Primary hyperaldosteronism is the most common cause of secondary hypertension. Elevated aldosterone levels cause heart damage and increase cardiovascular morbidity and mortality. Early diagnosis could change the course of this entity. The objective of this report was to study the clinical characteristics, cardiac damage and cardiovascular risk associated with primary hyperaldosteronism.
MethodsWe studied 157 patients with this diagnosis. We analyzed the reason for etiological investigation, and the routinely performed tests, including echocardiography. We used a cohort of 720 essential hypertensive patients followed in our unit for comparison.
ResultsCompared with essential hypertensive patients, those with hyperaldosteronism were younger (56.9 [11.7] years vs 60 [14.4] years; P<.001), had higher blood pressure prior to the etiological diagnosis (136 [20.6] mmHg vs 156 [23.2] mmHg), more frequently had a family history of early cardiovascular disease (25.5% vs 2.2%; P<.001), and had a higher prevalence of concentric left ventricular hypertrophy (69% vs 25.7%) and higher cardiovascular risk. Specific treatment resulted in optimal control of systolic and diastolic blood pressures (from 150.7 [23.0] mmHg and 86.15 [14.07] mmHg to 12.69 [15.3] mmHg and 76.34 [9.7] mmHg, respectively). We suspected the presence of hyperaldosteronism because of resistant hypertension (33.1%), hypokalemia (38.2%), and hypertensive crises (12.7%). Only 4.6% of these patients had been referred from primary care with a suspected diagnosis of hyperaldosteronism.
ConclusionsHyperaldosteronism should be suspected in cases of resistant hypertension, hypokalemia and hypertensive crises. The diagnosis of hyperaldosteronism allows better blood pressure control. The most prevalent target organ damage is left ventricular hypertrophy.
Keywords
.
IntroductionAldosterone, the major and most powerful mineralocorticoid hormone in humans, is synthesized from cholesterol in the adrenal cortex1 and governs the physiological control of renal electrolyte balance.2 In healthy individuals, one of the effects of aldosterone is to maintain an optimal balance, which helps to preserve a healthy vascular system. However, when this balance is upset, the beneficial effects of aldosterone are lost and those that are detrimental to the vascular system predominate, leading to organ dysfunction3 and hypertension (HT).
Currently, the prevalence of primary hyperaldosteronism (PHA) is much higher than the previously accepted rate, less than 1% of the hypertensive population, and in the specialist setting, may be over 10%.4 The prevalence of PHA reported in Spain is between 5.1% and 6%5,6 in internal medicine and cardiology units.
PHA is the most common endocrine cause of secondary HT.7 This condition includes a variety of disorders characterized by an overproduction of aldosterone that is relatively independent of the renin-angiotensin-aldosterone system and is not suppressed with sodium overload.8 This overproduction of aldosterone causes damage to the cardiovascular system, suppression of plasma renin, HT, sodium retention, and potassium excretion, which leads to hypokalemia. Prolonged exposure to high plasma aldosterone concentrations is associated with greater oxidative stress, cardiovascular remodeling, hypertrophy, and fibrosis.9 This hormone is also involved in collagen synthesis and produces vascular remodeling and myocardial fibrosis in a process that is independent of its effect on arterial blood pressure (BP).10,11
All of the above translates into higher rates of cardiovascular morbidity and mortality in patients with PHA compared with essential hypertensive patients matched for age, sex, and BP.12
Moreover, aldosterone plays an important role in carbohydrate metabolism, with direct effects on pancreatic beta cells13 and on insulin signaling,14 and thus contributes, through its role in endothelial dysfunction, to the metabolic syndrome which, in turn, exerts its effect on the development of refractory HT and cardiovascular disease.15 Indeed, a higher incidence of cases of metabolic syndrome has been documented in PHA.16
For all these reasons, some authors have postulated that screening should be performed in all hypertensive patients,17 even in the context of mild HT, when there is no hypokalemia or family history, since the later the diagnosis, the longer the exposure to high aldosterone concentrations and the greater the likelihood of irreversible morphological changes.
PHA has classically been characterized as an uncommon disease with a benign course that is suspected only in the presence of hypokalemia. This profile has changed substantially due to findings published in recent years. The prevalence of PHA can be as high as 10% among hypertensive patients,4 and some reports show that hyperkalemia is present in approximately half of cases of PHA and that the cardiovascular risk of these patients is higher.10,11
ObjectivesGiven the importance of this secondary cause of HT and the small number of published studies on the subject, we proposed to:
- •
Determine the characteristics of patients with PHA in terms of the reason for performing etiological study, as well as the clinical and biochemical picture, morphological pattern, and profile of the disease course in those individuals diagnosed with this disease in the Hypertension Unit (HTU) of Hospital Clínico San Carlos in Madrid, Spain.
- •
Assess the cardiac and renal damage and cardiovascular risk of patients with PHA.
- •
Compare the clinical profile of patients with PHA with that of a cohort with essential HT from our HTU.
For this retrospective study, we selected 172 patients from the HTU who had been diagnosed with PHA between 1983 and March 2010. Of the 172 patients selected, we enrolled 157 who met the following inclusion criteria for the diagnosis of PHA: a) aldosterone concentration above the mean, with suppressed plasma renin activity and an aldosterone (ng/dL) to renin (pg/dL) ratio greater than 38, and evidence of abnormal adrenal anatomy (hyperplasia or adenoma) according to imaging techniques; b) demonstration, by means of scintigraphy with radiolabeled cholesterol, of functional activity, despite pharmacological suppression, and/or c) in those in whom the possibility of surgical treatment was evaluated, demonstration of the lateralization of aldosterone secretion in adrenal vein sampling, with determination of the aldosterone and cortisol concentrations, prior to the invasive procedure.
For comparison, we used a population of 720 patients with essential HT18 in representation of the hypertensive population being followed in our HTU.
Patients with incomplete data on the results of circulating renin:aldosterone determination or missing results of imaging study were excluded (n=15).
Office BP was measured using validated devices and by personnel trained according to the guidelines proposed by the European Society of Hypertension.19 BP was recorded both on arrival at the HTU and during the visit following diagnosis and the initiation of specific treatment. The personal data and anthropometric measurements, personal and family history of cardiovascular disease and risk factors, and time interval since the diagnosis of HT were collected from the medical records. In addition, we recorded the reason for investigating secondary HT that led to the diagnosis of PHA and whether the patient had been referred to the HTU by his or her general physician due to suspected PHA.
The results of complementary studies performed in the HTU using routine analytical techniques for renal function, blood glucose profile, lipid profile, and serum and 24-h urinary sodium and potassium determination were also recorded. In addition, we collected data from the ultrasound scan performed in the Echocardiography Laboratory of Hospital Clínico San Carlos, with determination of the left ventricular morphology. Concentric left ventricular hypertrophy was diagnosed when the echocardiogram revealed a relative wall thickness greater than 0.44 and left ventricular mass index greater than 125g/m2 (normal left ventricular mass index less than 125g/m2 and normal relative wall thickness less than 0.44). Concentric remodeling was established when left ventricular mass index was less than 125g/m2 and relative wall thickness was greater than 0.44; finally, eccentric left ventricular hypertrophy (LVH) was identified by left ventricular mass index greater than 125g/m2 and relative wall thickness less than 0.44.20
The plasma aldosterone and renin concentrations were determined in all patients after discontinuation of antialdosterone therapy at least 4 weeks before the samples were obtained. When possible, the determination was carried out without drug therapy, and when this was not possible (refractory HT), drugs that increased the positive predictive value of the ratio (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers) were administered. The blood samples for these determinations were obtained from outpatients under baseline conditions after a 15-min rest. Prior to analysis, oral potassium supplementation was administered to patients with hypokalemia. If the ratio of plasma aldosterone to plasma renin was greater than 38, in the presence of elevated aldosterone levels, an etiological study was performed.
Subsequently, the morphological study of the adrenal glands was carried out by means of computed tomography and/or scintigraphy with NP-59, that is, 6β-[I-131] iodomethyl-19-norcholesterol, after suppression with dexamethasone. Based on the results of the imaging studies and in accordance with the indications of the consensus guidelines of the Endocrine Society,4 the population with PHA was subdivided into 3 categories: adenoma, simple hyperplasia, and nodular hyperplasia.
After the morphological study, venography was performed in samples obtained from the adrenal veins when lateralization was suspected, and the possibility of invasive treatment (surgery or radiofrequency ablation) was assessed. The requirements for drug therapy and normokalemia were similar to those applied prior to the hormone analysis.
For cardiovascular risk stratification, we used the table provided by the European Society of Hypertension/European Society of Cardiology,19 and the Adult Treatment Panel III criteria were applied for the diagnosis of the metabolic syndrome.21
This study was approved by the ethics committee of Hospital Clínico San Carlos and the guidelines for good clinical practice were followed.
Statistical AnalysisIn the statistical analysis, the categorical variables were expressed as rates or percentages and the continuous variables as the mean (standard deviation) or the mean (standard error).
The comparison of the categorical variables was carried out using the chi-square test. The continuous variables were compared by means of: a) Student's t test when the variable had 2 categories, and b) ANOVA when it had more than 2. Student's t test and ANOVA assume normal distributions and homogeneity of variance. These assumptions were assessed using the Kolmogorov-Smirnov and Levene's test, respectively. When these assumptions were not met, the variable was categorized into tertiles and the Kruskal-Wallis nonparametric test was applied.
The statistical analysis was performed using the SPSS software package (version 15.0). In all the comparisons, the null hypothesis was rejected with a type I error or an α level less than .05.
ResultsThe general characteristics of our population with PHA are shown in Table 1.
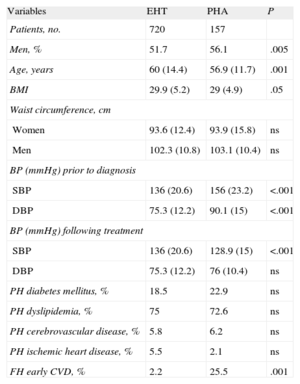
General Characteristics of the Population With Primary Hyperaldosteronism and Comparison With Essential Hypertensive Patients.
| Variables | EHT | PHA | P |
| Patients, no. | 720 | 157 | |
| Men, % | 51.7 | 56.1 | .005 |
| Age, years | 60 (14.4) | 56.9 (11.7) | .001 |
| BMI | 29.9 (5.2) | 29 (4.9) | .05 |
| Waist circumference, cm | |||
| Women | 93.6 (12.4) | 93.9 (15.8) | ns |
| Men | 102.3 (10.8) | 103.1 (10.4) | ns |
| BP (mmHg) prior to diagnosis | |||
| SBP | 136 (20.6) | 156 (23.2) | <.001 |
| DBP | 75.3 (12.2) | 90.1 (15) | <.001 |
| BP (mmHg) following treatment | |||
| SBP | 136 (20.6) | 128.9 (15) | <.001 |
| DBP | 75.3 (12.2) | 76 (10.4) | ns |
| PH diabetes mellitus, % | 18.5 | 22.9 | ns |
| PH dyslipidemia, % | 75 | 72.6 | ns |
| PH cerebrovascular disease, % | 5.8 | 6.2 | ns |
| PH ischemic heart disease, % | 5.5 | 2.1 | ns |
| FH early CVD, % | 2.2 | 25.5 | .001 |
BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; DBP, diastolic blood pressure; EHT, essential hypertension; FH, family history; ns, not significant; PH, personal history; PHA, primary hyperaldosteronism; SBP, systolic blood pressure.
Unless otherwise indicated, the data are expressed as mean (standard deviation).
The mean time since onset of HT in these patients was 13.2 years and the prevalence of chronic atrial fibrillation was 4.8%. There was a considerable incidence of a family history of HT (56.9%) and of diabetes mellitus (15.4%). Among family members, 16.8% had a history of early cerebrovascular events and 15.4% had a history of early ischemic heart disease.
Among the echocardiographic results, available for 126 patients of the series, the most common finding was concentric LVH, which was observed in 69% of the patients; in 25.4%, the results were normal; 1.4% of the patients exhibited remodeling, and 4.2%, eccentric hypertrophy; that is, 74.6% had an abnormal left ventricular morphology.
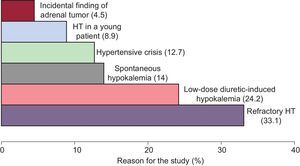
The most common cause for etiological investigation was refractory HT in 33.1%, followed by hypokalemia in 38.2% (14% spontaneous and 24.2% induced by low-dose diuretics [12.5mg or 25mg of hydrochlorothiazide]) (Figure).
Of the diagnosed patients, only 4.6% had been referred from primary care due to suspected PHA.
The mean potassium level prior to the etiological diagnosis was 3.42 (0.61) mmol/L (normal, 3.5-5.1 mmol/L; the serum potassium after specific treatment was 4.33 [0.51] mmol/L; serum sodium, 140 (3) mmol/L (normal, 135-145 mmol/L); urinary sodium, 136 (73) mmol/L/24 h; and urinary potassium, 65.7 (28) mmol/24 h. The mean plasma renin concentration was 4.4 (3.0) pg/mL (normal, 3.0-33 pg/mL), the mean serum aldosterone concentration, 305 (260.7) pg/mL (normal, 97-626 pg/mL), and the mean aldosterone:renin ratio was 425 (1301.4) (normal, <38).
For the imaging diagnosis, the most frequently used technique was computed tomography, which was performed in 83.4% of the patients. According to the results of this technique, the most common finding was nodular hyperplasia, which was diagnosed in 38.9%, followed by adenoma (28.3%), and simple hyperplasia (25.9%).
In all, 77 scintigraphies (49%) were carried out with radiolabeled cholesterol, following dexamethasone suppression, and the most common diagnosis with this technique was bilateral simple hyperplasia (51.9% of the patients).
For the final diagnosis, in more than one third of the population (34.4%), the workup was completed with adrenal venography and collection of blood samples to determine aldosterone and cortisol levels. Once the diagnostic workup had been completed (imaging techniques, scintigraphy, and venography), the diagnoses accepted as definitive were: adenoma (17.4%), unilateral simple hyperplasia (5.8%), bilateral simple hyperplasia (31.6%), unilateral nodular hyperplasia (10.9%), and bilateral nodular hyperplasia (34.2%).
The diagnosis was modified by venography in 46.3%. Thus, in the final diagnosis, there was a higher prevalence of bilateral involvement (65.8%), with an incidence of adenomas of 17.4%.
In Table 1, we compare the clinical characteristics of the patients with PHA and those of a cohort of patients with essential HT. When compared with the essential hypertensive population followed in the HTU, the patients with PHA were younger, had higher BP prior to the etiological diagnosis, achieved better BP control after initiation of specific treatment, and had a more extensive family history of early cardiovascular disease, with statistically significant differences.
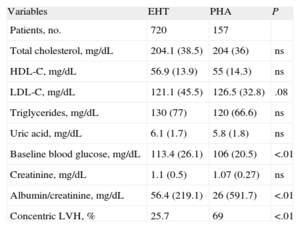
Table 2 shows the comparison of the biochemical profile and of the target organ damage of the two cohorts. The PHA patients had a significantly higher prevalence of cardiac target organ damage.
Comparison of the Biochemical Profile and Target Organ Damage in the Cohort of Essential Hypertensive Patients and Those With Primary Hyperaldosteronism.
| Variables | EHT | PHA | P |
| Patients, no. | 720 | 157 | |
| Total cholesterol, mg/dL | 204.1 (38.5) | 204 (36) | ns |
| HDL-C, mg/dL | 56.9 (13.9) | 55 (14.3) | ns |
| LDL-C, mg/dL | 121.1 (45.5) | 126.5 (32.8) | .08 |
| Triglycerides, mg/dL | 130 (77) | 120 (66.6) | ns |
| Uric acid, mg/dL | 6.1 (1.7) | 5.8 (1.8) | ns |
| Baseline blood glucose, mg/dL | 113.4 (26.1) | 106 (20.5) | <.01 |
| Creatinine, mg/dL | 1.1 (0.5) | 1.07 (0.27) | ns |
| Albumin/creatinine, mg/dL | 56.4 (219.1) | 26 (591.7) | <.01 |
| Concentric LVH, % | 25.7 | 69 | <.01 |
EHT, essential hypertension; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVH, left ventricular hypertrophy; ns, not significant; PHA, primary hyperaldosteronism.
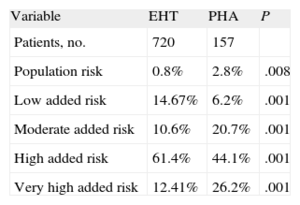
Table 3 compares the cardiovascular risk profile of the 2 cohorts. As can be seen, the patients with PHA are included in the highest stratum in the risk stratification.
Comparison of the Distribution of Cardiovascular Risk in Essential Hypertensive Patients Followed in the Hypertension Unit and Patients Diagnosed With Primary Hyperaldosteronism (Evaluated According to the 2007 ESC/ESH20).
| Variable | EHT | PHA | P |
| Patients, no. | 720 | 157 | |
| Population risk | 0.8% | 2.8% | .008 |
| Low added risk | 14.67% | 6.2% | .001 |
| Moderate added risk | 10.6% | 20.7% | .001 |
| High added risk | 61.4% | 44.1% | .001 |
| Very high added risk | 12.41% | 26.2% | .001 |
EHT, essential hypertension; PHA, primary hyperaldosteronism.
In recent years, the number of publications on the most common cause of secondary HT, PHA,7 has multiplied, possibly because the availability of simpler diagnostic methods has significantly increased the diagnosis of this condition.4
In what proves to be one of its strengths, our study provides a comprehensive assessment of the series corresponding to the largest single-center registry of those published to date.12,22,23 In Spain, few studies have been published on this subject5,6 and, thus, our cohort provides information of great interest for understanding not only the clinical characteristics and organ damage in these patients, but also the presentation of the disease and assessment of the cardiovascular risk.
The hypertensive population that should be evaluated to exclude PHA is a matter of debate.7 According to some experts, certain groups of patients have a higher prevalence of PHA and should undergo investigation: those with moderate/severe HT,22 resistant HT,24 HT associated with spontaneous or diuretic-induced hypokalemia,8,25,26 HT associated with a family history of early-onset HT or stroke at an early age (under 50 years),25 and the first-degree relatives of patients diagnosed with PHA.4 In our series, PHA was confirmed to be a highly common cause of resistant HT (33.1%). This entity is also a cause that should be excluded in any patient with spontaneous or diuretic-induced hypokalemia (38.2%).
One important finding is that 12.7% of these patients develop hypertensive crises, an occurrence that had not previously been reported in the literature, although the association of PHA with hypertensive emergencies was described in a series of only 7 patients.27
The development of PHA in hypertensive patients under 40 years of age (8.9%) indicates that this entity should also be suspected in these population groups with no clinical signs that would lead clinicians to suspect some other underlying cause. We agree with the observation of Mosso et al.22 that hypertensive patients with PHA are younger than those with essential HT. This observation is important since, as this population has a long life expectancy, the clinical benefits of a cure or, at least, the initiation of specific treatment, would be substantial.
Scheuner et al.28 recently demonstrated that assessment of the family history in patients with ischemic heart disease can improve the positive predictive value of certain measurements of risk, arguing that there is an underlying set of genetic risk factors that, once identified, would enable the application of genetic tests. Thus, in our population, a family history of early cardiovascular disease becomes more relevant since it may indicate a common genetic stratum.
Despite the increase in the prevalence of PHA, in Spain, the index of suspicion continues to be low, which is reflected in the low percentage of patients (4.6%) referred from primary care to the HTU with suspected PHA. This circumstance could be explained by the difficulty of carrying out systematic screening in primary care,29 variability in the clinical presentation of PHA, the high cost of the study, and the general acceptance of spironolactone as the fourth most widely administered drug in patients with resistant HT, the use of which would lead to control of PHA in many cases.30
In our study, despite the younger age of the patients with PHA, BP prior to etiological diagnosis was higher than that of patients with essential HT and the control achieved after diagnosis was better in the former than in the latter, a finding that agrees with the data published in other series.22,31,32
A notable finding concerning target organ damage in PHA patients was the high prevalence of echocardiographic evidence of LVH, a result that is consistent with that reported by Morillas et al.6 This result may be explained by the deleterious effect of high aldosterone concentrations which, acting in myocardial tissue, produce hypertrophy and fibrosis.11 According to these observations, there is a very close association between LVH and PHA. Thus, from our point of view, the presence of LVH not explained by BP should suggest the need to perform an etiological workup.
The deleterious role of aldosterone in carbohydrate metabolism has been reported elsewhere.14 Our PHA patients had a higher baseline blood glucose level than those with essential HT, a finding that coincides with the results of Matrozova et al.33 and contrasts with previously published data.34,35
Our data on the similarity in the lipid profile coincide with those published by other authors.33,35,36 Holaj et al.35 did find significantly lower high-density lipoprotein cholesterol levels in patients with PHA.
Patients with PHA have a clear increase in cardiovascular risk, as did the patients in our cohort, when compared with patients with essential HT, a datum that is accepted in the literature.4,12,37 This difference was found even though the population with PHA was younger and was compared with a cohort of hypertensive patients followed in a tertiary HTU, who have a higher cardiovascular risk than that of other hypertensive populations.
This increase in risk is probably related to the deleterious effects produced by prolonged exposure to high aldosterone concentrations,12 and takes place despite the lower prevalence of the metabolic syndrome in the PHA population than in essential hypertensive patients18 (data not shown).
Strengths and LimitationsThis report shows the limitations inherent to retrospective studies. Due to the low prevalence of the disease, the patients in the series were enrolled over a 25-year period. The strength of the study is the large number of cases collected. In the comparison between essential hypertensive patients and the PHA patients, the results would be more robust if the design had been that of a case-control study; nevertheless, the 720 patients with essential HT are representative of the population followed in our HTU.
ConclusionsA more active approach to the etiological study of patients with HT with spontaneous or diuretic-induced hypokalemia or resistant HT is essential. This is also true for hypertensive patients who have had repeated hypertensive crises over the course of their disease.
More than 70% of the patients with PHA have LVH.
The treatment of the underlying causes of PHA, which reduces exposure to high aldosterone concentrations, enables us to achieve optimal BP control, which probably reduces LVH and cardiovascular risk.
Conflicts of interestNone declared.