.
IntroductionCardiac resynchronization therapy (CRT) is a nonpharmacological treatment alternative for patients with drug-refractory mild, moderate or severe heart failure (HF); New York Heart Association (NYHA) class I, II, III, and IV; prolonged QRS duration; and severely depressed left ventricular ejection fraction (LVEF). Clinical trials1, 2, 3, 4, 5, 6, 7, 8, 9, 10 showed that CRT reduces HF hospitalizations, decreases mortality, and improves the quality of life and cardiac function, described as left ventricular (LV) reverse remodeling. However, the number of patients who do not respond to this therapy remains as high as 30% to 35%.11
Clinical trials evaluating the effects of CRT have used different outcome measures throughout time. Early clinical trials used clinical parameters such as NYHA functional class, 6-min walk test, and quality of life assessments. Clinical end point was the decrease in HF hospitalizations.7 The clinical parameters are usually assessed subjectively by physicians and may not be related to long-term mortality benefit. The NYHA functional class assessment seems to be a reliable measure of functional status in patients with cardiac disease; however, its reproducibility is not yet established.12 Evaluating the improvement of echocardiography parameters, and assessing LVEF and the decrease in end-diastolic and end-systolic LV volumes are measures that are more objective and are highly correlated with long-term mortality benefit.13, 14 It is accepted to use 15% reduction in LV end-systolic volume decrease to define responders; however, the number of nonresponders may be as high as 43%.13 It is important to stress that clinical and echocardiographic response do not always follow the same line, eg, patients with improvement of their clinical status might not always show significant reverse remodeling. Furthermore, individual changes in LVEF are often difficult to detect due to the high measurement variability. The Table summarizes the main characteristics of the major randomized CRT clinical trials.1, 2, 3, 4, 5, 6, 7, 8, 9, 10
Randomized Cardiac Resynchronization Therapy Clinical Trials
| Trials | Patients, no. | Female, % | Primary end points | Secondary end points | Etiology, ischemic % | LVEF, % | QRS, ms |
| PATH-CHF | 41 | 50 | 6MWT, peak VO2 | NHYA class, QOL, hospitalizations | 29 | 21±7 | 175 |
| MUSTIC-SR | 58 | 26 | 6MWT | NYHA, QOL, Peak VO2, MR, LV, hospitalizations, total mortality | 37 | 23±7 | 174 |
| MIRACLE | 453 | 32 | 6MWT, NHYA, QOL | Peak VO2, LVEF, LVEDD, MR, clinical composite response | 54 | 22±6 | 166 |
| MIRACLE ICD | 555 | 23 | 6MWT, NYHA, QOL | Peak VO2, LVEF, LV volumes, MR, clinical composite score | 70 | 24±6 | 164 |
| COMPANION | 1520 | 22 | All-cause mortality or hospitalization | All-cause mortality and cardiac mortality | 56 | 21 | 159 |
| CARE-HF | 814 | 26 | All-cause mortality | NYHA, QOL, LVEF, LVESV, hospitalization for heart failure | 38 | 25 | 160 |
| REVERSE | 610 | 21 | HF clinical composite score | LVESV | 54 | 27±7 | 153 |
| MADIT-CRT | 1820 | 25 | HF or death | LVESV, LVEDV change, multiple HF events | 57 | 24±5 | 162 |
| RAFT | 1798 | 17 | All-cause mortality or HF hospitalization | All-cause mortality, cardiac mortality, HF hospitalization | 67 | 23±5 | 158 |
6MWT, 6-min walk test; CARE-HF, Cardiac Resynchronization-Heart Failure; COMPANION, Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure; HF, heart failure; LV, left ventricular; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MADIT-CRT, Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy; MIRACLE, Multicenter InSync Randomized Clinical Evaluation; MIRACLE ICD, Multicenter InSync Implantable Cardioverter Defibrillator trial; MR, mitral regurgitation; MUSTIC, Multisite Simulation in Cardiomyopathies; NYHA, New York Heart Association; PATH-CHF, Pacing Therapies in Congestive Heart Failure trial; QOL, quality-of-life score; RAFT, Resynchronization-Defibrillation for Ambulatory Heart Failure; REVERSE, Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction; VO2, volume of oxygen.
With the following article, we would like to provide practical and comprehensive guidance for the clinician on how to manage the patient who does not improve after initiation of CRT and how to identify the reasons for nonresponse. However, a complete and comprehensive review of all the factors that are associated with nonresponse to CRT (eg, baseline clinical characteristics like age, sex, ischemic etiology, atrial fibrillation; comorbidities; lead placement, etc.)6, 8, 9 is beyond the scope of this manuscript and has been discussed in detail elsewhere.15
Clinical assesment of a CARDIAC RESYNCHRONIZATION THERAPY nonresponder patient Step 1. Check the Electrocardiogram With and Without PacingWhen assessing the patient with an implanted CRT device and an unsatisfactory clinical response to CRT, the first recommended step is to perform and analyze the 12-lead electrocardiogram (ECG), possibly with baseline ECG for direct comparison. If baseline ECG is not available, the acute effect of CRT on the electrical conduction sequence during active and inactive pacing can be compared, unless the patient is pacemaker-dependent. In pacemaker-dependent patients, the ECG with CRT pacing should be compared to conventional right ventricular apical pacing.
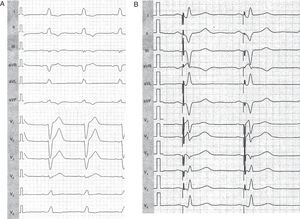
The first assessment should include the baseline QRS duration and morphology. If the baseline QRS duration without pacing is less than 150ms the patient is less likely to suffer from mechanical dyssynchrony and therefore also less likely to respond. Current guidelines recommend a relatively short QRS width of 120ms or above as an inclusion criterion for CRT. However, the mean QRS duration in the large randomized clinical trials was approximately 160ms (Table). Several studies showed that CRT is more effective in patients with broader QRS complex as they exhibit higher degrees of dyssynchrony. With respect to QRS morphology, a typical left bundle branch block (LBBB) pattern is associated with better clinical outcome, as reported in a recently published MADIT-CRT sub-analysis.16 LBBB patients experienced more benefit in terms of reduction of HF events or death with CRT (hazard ratio [HR]=0.047; P<.001) than non-LBBB patients (HR = 1.24; P=.257). The risk of ventricular arrhythmias or death was also significantly reduced by CRT in defibrillator patients with LBBB, but not in non-LBBB (right bundle branch block [RBBB] and intraventricular conduction delay). Corresponding to the previous findings, LV reverse remodeling and the improvement in LVEF were significantly greater among patients with LBBB compared to non-LBBB. Figure 1A shows typical LBBB in a 45-year-old female with nonischemic origin of dilated cardiomyopathy. After CRT, effective resynchronization is evident by the QRS axis change and the reduction of the QRS width (Figure 1B).
Figure 1. Electrocardiography of a 45-year-old female cardiac resynchronization therapy recipient with left bundle branch block A, before resynchronization; B, after cardiac resynchronization therapy implantation. Note the changes in leads I (new S wave) and V1 (new R wave) indicating initial activation from a left ventricular site.
The baseline and paced QRS width are easy to measure and serve as a basic parameter of CRT resynchronization. The more the QRS width is reduced by CRT, the more beneficial CRT therapy is. A recent substudy of the PROSPECT study showed that the difference in baseline vs paced QRS width predicted clinical outcome in CRT patients.17 However, it should be noted that reduction of QRS width by CRT is a specific but not very sensitive marker for CRT response. Many patients with no significant changes in QRS width might still favorably respond to CRT.
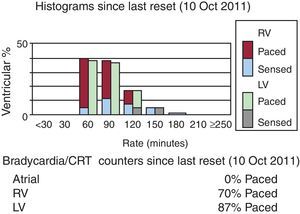
It is also important to evaluate whether the patient is in normal sinus rhythm or in atrial fibrillation. Atrial fibrillation is common in HF patients and in patients with implanted CRT. Newly onset episodes of paroxysmal or persistent atrial fibrillation are associated with worse outcome in CRT patients. Atrial fibrillation often leads to tachycardia with loss of LV capture or fusion/pseudofusion beats with ineffective resynchronization (Figure 2). If rhythm control is failing with cardioversion and/or antiarrhythmic therapy, it is crucial to control the ventricular rate in order to ensure biventricular capture. Some patients require atrioventricular (AV) node ablation to ensure 100% ventricular pacing and this aggressive approach has been shown to improve exercise tolerance, LV reverse remodeling, and LVEF with a significant survival benefit in this patient population. The role of pulmonary vein isolation (PVI) is still under debate. Some smaller studies showed benefit of this procedure in HF patients; however, none of them was conducted in CRT patients. Two large randomized trials, AMICA (NCT00652522) and CASTLE-AF (NCT00643188), are currently enrolling patients to evaluate the effects of pulmonary vein isolation in CRT recipients.
Figure 2. Cardiac resynchronization therapy in a patient with atrial fibrillation and evidence of loss of left ventricular capture resulting in ineffective resynchronization. Atrial lead was inactivated in this patient due to permanent atrial fibrillation. Ventricular histogram represents high ventricular rates and 87% of left ventricular pacing only as shown in brady/cardiac resynchronization theraphy counters at the bottom of the image. CRT, cardial resynchronization therapy; LV, left ventricular; RV, right ventricular.
Frequent ventricular ectopic beats, more frequently observed in patients with an ischemic origin of the cardiomyopathy, inhibit LV pacing and reduce the efficacy of CRT. Patients with a high burden of ventricular unifocal ectopic beats might be considered for catheter ablation; however, clinical data are still limited.
After determining the underlying rate and rhythm, we need to assess LV capture. LV noncapture is a common cause of CRT nonresponders. Beats with biventricular pacing are showing frontal plane QRS axis in the right superior quadrant and a dominant R wave in lead V1. If V1 has a negative QRS complex, LV loss of capture or a suboptimal LV lead position is suspected.
Fusion and pseudofusion beats are important to be recognized and corrected by shortening of the AV-delay to ensure 100% ventricular pacing.
Step 2. Check the Device (Device Interrogation)Device interrogation provides broad spectrum of information on the HF status of the patient. Atrial, right ventricular, and LV sensing and pacing parameters have to be checked. Noncapture is a common late complication of CRT and may result in no response. Phrenic nerve stimulation is also often observed in CRT recipients and in some cases might be associated with LV lead dislocation.
The percentage of LV pacing must be as high as 90% to ensure optimal CRT delivery. It might be lower in case of LV lead dislocation, paroxysmal or permanent atrial fibrillation, and frequent ventricular ectopic beats. Fusion and pseudofusion is often not detected by the device counter and the percentage of LV pacing is falsely reported as being normal (ie, >90%) by the device.
AV-delay and ventriculoventricular (VV)-delay assessment and optimization is essential in CRT nonresponders. It is recommended to perform AV-delay optimization in all patients, guided either by the device or by echocardiography; this will be discussed more in detail in Step 4. Data on VV-delay optimization and its role in CRT patients are limited and controversial. Most studies suggest LV pre-activation or simultaneous LV-right ventricular pacing to be optimal in CRT patients. CRT nonresponders should always be assessed and optimized with regard to VV-timing.
Integrated device sensors are continuously monitoring physical activity, heart rate variability, and parameters like the change in minute ventilation reflecting thoracic impedance that have been shown to provide clinically relevant information on the HF status of CRT patients. Heart rate variability is an effective measure representative of the severity of HF. When it is compared to a baseline reference, it reflects changes in LVEF and in LV filling pattern. Therefore, improvement in heart rate variability provides evidence of favorable CRT response.
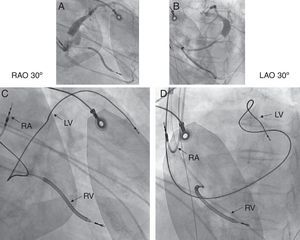
Step 3. Check the Lead PositionLV lead location is probably one of the most important contributing factors for CRT response; therefore it is crucial to assess the LV lead position in all CRT nonresponders. Chest X-ray images (posterior-anterior and lateral projection) or fluoroscopy are preferred to evaluate the LV lead location. The left anterior oblique view, representative of the short-axis view of the heart, helps to classify the LV wall into anterior, anterolateral, lateral, posterolateral, and posterior LV lead positions. The right anterior oblique view, which represents the long axis, is used to define basal, mid-ventricular, and apical lead positions. It is generally recommended to implant the LV lead in a basal to mid-lateral or posterolateral side-branch of the coronary sinus, if there is an eligible vein. Position of the anterior LV lead is associated with worse prognosis and nonresponse to CRT18 (Figure 3). However, a recent substudy published by Singh et al.19 has shown that LV lead location defined as lateral, posterior or anterior did not influence the clinical outcome in mild HF patients receiving CRT. Additionally, in this study, any apical LV lead location was associated with significantly higher risk of HF or death when compared to basal or mid-ventricular LV lead locations. This helps to understand that apical pacing in CRT might induce more heterogeneity in the LV activation and more dyssynchrony, and therefore should be avoided. However, the individual activation pattern might be highly variable even in LBBB patients and also in patients with RBBB or intraventricular conduction delay; therefore, there is a rationale for individually optimized CRT during the implantation.20
Figure 3. Cardiac resynchronization therapy patient with suboptimal, mid-anterior left ventricular lead position. A, coronary sinus venogram in right anterior oblique 30° view. B, coronary sinus venogram in left anterior oblique 30° view. C, final lead locations in right anterior oblique 30° view. D, final lead locations in left anterior oblique 30° view. Position of the right atrial lead is at the right atrial appendage, right ventricular lead at the right ventricular apex, left ventricular lead in the mid-anterior vein; external electrocardiogram electrodes are seen on the chest wall. LAO, left anterior oblique; LV, left ventricular lead; RA, right atrial lead; RAO, right anterior obliquer; RV, right ventricular lead. With kind permission of Prof. Helmut Klein, University of Rochester, New York, United States.
If the nonresponder patient has a suboptimal LV lead location, a second LV lead implantation is to be considered. Computed tomography scan might be a useful method to evaluate coronary sinus side-branches for the second LV lead implantation. If transvenous LV lead implantation is not possible, an epicardial approach via mini-thoracotomy might be considered. Some authors even suggest transseptal endocardial LV lead implantation. Transseptal endocardial LV lead implantation is performed with endocardial screw-in leads, which are passing by the interatrial septum and the mitral valve and are attached to the LV wall. Endocardial pacing has been demonstrated to be more physiologic in experimental and clinical observations as compared to epicardial pacing. Additionally, this technique has the advantage of positioning the LV lead within the LV cavity unrestricted by the coronary sinus branches. However, there are very limited data on the long-term safety and efficacy of this method. Patients require long-term anticoagulation and there is limited data on any risk of worsening mitral regurgitation (due to the transmitral lead position).
Some smaller studies suggested right ventricular lead location to play a role in CRT response. The right ventricular apical lead position has performed worse than higher right ventricular septal or outflow tract positions in some trials, which might be explained by the fact that apical pacing creates more heterogeneous activation and thus more dyssynchrony. Larger scale data are needed to further explore this.
Step 4. Check the Mechanical Resynchronization Effect by EchocardiographyEchocardiographic assessment immediately after the device implantation or during the follow-up procedures provides further information on the response and the nonresponders to CRT. The transmitral filling profile improves acutely in most patients with the initiation of CRT. However, if it remains too short (below 40%-45% of the corresponding cycle length) or altered, it can be optimized by changing the AV-delay of the device programming. The SMART AV trial showed that patients with normal AV-delay did not derive benefit from echo-guided or device-guided AV-optimization compared to the empiric settings; however, patients with prolonged AV-conduction were not included in this prospective, randomized study.21 Other data suggest patients with prolonged AV-interval to derive benefit from AV-delay optimization.22 If we have a nonresponder patient and pathologic transmitral filling parameters, AV-delay optimization is suggested to be done with the use of echocardiography, preferably. Of note, optimal AV-delay changes throughout time; therefore, additional assessment of transmitral filling pattern and AV-delay is recommended every 6 months.
Other important markers are the immediate decrease of functional mitral regurgitation and the acute increase of LV dP/dt.23 Acute hemodynamic changes with CRT can be monitored on a beat-to-beat basis by the initiation or cessation of LV pacing.
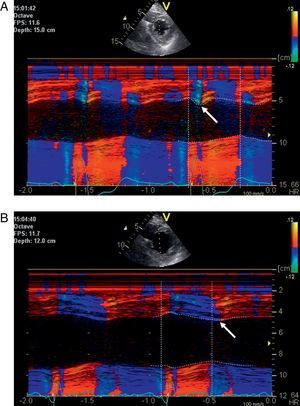
Presystolic septal flash is a sensitive marker of dyssynchrony and predicts a favorable response in CRT (Figure 4). If septal flash is seen in a CRT nonresponder that gives the diagnosis of severe dyssynchrony, which might be due to suboptimal lead positioning or suboptimal device settings; VV-optimization is recommended.
Figure 4. Echocardiography images of the patient from Figure 1 with (A) early systolic inward motion of the septum (“septal flash”, white arrow) before resynchronization therapy during left bundle branch block and (B) normalization of septal motion with disappearance of the septal flash and late maximal inward motion of the septum (white arrow) during active cardiac resynchronization therapy with slight left ventricular preactivation (−20ms).
Additional assessment includes the presence or elimination of LV interventricular and intraventricular mechanical dyssynchrony. Interventricular mechanical dyssynchrony is measured as the difference of the aortic and pulmonary pre-ejection interval. Its immediate decrease shows effective resynchronization. Intraventricular dyssynchrony can be evaluated by color-coded tissue Doppler imaging methods, measured as the standard deviation of the time from the onset of QRS to peak longitudinal velocities (Ts), recorded in the basal and mid segments of the inferoseptal, lateral, inferior, anterior, anteroseptal, and posterior walls in a 12-segment model. Some data suggest that 2-dimensional (2D) strain dyssynchrony based on 2D speckle tracking is a better and more reproducible measurement of dyssynchrony compared to tissue velocity imaging for monitoring changes in mechanical dyssynchrony with CRT. Three-dimensional (3D) echocardiographic techniques analyze regional wall motion with the use of regional displacements in 3D and consequently calculating 3D strain. Dyssynchrony is assessed with parametric imaging qualitatively or quantitatively as the sum of regional LV wall displacements.24 The 3D dyssynchrony measurement evaluates all segments of the LV and allows us to show isolated apical dyssynchrony patterns as well.
The presence or even worsening of intraventricular dyssynchrony is a common problem in CRT nonresponders. VV-optimization is a useful tool to correct intraventricular dyssynchrony by device programming. As optimal VV-delay settings are highly variable in CRT patients, echocardiography-guided VV-optimization is recommended. Common tissue Doppler imaging dyssynchrony indexes do not clearly reflect the changes of the hemodynamic parameters. Thus, hemodynamic parameters (LV stroke volume) are preferred when guiding VV-optimization with echocardiography. If the elimination of intraventricular dyssynchrony is not possible in CRT nonresponders, it may require LV lead revision or even discontinuation of CRT in selected cases.25
A new therapeutic option in CRT nonresponders is the cardiac contractility modulation, as reported by Nagele et al.26 Cardiac contractility modulation delivers nonexcitatory high-energy stimulation during the absolute refractory period, increasing Ca-influx and therefore improving contractility in HF patients, especially in those with narrow QRS and depressed LVEF. Sixteen nonresponder CRT patients were successfully treated with cardiac contractility modulation; however, a second procedure might have additional risk to the patients.26
Step 5. Check the MedicationNonresponder patients often have inadequate medical therapy. Mullens et al. reported that up to 24% of patients did not take one of the indicated HF therapy drugs.25 Drug discontinuation often occurs with worsening HF, progressive renal dysfunction or when experiencing aside-effect. It is of high importance to re-initiate medical therapy and sufficiently increase the dosage as suggested by the current guidelines. Patients with newly onset atrial or ventricular arrhythmias might additionally need antiarrhythmic agents. Not only drug prescriptions, but careful evaluation of patient compliance with regard to medication or fluid restriction is essential.
Step 6. Check ComorbiditiesElderly patients have several comorbidities, like diabetes, ischemic heart disease, and vascular and cerebral diseases, all leading to higher risk of all-cause mortality and potentially attenuate the beneficial effects of CRT. Decreased renal function, anemia, and hypotension are associated with poor prognosis in CRT recipients.
The etiology of the underlying disease additionally plays an important role. The MADIT-CRT trial showed that ischemic and nonischemic patients derived similar clinical benefit in mildly symptomatic or asymptomatic HF; however, nonischemic patients showed more pronounced reverse remodeling than ischemic patients. The clinical benefit was significantly different within risk subsets in ischemic and nonischemic patients, suggesting that risk assessment should be based on the underlying etiology of HF.27
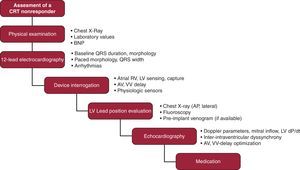
ConclusionsNonresponse to CRT is a frequent problem, which necessitates the complex evaluation of baseline and post-CRT electrocardiographic and echocardiographic data, as well as device interrogation, evaluation of lead location, and careful optimization of medical therapy (Figure 5). In case of worsening hemodynamic status, the cessation of CRT might be considered. Optimizing the individual response in all terms, the beneficial effects of CRT will be maximized.
Figure 5. Proposal for a stepwise assessment in nonresponders to cardiac resynchronization therapy. AP, anteroposterior; AV, atrioventricular; BNP, brain natriuretic peptide; CRT, cardiac resynchronization therapy; LV, left ventricular lead; RV, right ventricular lead; VV, ventriculoventricular.
Conflicts of interestValentina Kutyifa has received consultant fees/honaria from Boston Scientific, Biotronik, and Servier. Ole A. Breithardt has received speaker honoraria from GE Healthcare, Germany.
Corresponding author: Medizinische Klinik 2, Universitätsklinikum Erlangen, Ulmenweg 18, DE-91054 Erlangen, Germany. ole.breithardt@uk-erlangen.de