard deviation or median [interquartile range].
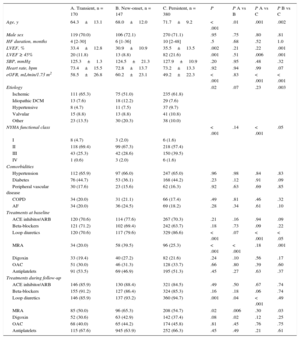
At baseline, anemia was present in 550 patients (46.9%). One quarter of anemic patients (14.5% of total patients) had normalized hemoglobin levels at 6 months (transient anemia), and a similar proportion of patients had developed new anemia (12.5% of the cohort) at 6 months, while 32.4% of patients were persistently anemic (Figure 1). Patients with any kind of anemia (n = 697, 59.4%) were older (P < .001), had a lower glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration formula) (P < .001), were in a worse functional class (P < .001), and had a lower duration of HF (Table 1). No significant differences were found between groups regarding the etiology of HF or associated comorbidities. On the other hand, the use of an angiotensin-converting enzyme inhibitor (P < .001) and beta-blockers (P = .03) was lower among patients with anemia during follow-up (Table 1). Table 2 shows the differences in demographic and clinical characteristics among anemic patients based on the prespecified categories of anemia. Remarkably, patients with persistent anemia were older and had a worse functional class, better LVEF, and lower estimated glomerular filtration rate.
Differences Among Categories of Anemia
| A. Transient, n = 170 | B. New-onset, n = 147 | C. Persistent, n = 380 | P | P A vs B | P A vs C | P B vs C | |
|---|---|---|---|---|---|---|---|
| Age, y | 64.3±13.1 | 68.0±12.0 | 71.7±9.2 | < .001 | .01 | .001 | .002 |
| Male sex | 119 (70.0) | 106 (72.1) | 270 (71.1) | .95 | .75 | .80 | .81 |
| HF duration, months | 4 [2-30] | 6 [1-36] | 10 [2-48] | .5 | .68 | .52 | 1.0 |
| LVEF, % | 33.4±12.8 | 30.9±10.9 | 35.5±13.5 | .002 | .21 | .22 | .001 |
| LVEF ≥ 45% | 20 (11.8) | 13 (8.8) | 82 (21.6) | .001 | .51 | .006 | .001 |
| SBP, mmHg | 125.3±1.3 | 124.5±21.3 | 127.9±10.9 | .20 | .95 | .48 | .32 |
| Heart rate, bpm | 73.4±15.5 | 72.8±13.7 | 73.2±13.3 | .92 | .94 | .99 | .07 |
| eGFR, mL/min/1.73 m2 | 58.5±26.8 | 60.2±23.1 | 49.2±22.3 | < .001 | .83 | < .001 | < .001 |
| Etiology | .02 | .07 | .23 | .003 | |||
| Ischemic | 111 (65.3) | 75 (51.0) | 235 (61.8) | ||||
| Idiopathic DCM | 13 (7.6) | 18 (12.2) | 29 (7.6) | ||||
| Hypertensive | 8 (4.7) | 11 (7.5) | 37 (9.7) | ||||
| Valvular | 15 (8.8) | 13 (8.8) | 41 (10.8) | ||||
| Other | 23 (13.5) | 30 (20.3) | 38 (10.0) | ||||
| NYHA functional class | < .001 | .14 | < .001 | .05 | |||
| I | 8 (4.7) | 3 (2.0) | 6 (1.6) | ||||
| II | 118 (69.4) | 99 (67.3) | 218 (57.4) | ||||
| III | 43 (25.3) | 42 (28.6) | 150 (39.5) | ||||
| IV | 1 (0.6) | 3 (2.0) | 6 (1.6) | ||||
| Comorbidities | |||||||
| Hypertension | 112 (65.9) | 97 (66.0) | 247 (65.0) | .96 | .98 | .84 | .83 |
| Diabetes | 76 (44.7) | 53 (36.1) | 168 (44.2) | .23 | .12 | .91 | .09 |
| Peripheral vascular disease | 30 (17.6) | 23 (15.6) | 62 (16.3) | .92 | .63 | .69 | .85 |
| COPD | 34 (20.0) | 31 (21.1) | 66 (17.4) | .49 | .81 | .46 | .32 |
| AF | 34 (20.0) | 36 (24.5) | 69 (18.2) | .28 | .34 | .61 | .10 |
| Treatments at baseline | |||||||
| ACE inhibitor/ARB | 120 (70.6) | 114 (77.6) | 267 (70.3) | .21 | .16 | .94 | .09 |
| Beta-blockers | 121 (71.2) | 102 (69.4) | 242 (63.7) | .18 | .73 | .09 | .22 |
| Loop diuretics | 120 (70.6) | 117 (79.6) | 329 (86.6) | < .001 | .07 | < .001 | < .05 |
| MRA | 34 (20.0) | 58 (39.5) | 96 (25.3) | < .001 | < .001 | .18 | .001 |
| Digoxin | 33 (19.4) | 40 (27.2) | 82 (21.6) | .24 | .10 | .56 | .17 |
| OAC | 51 (30.0) | 46 (31.3) | 128 (33.7) | .66 | .80 | .39 | .60 |
| Antiplatelets | 91 (53.5) | 69 (46.9) | 195 (51.3) | .45 | .27 | .63 | .37 |
| Treatments during follow-up | |||||||
| ACE inhibitor/ARB | 146 (85.9) | 130 (88.4) | 321 (84.5) | .49 | .50 | .67 | .74 |
| Beta-blockers | 155 (91.2) | 127 (86.4) | 324 (85.3) | .16 | .18 | .06 | .74 |
| Loop diuretics | 146 (85.9) | 137 (93.2) | 360 (94.7) | .001 | .04 | < .001 | .49 |
| MRA | 85 (50.0) | 96 (65.3) | 208 (54.7) | .02 | .006 | .30 | .03 |
| Digoxin | 52 (30.6) | 63 (42.9) | 142 (37.4) | .08 | .02 | .12 | .25 |
| OAC | 68 (40.0) | 65 (44.2) | 174 (45.8) | .81 | .45 | .76 | .75 |
| Antiplatelets | 115 (67.6) | 945 (63.9) | 252 (66.3) | .45 | .49 | .21 | .61 |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation/flutter; ARB, angiotensin blockers; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineral-corticoid receptor antagonist; NYHA, New York Heart Association; OAC, oral anticoagulants; SBP, systolic blood pressure.
Data are expressed as no. (%), mean±standard deviation or median [interquartile range].
Patients with new-onset anemia differed from those with transient anemia only in that they were older and received more furosemide, mineralcorticoid receptor antagonists, and digoxin, the latter 3 probably reflecting worse clinical status despite similar NYHA functional class.
Anemia Category and SurvivalA total of 494 (42.1%) patients died during a mean follow-up of 3.7±2.8 years after the 6-month visit. Causes of death were worsening HF in 147 patients (29.8%), sudden death in 58 patients (11.7%), acute myocardial infarction in 36 patients (7.3%), stroke in 16 (3.2%), cardiovascular procedure in 8 (1.6%), other cardiovascular causes in 28 patients (5.7%), noncardiovascular causes in 168 patients (34.0%), and unknown cause in 33 patients (6.7%). Mortality was significantly higher in the presence of anemia (any type) (hazard ratio [HR] = 2.08; 95% confidence interval [95%CI], 1.71-2.53; P < .001). In a sensitivity analysis, the presence of anemia at baseline based on the WHO criteria showed an independent association with mortality in the comprehensive multivariable analysis: HR = 1.39; 95%CI, 1.15-1.67; P = .001.
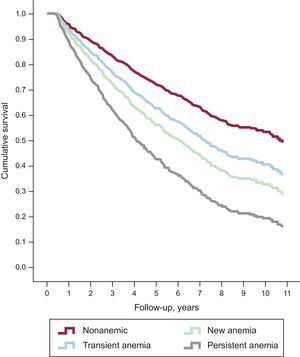
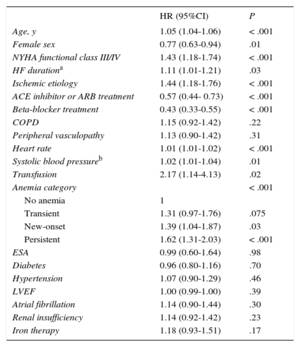
When anemia strata were taken into account, the worst prognosis was for persistent anemia (HR = 2.59; 95%CI, 2.09-3.20; P < .001), followed by new-onset anemia (HR = 1.75; 95%CI, 1.31-2.34; P < .001) and transient anemia (HR = 1.42; 95%CI, 1.06-1.91; P = .02) (Figure 2). On comprehensive multivariable Cox regression analysis, including as covariates age, sex, NYHA functional class, HF duration, HF etiology, LVEF, diabetes mellitus, hypertension, atrial fibrillation, renal failure, chronic obstructive pulmonary disease, peripheral vasculopathy, heart rate, blood systolic pressure and treatments during follow-up, the type of anemia was an independent predictor of all-cause mortality (P < .001) (Table 3).
Nonadjusted survival curves according to anemia strata. The worst prognosis was observed for persistent anemia (HR = 2.06; 95%CI, 2.10-3.21; P < .001), followed by new-onset anemia (HR = 1.76; 95%CI, 1.32-2.35; P < .001) and transient anemia (HR = 1.42; 95%CI, 1.06-1.91; P = .02). 95%CI, 95% confidence interval; HR, hazard ratio.
Multivariable Cox Regression Analysis for Risk of All-cause Death
| HR (95%CI) | P | |
|---|---|---|
| Age, y | 1.05 (1.04-1.06) | < .001 |
| Female sex | 0.77 (0.63-0.94) | .01 |
| NYHA functional class III/IV | 1.43 (1.18-1.74) | < .001 |
| HF durationa | 1.11 (1.01-1.21) | .03 |
| Ischemic etiology | 1.44 (1.18-1.76) | < .001 |
| ACE inhibitor or ARB treatment | 0.57 (0.44- 0.73) | < .001 |
| Beta-blocker treatment | 0.43 (0.33-0.55) | < .001 |
| COPD | 1.15 (0.92-1.42) | .22 |
| Peripheral vasculopathy | 1.13 (0.90-1.42) | .31 |
| Heart rate | 1.01 (1.01-1.02) | < .001 |
| Systolic blood pressureb | 1.02 (1.01-1.04) | .01 |
| Transfusion | 2.17 (1.14-4.13) | .02 |
| Anemia category | < .001 | |
| No anemia | 1 | |
| Transient | 1.31 (0.97-1.76) | .075 |
| New-onset | 1.39 (1.04-1.87) | .03 |
| Persistent | 1.62 (1.31-2.03) | < .001 |
| ESA | 0.99 (0.60-1.64) | .98 |
| Diabetes | 0.96 (0.80-1.16) | .70 |
| Hypertension | 1.07 (0.90-1.29) | .46 |
| LVEF | 1.00 (0.99-1.00) | .39 |
| Atrial fibrillation | 1.14 (0.90-1.44) | .30 |
| Renal insufficiency | 1.14 (0.92-1.42) | .23 |
| Iron therapy | 1.18 (0.93-1.51) | .17 |
95%CI, 95% confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; COPD, chronic obstructive pulmonary disease; ESA, erythropoiesis-stimulating agents; HF, heart failure; HR, hazard ratio; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Anemia was studied in two thirds of the patients. Fifty percent of the patients with baseline anemia had an iron deficiency component (defined as ferritin < 100μg/dL or < 300μg/dL with transferrin saturation index < 20%) while the rest had chronic disease or renal failure-associated anemia. Only 199 (17%) patients of the total cohort (one third of anemic patients) received any treatment to resolve the anemia or to restore hemoglobin levels during the first 6 months between the 2 hemoglobin samples, and iron supplementation was the most common treatment (82% of the treated patients). Erythropoietin was used in 6.5%, and transfusion was needed in 4.5% of anemic patients. Of interest, iron supplementation and the use of erythropoietin were not independently related to survival, but blood transfusion doubled the risk of death in the multivariable analysis (Table 3).
DISCUSSIONTreatment of HF has shown a remarkable spectrum of improvement, tackling the myocardium, electrical system, and neurohormonal activation, with eventually limited room for further optimization if attention is restricted to the heart only. Thus, we must now look into the comorbidities that also affect prognosis for HF patients. These patients have a high prevalence of anemia,2 with variable numbers according to the cutpoint used to define anemia and the setting where anemia is studied (chronic or acutely decompensated HF).3,22 In our cohort of 1173 consecutive real-life ambulatory patients, we found an anemia prevalence of 47%, using the WHO definition of anemia.
In the last 2 decades, the relationship between anemia and HF prognosis has been amply studied, yet hemoglobin kinetics and outcomes during follow-up have remained unexplored. In this work, the sole presence of anemia was associated with higher mortality, but hemoglobin kinetics and thus the type of anemia were also relevant for outcomes. The anemia strata as defined by hemoglobin measurements 6 months apart remained statistically significant with prognosis, even accounting for known confounders and medical therapy in the multivariable analysis. A single-center retrospective study also evaluated the long-term prognosis of consecutive patients with HF according to the presence and dynamics of anemia.2 Anemia was found in only 17.2% of patients, although the criteria used for anemia definition (hemoglobin < 12g/dL for men and < 11g/dL for women) were stricter than ours (WHO criteria). Of interest, in that study 43% of the patients had experienced resolution of anemia at 6 months while 16% of nonanemic patients at baseline developed anemia in the first 6 months. When the 2 studies were compared, beyond differences in anemia prevalence, in our study, fewer patients had transient anemia, probably because of differences in population clinical characteristics (older and with more comorbidities, especially renal insufficiency in our cohort). In contrast, the proportion of patients who developed new-onset anemia in the first 6 months of follow-up in our cohort was quite similar to that described by Tang et al.2 As expected, persistent anemia was related to the highest mortality rates in both studies.
Tang et al2 also found that patients with transient anemia showed similar outcomes to patients without anemia, whereas in our study, transient anemia was associated with increased mortality. No clear-cut explanation emerges for this divergence, but it may well be that transient anemia, despite current recommended treatment, acts as a mirror of more severe underlying disease.
Although anemia is widely studied, its origin in HF is not completely understood,11,23–25 and most HF patients with anemia are not completely characterized. Anemia is considered to develop because of a complex interaction between iron deficiency, kidney disease, cytokine production, and blood loss, which may interact with absorption and lead to a nutrient insufficiency.10 In this study, the most common cause of anemia was iron deficiency. Moreover, the question of whether treating anemia may improve outcomes for these patients remains controversial, and several trials have failed to show improvement in mortality rates in different HF populations, although some showed clinical benefits in selected populations.15,26,27 Recently, treating iron deficiency with intravenous iron was reported to have a clinical benefit, although the effect on survival has not been evaluated.28 In our study (started before the current “intravenous iron treatment era”), less than a fifth of anemic patients received specific treatment to restore hemoglobin levels. Remarkably, most of the patients whose anemia resolved received only conventional HF treatment. On the other hand, patients receiving blood transfusion showed worse outcomes, whereas no increased risk of death was detected as associated with the other anemia treatments.
Study LimitationsWe used only 2 measures of hemoglobin 6 months apart without more information about changes in hemoglobin levels during the remaining follow-up. Furthermore, we used a fixed cutoff value for anemia definition (even with sex-specific cutoffs). As in all published studies based on 2 clinical or analytical determinations, our analyses were performed in “completers,” that is, patients with both baseline and 6-month hemoglobin data available for analysis. Therefore, those patients that died before the 6 month visit were not included in the study, which could have introduced an unavoidable bias in the analysis performed. It is not possible to predict the association of changes in hemoglobin with prognosis in “noncompleters”.
Although our study cohort was formed by an unselected population of HF patients treated in a specific multidisciplinary HF unit of a tertiary hospital, most of them were referred from the cardiology ward. Patients were predominantly male, and the most common cause of HF was ischemic heart disease. Therefore, the results of this study must be interpreted carefully when considering the overall population.
CONCLUSIONSIn a large cohort of consecutive real-life ambulatory HF patients, persistent, new, and even transient anemia was deleterious in HF during a long-term follow-up. More awareness in the treatment of anemia in HF seems recommended, and further studies are needed to clarify this issue.
CONFLICTS OF INTERESTNone declared.
- –
Anemia is a frequent comorbidity among HF patients, ranging from 5% to 55%, depending on clinical setting and the cutpoint used to define anemic status.
- –
Anemia carries worse outcome in HF patients: a two-fold higher risk of all-cause mortality with a linear relationship between lower values of hemoglobin and mortality have been described.
- –
Little is known about hemoglobin kinetics in HF, and at present whether changes in anemic status influence outcomes during long-term follow-up is incompletely understood.
- –
The anemia strata based on hemoglobin measurements (no anemia, transient anemia, new-onset anemia, and persistent anemia) remained statistically significant with prognosis, even accounting for known confounders and medical therapy.
- –
The worst prognosis was observed in persistently anemic patients followed by patients with new-onset anemia.
- –
Transient anemia was also associated with increased mortality, although in the multivariable analysis only achieved borderline significance.
- –
Anemia resolved in 24% of anemic patients. Most of them received only conventional HF treatment. Patients receiving blood transfusion showed worse outcomes whereas no increased risk of death was associated with the other anemia treatments.