Coronavirus disease (COVID-19) has been designated a global pandemic by the World Health Organization. It is unclear whether previous treatment with angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) affects the prognosis of COVID-19 patients. The aim of this study was to evaluate the clinical implications of previous treatment with ACEI/ARB on the prognosis of patients with COVID-19 infection.
MethodsSingle-center, retrospective, observational cohort study based on all the inhabitants of our health area. Analyses of main outcomes (mortality, heart failure, hospitalization, intensive care unit [ICU] admission, and major acute cardiovascular events [a composite of mortality and heart failure]) were adjusted by multivariate logistic regression and propensity score matching models.
ResultsOf the total population, 447 979 inhabitants, 965 patients (0.22%) were diagnosed with COVID-19 infection, and 210 (21.8%) were under ACEI or ARB treatment at the time of diagnosis. Treatment with ACEI/ARB (combined and individually) had no effect on mortality (OR, 0.62; 95%CI, 0.17-2.26; P=.486), heart failure (OR, 1.37; 95%CI, 0.39-4.77; P=.622), hospitalization rate (OR, 0.85; 95%CI, 0.45-1.64; P=.638), ICU admission (OR, 0.87; 95%CI, 0.30-2.50; P=.798), or major acute cardiovascular events (OR, 1.06; 95%CI, 0.39-2.83; P=.915). This neutral effect remained in a subgroup analysis of patients requiring hospitalization.
ConclusionsPrevious treatment with ACEI/ARB in patients with COVID-19 had no effect on mortality, heart failure, requirement for hospitalization, or ICU admission. Withdrawal of ACEI/ARB in patients testing positive for COVID-19 would not be justified, in line with current recommendations of scientific societies and government agencies.
Keywords
By 1 April 1, 2020, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),1–4 known as COVID-19 (coronavirus disease 2019), had spread to over 200 countries, infecting more than 1 million people, and was designated a global pandemic by the World Health Organization.5
Although the infection can cause severe disease in anyone, one of the largest analyses conducted to date,6 reported more severe outcomes (intensive care unit [ICU] admission, mechanical ventilation, and death) in patients with hypertension, coronary artery disease, and diabetes.
The interaction of SARS-CoV-2 and the renin-angiotensin-aldosterone system is a cornerstone in COVID-19 infection. The virus links to membrane-bound angiotensin-converting enzyme 2 (ACE2) and is internalized in the cells by transmembrane proteases.7,8 Angiotensin I upregulates a metalloprotease (ADAM-17) that increases the solubility of ACE2 (a form not valid for SARS-CoV-2 binding)9,10 and also releases proinflammatory cytokines that downregulate ACE2 cell surface expression, reducing the ability of SARS-CoV-2 to cause damage.11
Given the possible increased susceptibility of patients taking ACEI in the COVID-19 pandemic, it has been proposed that they should discontinue this medication.12 At this moment, the impact of angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) on the clinical course of COVID-19 is controversial.3,7,13–17 When the current study was being performed, scientific societies and government agencies were recommending continuing with this treatment, due to the lack of evidence for the hypothesis.18,19
There is a lack of evidence in this field, and most of the available data are based on observational studies conducted in China; none have been based on a whole population, and there is little information on how the virus affects the western population.
METHODSStudy design and participantsWe performed a single-center, retrospective, observational cohort study at a university hospital covering a city and its metropolitan area, whose population has been confined during the study period under the state of alarm beginning on 14 March, 2020. We included all cases of laboratory-confirmed SARS-CoV-2 infection in the area, according to the interim guidance of the World Health Organization,20 independently of their outcome. Diagnosis of COVID-19 was based on nasopharyngeal swab real time-polymerase chain reaction, which has high sensitivity for virus detection; however, the sensitivity can decrease if the patient's viral load is low or if there are deficiencies in sample collection. This could affect the sample size, but probably not the results, since its distribution is homogeneous in the population.
This study complied with the edicts of the 1975 Declaration of Helsinki and was approved by the Galician Medication Research Ethics Committee.
Data collectionStandardized forms were used to set up the database, including demographic information, epidemiological data, tests performed, drugs received during hospital admission and at discharge according to physician criteria, and other relevant clinical information. Clinical information was collected from electronic medical records, providing access to the entire clinical history from primary to hospital care, as well as to electronic prescriptions, to confirm treatment adherence.
OutcomesThis work was conducted to properly typify the clinical implications of previous chronic treatment with ACEI/ARB on the prognosis of COVID-19. The main outcome was the impact of previous chronic treatment with ACEI and ARB on prognosis, evaluated through mortality, heart failure, the need for hospitalization and intensive care unit (ICU) stay, and major adverse cardiovascular events (a composite of death and heart failure). Heart failure was defined following the current guidelines,21 based on clinical, analytical, and radiological data. We also studied whether renin-angiotensin-aldosterone system inhibition maintained for more than 1 year could change the results.
Statistical analysisFor the comparison of patients treated and not treated with ACEI/ARB, continuous variables (expressed as mean± standard deviation) were compared with the Student t test, and discrete variables (expressed as percentages) were assessed with the chi-square or Fisher's exact test, as necessary.
Logistic regression models were performed to explain the independent association between ACEI/ARB treatment and hospital admission, ICU admission, mortality, and heart failure. For the multivariate adjustment, all variables showing a significant association (P<.05) with events in the univariate analyses were included (see table 1 and table 2 of the supplementary data), with no selection. All selected multivariate models had good discrimination (c-statistic >0.85 for the total population, and >0.80 for hospitalized patients) and good calibration (P value for the Hosmer-Lemsehow test >.6 for the total population, and> .40 for hospitalized patients). The results are expressed as odds ratios (OR), with their 95% confidence intervals (95%CI). In all hypothesis tests, the null hypothesis was rejected with a type I error or alpha error<.05.
Due to the substantial differences in baseline characteristics between patients receiving and not receiving ACEI/ARB, the analysis was complemented with a propensity score matching analysis. Patients were matched according to ACEI/ARB therapy based on propensity scores. We applied a greedy 1:1 matching algorithm without replacement, with a caliper of 0.1. Propensity scores were estimated using a nonparsimonious multivariable logistic regression model, with ACEI/ARB therapy as the dependent variables and those characteristics that differed (P <.05) between patients treated and not treated with ACEI/ARB (table 1) as covariates. Propensity score matching was performed for all patients and was repeated only for those patients with hospital admissions. After propensity score matching, 164 paired patients were identified with balanced baseline characteristics and there were no significant differences according to ACEI/ARB therapy (standard deviation <0.1 for all variables). In the propensity score-matched population, outcomes were compared using a stratified logistic regression model.
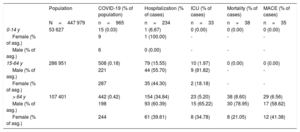
Age and sex distribution of the population affected by COVID-19
| Population | COVID-19 (% of population) | Hospitalization (% of cases) | ICU (% of cases) | Mortality (% of cases) | MACE (% of cases) | |
|---|---|---|---|---|---|---|
| N=447 979 | n=965 | n=234 | n=33 | n=38 | n=35 | |
| 0-14 y | 53 627 | 15 (0.03) | 1 (6.67) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Female (% of asg.) | 9 | 1 (100.00) | - | - | - | |
| Male (% of asg.) | 6 | 0 (0.00) | - | - | - | |
| 15-64 y | 286 951 | 508 (0.18) | 79 (15.55) | 10 (1.97) | 0 (0.00) | 0 (0.00) |
| Male (% of asg.) | 221 | 44 (55.70) | 9 (81.82) | - | - | |
| Female (% of asg.) | 287 | 35 (44.30) | 2 (18.18) | - | - | |
| > 64 y | 107 401 | 442 (0.42) | 154 (34.84) | 23 (5.20) | 38 (8.60) | 29 (6.56) |
| Male (% of asg.) | 198 | 93 (60.39) | 15 (65.22) | 30 (78.95) | 17 (58.62) | |
| Female (% of asg.) | 244 | 61 (39.61) | 8 (34.78) | 8 (21.05) | 12 (41.38) |
Asg., age subgroup; ICU, intensive care unit; MACE, major adverse cardiovascular events (acute coronary syndrome, myocarditis, arrhythmic cardiac arrest, stroke, pulmonary emboli, heart failure, and cardiovascular mortality).
The data are expressed as No. (%).
Similar analyses were repeated only for hospitalized patients, using both logistic regression and propensity score matching (with 58 paired patients). The statistical analysis was performed with SPSS 25.0.
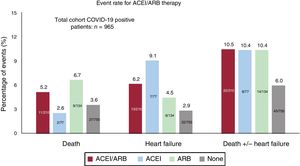
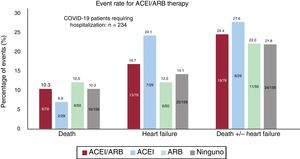
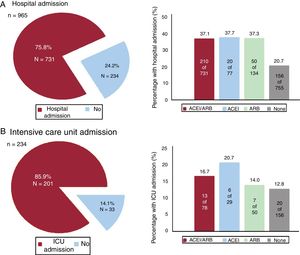
RESULTSFrom 10 March to 6 April, 965 patients (0.22%) were diagnosed with COVID-19 out of the 447 979 inhabitants of the area covering the university hospital. Of COVID-19 patients, 234 (24.25%) needed hospitalization; among these patients, 33 (14.1%) required ICU admission. During the study period, 38 patients died (3.94%), of whom 35 (3.6%) had heart failure (figure 1, figure 2 and figure 3).
Of the total population, 72 527 (16.19%) were under chronic treatment with ACEI (26 617 [36.7%] or ARB (48 085 [66.3%]). Of the COVID-19 patients, 210 (21.8%) were under ACEI or ARB treatment at the time of diagnosis; of these, 165 (78.57%) were taking them for more than 1 year. The age and sex distribution of the population with COVID-19 involvement are summarized in table 1.
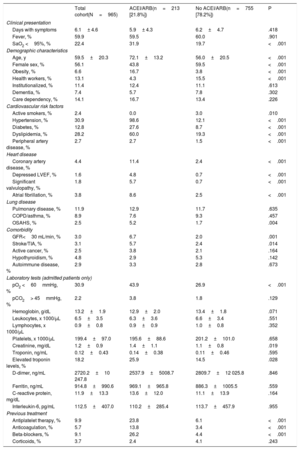
Table 2 summarizes the baseline characteristics of COVID-19 patients and provides a comparison of the cohort under ACEI/ARB treatment and that not receiving this treatment. The cohort of patients under ACEI/ARB was older (72.1± 13.2 vs 56.0± 20.5; P <.01) and had more cardiovascular risk factors (hypertension, diabetes, smoking, and dyslipidemia) and cardiovascular comorbidities (coronary artery diseases and ventricular dysfunction) than the cohort without ACEI/ARB. There were fewer women in the ACEI/ARB group (43.8% vs 59.5%; P <.01). Renal impairment and peripheral vasculopathy were also more prevalent in patients taking ACEI/ARB. On admission, patients with previous treatment with ACEI/ARB had lower oxygenation (peripheral O2 saturation under 95% in 31.9% vs 19.7%; P <.01; respiratory insufficiency in 43.9% vs 26.9%, P <.01) and had higher creatinine and troponin levels. Figure 1 and figure 2 show the events in all COVID-19 patients and in those who were admitted. Figure 3 shows hospital admissions according to type of treatment.
Baseline characteristics
| Total cohort(N=965) | ACEI/ARB(n=213 [21.8%]) | No ACEI/ARB(n=755 [78.2%]) | P | |
|---|---|---|---|---|
| Clinical presentation | ||||
| Days with symptoms | 6.1± 4.6 | 5.9± 4.3 | 6.2±4.7 | .418 |
| Fever, % | 59.9 | 59.5 | 60.0 | .901 |
| SaO2 <95%, % | 22.4 | 31.9 | 19.7 | <.001 |
| Demographic characteristics | ||||
| Age, y | 59.5±20.3 | 72.1±13.2 | 56.0±20.5 | <.001 |
| Female sex, % | 56.1 | 43.8 | 59.5 | <.001 |
| Obesity, % | 6.6 | 16.7 | 3.8 | <.001 |
| Health workers, % | 13.1 | 4.3 | 15.5 | <.001 |
| Institutionalized, % | 11.4 | 12.4 | 11.1 | .613 |
| Dementia, % | 7.4 | 5.7 | 7.8 | .302 |
| Care dependency, % | 14.1 | 16.7 | 13.4 | .226 |
| Cardiovascular risk factors | ||||
| Active smokers, % | 2.4 | 0.0 | 3.0 | .010 |
| Hypertension, % | 30.9 | 98.6 | 12.1 | <.001 |
| Diabetes, % | 12.8 | 27.6 | 8.7 | <.001 |
| Dyslipidemia, % | 28.2 | 60.0 | 19.3 | <.001 |
| Peripheral artery disease, % | 2.7 | 2.7 | 1.5 | <.001 |
| Heart disease | ||||
| Coronary artery disease, % | 4.4 | 11.4 | 2.4 | <.001 |
| Depressed LVEF, % | 1.6 | 4.8 | 0.7 | <.001 |
| Significant valvulopathy, % | 1.8 | 5.7 | 0.7 | <.001 |
| Atrial fibrillation, % | 3.8 | 8.6 | 2.5 | <.001 |
| Lung disease | ||||
| Pulmonary disease, % | 11.9 | 12.9 | 11.7 | .635 |
| COPD/asthma, % | 8.9 | 7.6 | 9.3 | .457 |
| OSAHS, % | 2.5 | 5.2 | 1.7 | .004 |
| Comorbidity | ||||
| GFR<30 mL/min, % | 3.0 | 6.7 | 2.0 | .001 |
| Stroke/TIA, % | 3.1 | 5.7 | 2.4 | .014 |
| Active cancer, % | 2.5 | 3.8 | 2.1 | .164 |
| Hypothyroidism, % | 4.8 | 2.9 | 5.3 | .142 |
| Autoimmune disease, % | 2.9 | 3.3 | 2.8 | .673 |
| Laboratory tests (admitted patients only) | ||||
| pO2 <60mmHg, % | 30.9 | 43.9 | 26.9 | <.001 |
| pCO2> 45mmHg, % | 2.2 | 3.8 | 1.8 | .129 |
| Hemoglobin, g/dL | 13.2±1.9 | 12.9±2.0 | 13.4±1.8 | .071 |
| Leukocytes, x 1000/μL | 6.5±3.5 | 6.3±3.6 | 6.6±3.4 | .551 |
| Lymphocytes, x 1000/μL | 0.9±0.8 | 0.9±0.9 | 1.0±0.8 | .352 |
| Platelets, x 1000/μL | 199.4±97.0 | 195.6±88.6 | 201.2±101.0 | .658 |
| Creatinine, mg/dL | 1.2±0.9 | 1.4±1.1 | 1.1±0.8 | .019 |
| Troponin, ng/mL | 0.12±0.43 | 0.14±0.38 | 0.11±0.46 | .595 |
| Elevated troponin levels, % | 18.2 | 25.9 | 14.5 | .028 |
| D-dimer, ng/mL | 2720.2±10 247.8 | 2537.9±5008.7 | 2809.7±12 025.8 | .846 |
| Ferritin, ng/mL | 914.8±990.6 | 969.1±965.8 | 886.3±1005.5 | .559 |
| C-reactive protein, mg/dL | 11.9±13.3 | 13.6±12.0 | 11.1±13.9 | .164 |
| Interleukin-6, pg/mL | 112.5±407.0 | 110.2±285.4 | 113.7±457.9 | .955 |
| Previous treatment | ||||
| Antiplatelet therapy, % | 9.9 | 23.8 | 6.1 | <.001 |
| Anticoagulation, % | 5.7 | 13.8 | 3.4 | <.001 |
| Beta-blockers, % | 9.1 | 26.2 | 4.4 | <.001 |
| Corticoids, % | 3.7 | 2.4 | 4.1 | .243 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; LVEF, left ventricle ejection fraction; OSAHS, obstructive sleep apnea-hypopnea syndrome; pCO2,partial pressure of carbon dioxide; pO2, partial pressure of oxygen; SaO2, arterial oxygen saturation; TIA, transient ischemic attack.
Values are expressed as No. (%) or mean±standard deviation.
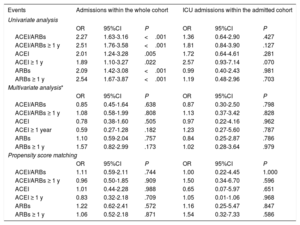
Table 3 shows that, on univariate analysis, treatment with ACEI/ARB (combined and individually) significantly increased in the risk of hospitalization, but this association disappeared both in the multivariate analysis with logistic regression and in that performed in the model created with propensity score matching to adjust for potential risk arguments. Hospitalized patients under ACEI/ARB showed more frequent need for more ICU admissions.
Association between ACEI/ARBs and hospital and ICU admissions
| Events | Admissions within the whole cohort | ICU admissions within the admitted cohort | ||||
|---|---|---|---|---|---|---|
| Univariate analysis | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACEI/ARBs | 2.27 | 1.63-3.16 | <.001 | 1.36 | 0.64-2.90 | .427 |
| ACEI/ARBs ≥ 1 y | 2.51 | 1.76-3.58 | <.001 | 1.81 | 0.84-3.90 | .127 |
| ACEI | 2.01 | 1.24-3.28 | .005 | 1.72 | 0.64-4.61 | .281 |
| ACEI ≥ 1 y | 1.89 | 1.10-3.27 | .022 | 2.57 | 0.93-7.14 | .070 |
| ARBs | 2.09 | 1.42-3.08 | <.001 | 0.99 | 0.40-2.43 | .981 |
| ARBs ≥ 1 y | 2.54 | 1.67-3.87 | <.001 | 1.19 | 0.48-2.96 | .703 |
| Multivariate analysis* | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACEI/ARBs | 0.85 | 0.45-1.64 | .638 | 0.87 | 0.30-2.50 | .798 |
| ACEI/ARBs ≥ 1 y | 1.08 | 0.58-1.99 | .808 | 1.13 | 0.37-3.42 | .828 |
| ACEI | 0.78 | 0.38-1.60 | .505 | 0.97 | 0.22-4.16 | .962 |
| ACEI ≥ 1 year | 0.59 | 0.27-1.28 | .182 | 1.23 | 0.27-5.60 | .787 |
| ARBs | 1.10 | 0.59-2.04 | .757 | 0.84 | 0.25-2.87 | .786 |
| ARBs ≥ 1 y | 1.57 | 0.82-2.99 | .173 | 1.02 | 0.28-3.64 | .979 |
| Propensity score matching | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACEI/ARBs | 1.11 | 0.59-2.11 | .744 | 1.00 | 0.22-4.45 | 1.000 |
| ACEI/ARBs ≥ 1 y | 0.96 | 0.50-1.85 | .909 | 1.50 | 0.34-6.70 | .596 |
| ACEI | 1.01 | 0.44-2.28 | .988 | 0.65 | 0.07-5.97 | .651 |
| ACEI ≥ 1 y | 0.83 | 0.32-2.18 | .709 | 1.05 | 0.01-1.06 | .968 |
| ARBs | 1.22 | 0.62-2.41 | .572 | 1.16 | 0.25-5.47 | .847 |
| ARBs ≥ 1 y | 1.06 | 0.52-2.18 | .871 | 1.54 | 0.32-7.33 | .586 |
95%CI, 95% confidence interval; ACEI, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; ICU, intensive care unit; OR, odds ratio.
Whole cohort: adjustment for those variables with a P <.05 in the univariate analysis (days with symptoms, fever, arterial oxygen saturation <95%, age, sex, health personnel, institutionalized, dependency status, dementia, hypertension, dyslipidemia, ventricular dysfunction, lung disease, previous cancer, hypothyroidism, antiplatelet therapy). ICU admitted cohort: adjustment for those variables with a P <.05 in the univariate analysis (arterial oxygen saturation <95%, diabetes mellitus, hypoxemia, hypercapnia, lymphocytes, creatinine, elevated troponin, ferritin, C-reactive protein, interleukin-6) (table 2 of the supplementary data).
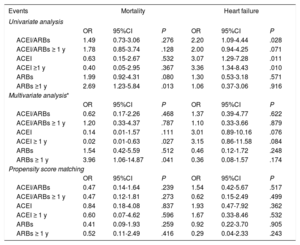
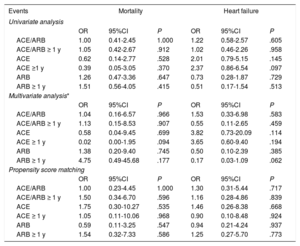
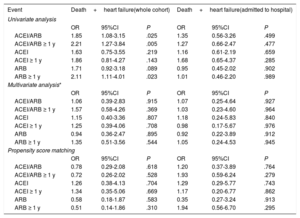
Previous treatment with ACEI/ARB (combined and individually) showed no impact on mortality or on heart failure, either in the multivariate analysis or in the propensity score-adjusted model. Taking the treatment for more than 1 year also had no effect (table 4). When we analyzed only the subgroup of patients requiring hospitalization, the absence of an impact on mortality and on heart failure remained both in the multivariate analysis and in the propensity score model, including in the evaluation of treatment taken for more than 1 year (table 5). The lack of effect remained when we applied the same models to the combined endpoint of mortality and heart failure (table 6).
Association between ACIE/ARBs, mortality and heart failure in the whole cohort of COVID-19 positive patients
| Events | Mortality | Heart failure | ||||
|---|---|---|---|---|---|---|
| Univariate analysis | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACEI/ARBs | 1.49 | 0.73-3.06 | .276 | 2.20 | 1.09-4.44 | .028 |
| ACEI/ARBs ≥ 1 y | 1.78 | 0.85-3.74 | .128 | 2.00 | 0.94-4.25 | .071 |
| ACEI | 0.63 | 0.15-2.67 | .532 | 3.07 | 1.29-7.28 | .011 |
| ACEI ≥1 y | 0.40 | 0.05-2.95 | .367 | 3.36 | 1.34-8.43 | .010 |
| ARBs | 1.99 | 0.92-4.31 | .080 | 1.30 | 0.53-3.18 | .571 |
| ARBs ≥1 y | 2.69 | 1.23-5.84 | .013 | 1.06 | 0.37-3.06 | .916 |
| Multivariate analysis* | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACEI/ARBs | 0.62 | 0.17-2.26 | .468 | 1.37 | 0.39-4.77 | .622 |
| ACEI/ARBs ≥ 1 y | 1.20 | 0.33-4.37 | .787 | 1.10 | 0.33-3.66 | .879 |
| ACEI | 0.14 | 0.01-1.57 | .111 | 3.01 | 0.89-10.16 | .076 |
| ACEI ≥ 1 y | 0.02 | 0.01-0.63 | .027 | 3.15 | 0.86-11.58 | .084 |
| ARBs | 1.54 | 0.42-5.59 | .512 | 0.46 | 0.12-1.72 | .248 |
| ARBs ≥ 1 y | 3.96 | 1.06-14.87 | .041 | 0.36 | 0.08-1.57 | .174 |
| Propensity score matching | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACEI/ARBs | 0.47 | 0.14-1.64 | .239 | 1.54 | 0.42-5.67 | .517 |
| ACEI/ARBs ≥ 1 y | 0.47 | 0.12-1.81 | .273 | 0.62 | 0.15-2.49 | .499 |
| ACEI | 0.84 | 0.18-4.08 | .837 | 1.93 | 0.47-7.92 | .362 |
| ACEI ≥ 1 y | 0.60 | 0.07-4.62 | .596 | 1.67 | 0.33-8.46 | .532 |
| ARBs | 0.41 | 0.09-1.93 | .259 | 0.92 | 0.22-3.70 | .905 |
| ARBs ≥ 1 y | 0.52 | 0.11-2.49 | .416 | 0.29 | 0.04-2.33 | .243 |
95%CI, 95% confidence interval; ACEI, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; OR, odds ratio.
Adjustment for those variables with a P <.05 in the univariate analysis (fever, oxygen saturation <95%, age, sex, obesity, health personnel, dependency status, hypertension, diabetes mellitus, dyslipidemia, arterial disease, heart disease, atrial fibrillation, pneumonia, chronic renal disease, cerebrovascular disease, autoimmune disease, anticoagulation, beta-blockers) (table 1 of the supplementary data).
Association between ACE/ARB and mortality and heart failure in hospitalized patients with COVID-19 infection
| Events | Mortality | Heart failure | ||||
|---|---|---|---|---|---|---|
| Univariate analysis | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACE/ARB | 1.00 | 0.41-2.45 | 1.000 | 1.22 | 0.58-2.57 | .605 |
| ACE/ARB ≥ 1 y | 1.05 | 0.42-2.67 | .912 | 1.02 | 0.46-2.26 | .958 |
| ACE | 0.62 | 0.14-2.77 | .528 | 2.01 | 0.79-5.15 | .145 |
| ACE ≥1 y | 0.39 | 0.05-3.05 | .370 | 2.37 | 0.86-6.54 | .097 |
| ARB | 1.26 | 0.47-3.36 | .647 | 0.73 | 0.28-1.87 | .729 |
| ARB ≥ 1 y | 1.51 | 0.56-4.05 | .415 | 0.51 | 0.17-1.54 | .513 |
| Multivariate analysis* | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACE/ARB | 1.04 | 0.16-6.57 | .966 | 1.53 | 0.33-6.98 | .583 |
| ACE/ARB ≥ 1 y | 1.13 | 0.15-8.53 | .907 | 0.55 | 0.11-2.65 | .459 |
| ACE | 0.58 | 0.04-9.45 | .699 | 3.82 | 0.73-20.09 | .114 |
| ACE ≥ 1 y | 0.02 | 0.00-1.95 | .094 | 3.65 | 0.60-9.40 | .194 |
| ARB | 1.38 | 0.20-9.40 | .745 | 0.50 | 0.10-2.39 | .385 |
| ARB ≥ 1 y | 4.75 | 0.49-45.68 | .177 | 0.17 | 0.03-1.09 | .062 |
| Propensity score matching | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACE/ARB | 1.00 | 0.23-4.45 | 1.000 | 1.30 | 0.31-5.44 | .717 |
| ACE/ARB ≥ 1 y | 1.50 | 0.34-6.70 | .596 | 1.16 | 0.28-4.86 | .839 |
| ACE | 1.75 | 0.30-10.27 | .535 | 1.46 | 0.26-8.38 | .668 |
| ACE ≥ 1 y | 1.05 | 0.11-10.06 | .968 | 0.90 | 0.10-8.48 | .924 |
| ARB | 0.59 | 0.11-3.25 | .547 | 0.94 | 0.21-4.24 | .937 |
| ARB ≥ 1 y | 1.54 | 0.32-7.33 | .586 | 1.25 | 0.27-5.70 | .773 |
95%CI, 95% confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; OR, odds ratio.
Adjusted for variables with P <.05 in the univariate analysis (fever, arterial oxygen saturation <95%, age, sex, obesity, health care worker, dependency, hypertension, diabetes mellitus, dyslipidemia, peripheral artery disease, coronary artery disease, atrial fibrillation, pulmonary disease, renal impairment, stroke/transient ischemic attack, hemoglobin, leukocytes, lymphocytes, creatinine, increased troponin, D-dimer, ferritin, ultrasensitive C-reactive protein, and interleukin-6 (table 1 of the supplementary data).
Association between ACEI/ARB with a composite endpoint of death and heart failure
| Event | Death+heart failure(whole cohort) | Death+heart failure(admitted to hospital) | ||||
|---|---|---|---|---|---|---|
| Univariate analysis | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACEI/ARB | 1.85 | 1.08-3.15 | .025 | 1.35 | 0.56-3.26 | .499 |
| ACEI/ARB ≥ 1 y | 2.21 | 1.27-3.84 | .005 | 1.27 | 0.66-2.47 | .477 |
| ACEI | 1.63 | 0.75-3.55 | .219 | 1.16 | 0.61-2.19 | .659 |
| ACEI ≥ 1 y | 1.86 | 0.81-4.27 | .143 | 1.68 | 0.65-4.37 | .285 |
| ARB | 1.71 | 0.92-3.18 | .089 | 0.95 | 0.45-2.02 | .902 |
| ARB ≥ 1 y | 2.11 | 1.11-4.01 | .023 | 1.01 | 0.46-2.20 | .989 |
| Multivariate analysis* | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACEI/ARB | 1.06 | 0.39-2.83 | .915 | 1.07 | 0.25-4.64 | .927 |
| ACEI/ARB ≥ 1 y | 1.57 | 0.58-4.26 | .369 | 1.03 | 0.23-4.60 | .964 |
| ACEI | 1.15 | 0.40-3.36 | .807 | 1.18 | 0.24-5.83 | .840 |
| ACEI ≥ 1 y | 1.25 | 0.39-4.06 | .708 | 0.98 | 0.17-5.67 | .976 |
| ARB | 0.94 | 0.36-2.47 | .895 | 0.92 | 0.22-3.89 | .912 |
| ARB ≥ 1 y | 1.35 | 0.51-3.56 | .544 | 1.05 | 0.24-4.53 | .945 |
| Propensity score matching | ||||||
| OR | 95%CI | P | OR | 95%CI | P | |
| ACEI/ARB | 0.78 | 0.29-2.08 | .618 | 1.20 | 0.37-3.89 | .764 |
| ACEI/ARB ≥ 1 y | 0.72 | 0.26-2.02 | .528 | 1.93 | 0.59-6.24 | .279 |
| ACEI | 1.26 | 0.38-4.13 | .704 | 1.29 | 0.29-5.77 | .743 |
| ACEI ≥ 1 y | 1.34 | 0.35-5.06 | .669 | 1.17 | 0.20-6.77 | .862 |
| ARB | 0.58 | 0.18-1.87 | .583 | 0.35 | 0.27-3.24 | .913 |
| ARB ≥ 1 y | 0.51 | 0.14-1.86 | .310 | 1.94 | 0.56-6.70 | .295 |
95%CI, 95% confidence interval; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; OR, odds ratio.
Whole cohort: adjustment of variables with P <.05 in univariate analysis (fever, arterial oxygen saturation <95%, age, sex, obesity, health care worker, care dependency, hypertension, diabetes, dyslipidemia, peripheral artery disease, heart disease, atrial fibrillation, lung disease, renal impairment, stroke/ transient ischemic attack). Cohort admitted to hospital: adjustment of variables with P <.05 in multivariate analysis (fever, arterial oxygen saturation <95%, age, sex, obesity, health care worker, care dependency, hypertension, diabetes, dyslipidemia, peripheral artery disease, heart disease, atrial fibrillation, lung disease, renal impairment, stroke/transient ischemic attack, hemoglobin, leukocytes, lymphocytes, creatinine, elevated troponin levels, D-dimer, ferritin, C-reactive protein, interleukin-6) (table 1 of the supplementary data).
To our knowledge, this is one of the few studies that analyzes the impact of ACEI/ARB on COVID-19 prognosis based on a large western-world population that includes all positive cases in a health area. This is also the largest cohort of patients studied to date.
The main findings are the neutral effect of ACEI/ARB on mortality, heart failure, and the combination of mortality and heart failure. Previous treatment with ACEI/ARB in patients with COVID-19 showed no association with the need for hospitalization or ICU admission. Taking ACEI/ARB for more than 1 year also had no effect. All these findings were confirmed, both in the overall analysis of the sample and in the propensity score model. Based on these data, withdrawal of chronic treatment with ACEI/ARB in patients testing positive for COVID-19 would not be justified. The available data on the effect of ACEI/ARB on mortality in patients with COVID-19 have focused mainly on cohorts of admitted patients and especially on hypertensive patients.22,23
SARS-CoV-2 enters the cells through a glycoprotein of its crown, which binds to the ACE2 of the alveoli pneumocytes,7,13 leading to its internalization after priming by the transmembrane protease, serine 2.7,8 After the virus enters the cell, it releases its RNA and proteins, leading to the development of new viral particles. ACE2 is 2% in a soluble form—not valid for SARS-CoV-2 binding—, after cleavage by ADAM-17. Angiotensin I upregulates ADAM-17, thus increasing soluble ACE2 levels.9,10 Furthermore, ADAM-17 also mediates the release of membrane-bound precursors of proinflammatory cytokines (tumor necrosis factor-α, interferon-γ, and interleukin-4) into the circulation. The interaction between SARS-CoV-2 and ACE2 triggers a massive production of proinflammatory cytokines, which attracts leucocytes and hyperactive macrophages that release more cytokines, inducing the obliteration of the alveoli and developing the characteristic hyaline membranes of acute respiratory distress syndrome.3,7,13,17 Therefore, downregulation of ACE2 cell surface expression reduces the ability of ARS-CoV-2 to cause damage.11 ACE inhibitors and ARB are highly recommended drugs for patients with cardiovascular diseases, such as refractory hypertension, heart failure, and coronary artery disease.18,19 Chronic treatment with these medications increases the expression of ACE.24–26 Based on this mechanism, some authors have hypothesized that persistent downregulation of the renin-angiotensin-aldosterone system may cause harm by increasing ACE2 expression.12,14 On the other hand, angiotensin II takes part in the immune response to acute respiratory distress syndrome, so its depuration though ACE2 may be beneficial.15–17 When this study was being performed, scientific societies and government agencies were recommending continuing with this treatment.18,19 This advice is supported by the results of this study, upgrading the expert-consensus recommendation to an evidence-based one according to a registry with all cases of COVID-19 infection from a western health area. An ongoing randomized clinical trial with losartan in these patients will help us to make a stronger recommendation (NCT04312009 and NCT04311177).27
To obtain an overall picture of the relevance of these results, we highlight that in the area where the study was conducted, 72 527 (16.19%) of the inhabitants were under chronic treatment with ACEI or ARB. Out of the COVID-19 patients, 210 (21.8%) were under ACEI or ARB at the time of diagnosis. According to the latest estimates, it is likely that by the end of the year, up to 70% of the Spanish population will have been infected by this new coronavirus,28 hence the importance of identifying the effects of these widely used drugs on the prognosis of this new disease.
Study limitationsBecause of its observational nature, unmeasured confounders could have constrained causal inference in the present study. False negatives in the polymerase chain reaction test could have affected the sample size, underestimating the number of individuals infected by the virus.
CONCLUSIONSWithdrawal of chronic treatment with ACEI/ARB in patients testing positive for COVID-19 would not be justified. In line with the recommendations of scientific societies and government agencies, this study supports continuation of this treatment.
It is unclear whether previous treatment with ACEI and ARB affects the prognosis of COVID-19 patients. Chronic treatment with these medications increases ACE expression. Based on this mechanism, there is a hypothesis postulating that persistent downregulation of the renin-angiotensin-aldosterone system may be harmful because it increases ACE2 expression.
WHAT DOES THIS STUDY ADD?Our data suggest that chronic administration of ACEI and ARB is safe in COVID-19 patients and therefore its withdrawal should not be recommended.
The authors declare that there is no conflict of interest regarding the publication of this article.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.05.018