The optimal antithrombotic therapy (AT) after left atrial appendage closure (LAAC) is debated. We assessed the impact of intensive vs nonintensive AT on the incidence of device-related thrombus (DRT) based on whether the device implantation was classified as optimal or suboptimal.
MethodsThis study included patients who underwent successful LAAC in 9 centers. Patients were classified according to the quality of device implantation: optimal (proximal implant without ≥3mm peridevice leak) or suboptimal (distal implant and/or ≥3mm peridevice leak). Postimplant AT was classified as either intensive (dual antiplatelet therapy, anticoagulants, or a combination of both) or nonintensive (no AT or a single antiplatelet therapy). The primary endpoint was the incidence of DRT between the 6th and 12th weeks postprocedure.
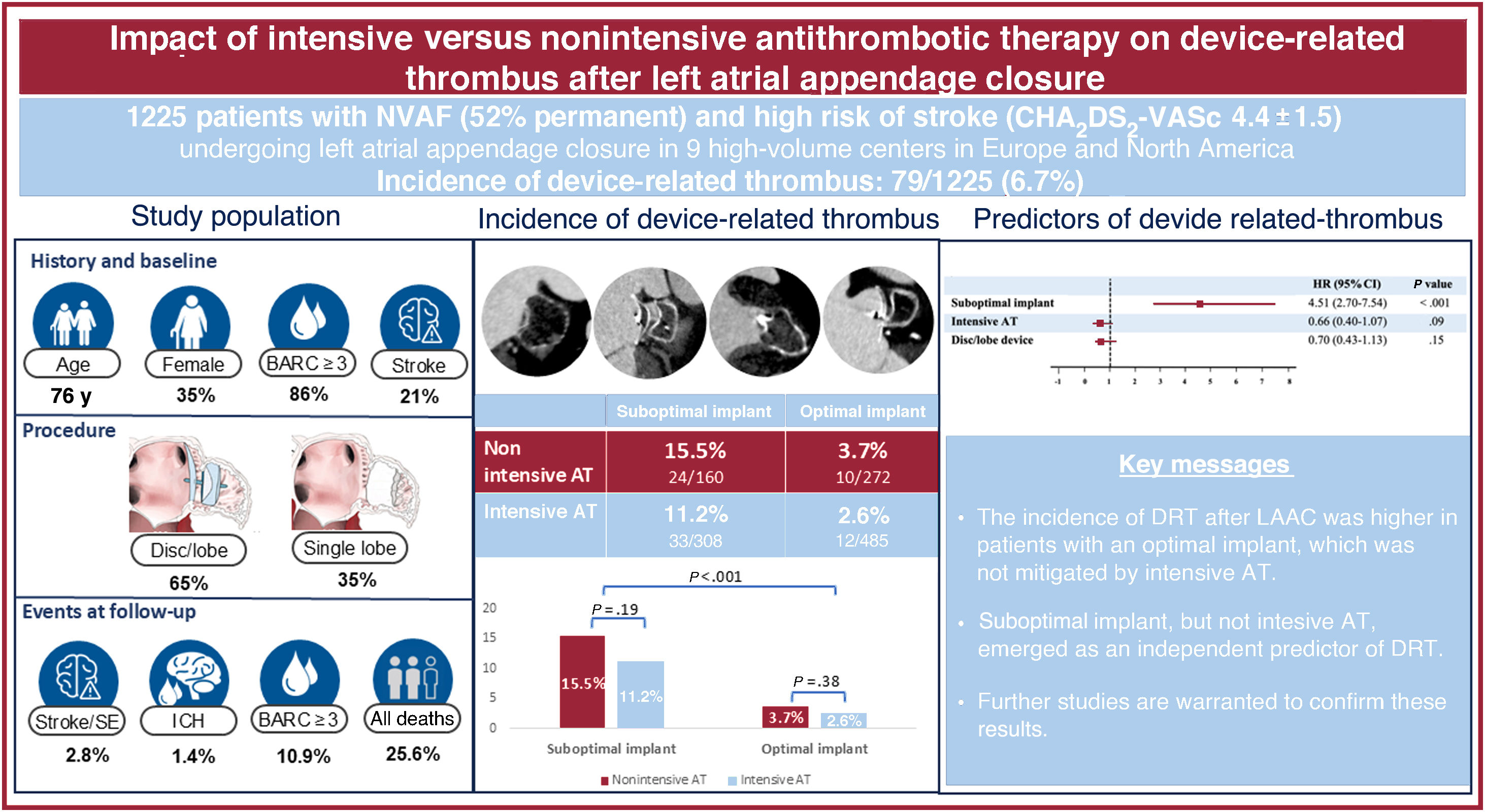
ResultsA total of 1225 patients underwent LAAC, with 757 (61.8%) achieving optimal device implantation and 468 (38.2%) classified as suboptimal. After a median follow-up of 20 months, the incidence of DRT in the optimal implant group was 2.6% with intensive AT and 3.7% with nonintensive AT (P=.38). In the suboptimal implant group, the incidence of DRT increased to 11.2% with intensive AT and 15.5% with nonintensive AT (P=.19). On multivariate analysis, suboptimal implantation (HR, 4.51; 95%CI, 2.70-7.54, P<.001) but not intensive AT (HR, 0,66; 95%CI, 0.40-1.07, P=.09) emerged as an independent predictor of DRT.
ConclusionsThe incidence of DRT after LAAC was higher in patients with suboptimal device implantation. In patients with optimal implantation, the incidence of DRT was low and similar between nonintensive and intensive AT strategies. Large, randomized trials are warranted to confirm these results.
Keywords
Growing evidence supports left atrial appendage closure (LAAC) in selected patients with nonvalvular atrial fibrillation.1,2 The adoption of the procedure has expanded at a remarkable rate, reflecting the major unmet need for an effective stroke prevention strategy in patients for whom long-term oral anticoagulation (OAC) is unacceptable.3 Recent refinements in procedural planning and technique have optimized the LAAC procedure and minimized complications.4,5 Device-related thrombus (DRT) is one of the remaining major concerns associated with LAAC, as it has been linked to higher rates of stroke and ischemic events.6,7 Postimplant antithrombotic therapy (AT) aims to mitigate the risk of DRT during the sealing process of the atrial surface of the device.8,9 The type and intensity of AT after LAAC have been arbitrarily proposed as either a temporary OAC regimen for 6 to 12 weeks,10 or dual antiplatelet therapy for 12 weeks followed by single antiplatelet therapy for up to 1 year.11 However, nonprocedural bleeding associated with intensive AT, particularly dual antiplatelet therapy, after left atrial appendage occlusion (LAAO) is not uncommon and has been linked to an increased risk of mortality,12 potentially diminishing the benefit of the procedure. More recently, less intensive protocols using single antiplatelet therapy or even no AT have been reported in selected high-risk bleeding patients undergoing LAAC, with an acceptable occurrence of ischemic adverse events.13,14
We have recently shown that LAAC device implant depth is an independent risk factor for DRT.15 Large proximal uncovered areas and uncovered pulmonary ridge (PR) observed with a deep implant have been considered equivalent to a neoappendage, thereby increasing the risk of postimplant DRT. In addition, peridevice leakage (PDL) may cause turbulence in blood flow adjacent to the device and stagnant blood in a residual neo-cavity, which is a well-known predisposing factor for DRT.16 Accordingly, an optimal device implant, in terms of minimizing the risk of postprocedure DRT, might be one with a proximal implant and without significant PDL. In contrast, a suboptimal device implant may result from a deeper device position and/or the presence of significant PDL.15 Although several studies have highlighted the negative impact of distal implants and significant PDL, none have evaluated these factors in combination. Furthermore, the role of intensive AT following optimal or suboptimal LAAC implants remains uncertain. Therefore, the aim of the present study was to evaluate the incidence of DRT in patients receiving either intensive or nonintensive AT after achieving an optimal or suboptimal LAAC device implant.
METHODSStudy cohortThe study cohort has been previously described in detail.15 In brief, baseline characteristics, procedural features, postimplant AT and outcomes of patients who underwent successful LAAC in 9 centers from Europe and North America were collected in a dedicated database. Device selection, pre- and postprocedural type of imaging, as well as postprocedural AT was left at the operator's discretion. LAAC procedures were performed in accordance with current expert recommendations.17 Follow-up (imaging) for the primary endpoint (DRT) was achieved in 96.7% of patients. Patients without cardiac imaging follow-up were not excluded in order to evaluate the adverse event rate as a function of implant type (optimal vs suboptimal) and AT (intensive vs nonintensive). The study was conducted in accordance with the institutional ethics committee of each participating center, and all patients provided signed informed consent for the procedures. The study conformed to the guiding principles of the Declaration of Helsinki.
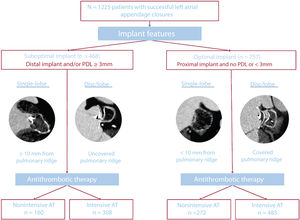
For the present study, patients were first classified into 2 groups (optimal and suboptimal implant) according to the achieved procedural result. An optimal implant was defined as a proximal implant in the absence of significant (≥3mm) PDL. Proximal implants were defined as PR coverage with disc/lobe devices and depth <10mm for single lobe devices. Conversely, suboptimal implant was defined as a distal implant (no PR coverage for disc/lobe devices and implant depth ≥10mm for single lobe devices) and/or existing significant (>3mm) PDL. These definitions were based on previous studies associating these cutoff points with an increased incidence of DRT and have been detailed elsewhere.15,16,18–22 All centers were trained to classify patients according to PR coverage and PDL measurements and obtain all previously described measurements. If there were doubts, patients were reclassified by the consensus of 2 expert physicians. Patients were also classified into 2 groups (intensive or nonintensive AT) according to the nature of postimplant AT. This classification adheres to the current LAAO consensus,17,23 which recommends intensive AT with dual antiplatelet therapy or OAC (vitamin K inhibitors, direct OAC, or low molecular weight heparin) following LAAO. Intensive AT was defined as any other AT after the procedure, which comprised dual antiplatelet therapy or any OAC (vitamin K inhibitors, direct OAC, or low molecular weight heparin). Nonintensive AT was defined as single antiplatelet therapy or the absence of any AT (either an antiplatelet agent or OAC) after device implantation. The incidence of DRT and clinical outcomes are described in 4 groups of patients, those with an optimal or suboptimal implant and intensive or nonintensive AT.
Endpoints and outcomesDefinitions of study data and outcomes have been previously described in detail.8 The first imaging follow-up test (transesophageal echocardiography [TEE] or computed tomography [CT], at the discretion of each center) was performed between the 6th and 12th week after the procedure. DRT was diagnosed on either TEE or CT and was defined as a thrombus adhering to the device's left atrial side, irrespective of its clinical consequences.7 Procedural adverse events, major adverse events (including death, stroke, systemic embolism, and major bleeding), and PDL were also reported, according to the Munich Consensus paper.18 Since the estimated hemorrhagic risk varied among patients, AT after LAAC was left to the individualized patient risk estimated by the treating physician. Major bleeding events were defined as type 3 or greater on the Bleeding Academic Research Consortium (BARC) scale.19
The primary outcome of the study was the incidence of DRT. The study objectives included: a) comparison of DRT rates according to the device implant achieved, defined as optimal or suboptimal, b) comparison of DRT rates according to the nature of the postimplant AT in the 2 groups. Additional analyses included clinical outcomes among groups of optimal or suboptimal device implant and postprocedural intensive or nonintensive AT.
Statistical analysisCategorical variables are presented as frequencies (percentages), and differences were assessed by the chi-square test (or Fisher test when necessary). Continuous variables are presented as mean±standard deviation (SD) or median [interquartile range]. The Kolmogorov-Smirnov test was applied to ensure normal distribution. Continuous variables were compared using the Student t-test or the Mann-Whitney U-test, as appropriate. The predictors of DRT were determined using a Cox regression analysis with a theory-driven prespecified variable selection approach. We obtained hazard ratios (HR) with associated 95% confidence intervals (95%CI). Prespecified variables with P values <.10 in the univariable analysis were included in the multivariable model. Prespecified variables included age, female sex, prior ischemic stroke, permanent atrial fibrillation, chronic kidney disease, suboptimal implantation, PDL, iatrogenic pericardial effusion, intensive AT, disc/lobe device, and CHADS-VASc score ≥3.7,24 Survival curves using all available follow-up data were constructed for the time-to-event variables using the Kaplan-Meier method. For all analyses, a 2-tailed P value <.05 was used as the criterion for statistical significance. Follow-up was considered to terminate at the date of the last follow-up. Analyses were performed using STATA software (V 14.0, StataCorp LP, College Station, TX).
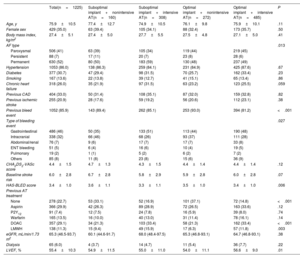
RESULTSA total of 1225 patients (35.0% female) undergoing successful transcatheter LAAC were included in the study (table 1 and figure 1). Among them, the achieved procedural result was optimal in 757 patients (61.8%) and suboptimal in 468 (38.2%) (figure 2). The AT in the study population is described in . The baseline characteristics of the 2 groups are summarized in . The proportion of patients with permanent nonvalvular atrial fibrillation was lower in the optimal implant group (48% vs 56%, P=.01). The baseline and clinical characteristics of the 2 groups according to postimplantation AT are summarized in table 1. Patients with nonintensive AT had a more frequent history of severe bleeding in both the optimal (93.0% vs 81.2% for those with intensive AT) and suboptimal implant groups (89.4% vs 85.1%, respectively). Compared with patients with intensive AT, the proportion of patients without intensive AT was higher among patients with a prior intracranial bleeding event and was lower among those with a prior gastrointestinal bleeding event (P=.027).
Baseline demographic and clinical characteristics according to optimal/suboptimal implantation and intensity of antithrombotic therapy after device implantation
| Total(n=1225) | Suboptimal implant+nonintensive AT(n=160) | Suboptimal implant+intensive AT(n=308) | Optimal implant+nonintensive AT(n=272) | Optimal implant+intensive AT(n=485) | P | |
|---|---|---|---|---|---|---|
| Age, y | 75.9±10.5 | 77.4±12.7 | 74.9±10.5 | 76.1±9.8 | 75.9±10.1 | .11 |
| Female sex | 429 (35.0) | 63 (39.4) | 105 (34.1) | 88 (32.4) | 173 (35.7) | .50 |
| Body mass index, kg/m2 | 27.4±5.1 | 27.4±5.0 | 27.7±5.5 | 27.5±4.8 | 27.1±5.0 | .41 |
| AF type | .013 | |||||
| Paroxysmal | 506 (41) | 63 (39) | 105 (34) | 119 (44) | 219 (45) | |
| Persistent | 88 (7) | 17 (11) | 20 (7) | 23 (8) | 28 (6) | |
| Permanent | 630 (52) | 80 (50) | 183 (59) | 130 (48) | 237 (49) | |
| Hypertension | 1053 (86.0) | 138 (86.3) | 259 (84.1) | 231 (84.9) | 425 (87.6) | .67 |
| Diabetes | 377 (30.7) | 47 (29.4) | 98 (31.5) | 70 (25.7) | 162 (33.4) | .23 |
| Smoking | 167 (13.6) | 22 (13.8) | 39 (12.7) | 41 (15.1) | 65 (13.4) | .86 |
| Chronic heart failure | 318 (26.0) | 35 (21.9) | 97 (31.5) | 63 (23.2) | 123 (25.5) | .059 |
| Previous CAD | 404 (33.0) | 50 (31.4) | 108 (35.1) | 87 (32.0) | 159 (32.8) | .82 |
| Previous ischemic stroke | 255 (20.9) | 28 (17.6) | 59 (19.2) | 56 (20.6) | 112 (23.1) | .38 |
| Previous bleed event | 1052 (85.9) | 143 (89.4) | 262 (85.1) | 253 (93.0) | 394 (81.2) | <.001 |
| Type of bleeding event | .027 | |||||
| Gastrointestinal | 486 (46) | 50 (35) | 133 (51) | 113 (44) | 190 (48) | |
| Intracranial | 338 (32) | 66 (46) | 68 (26) | 93 (37) | 111 (28) | |
| Abdominal/renal | 76 (7) | 9 (6) | 17 (7) | 17 (7) | 33 (8) | |
| ENT bleeding | 51 (5) | 6 (4) | 16 (6) | 10 (4) | 19 (5) | |
| Pulmonary | 19 (2) | 1 (1) | 5 (2) | 6 (2) | 7 (2) | |
| Others | 85 (8) | 11 (8) | 23 (8) | 15 (6) | 36 (9) | |
| CHA2DS2-VASc score | 4.4±1.5 | 4.7±1.3 | 4.3±1.5 | 4.4±1.4 | 4.4±1.4 | .12 |
| Baseline stroke risk | 6.0±2.8 | 6.7±2.8 | 5.8±2.9 | 5.9±2.8 | 6.0±2.8 | .07 |
| HAS-BLED score | 3.4±1.0 | 3.6±1.1 | 3.3±1.1 | 3.5±1.0 | 3.4±1.0 | .006 |
| Previous AT treatment | ||||||
| None | 278 (22.7) | 53 (33.1) | 52 (16.9) | 101 (37.1) | 72 (14.8) | <.001 |
| Aspirin | 366 (29.9) | 42 (26.3) | 89 (28.9) | 72 (26.5) | 163 (33.6) | .12 |
| P2Y12 | 91 (7.4) | 12 (7.5) | 24 (7.8) | 16 (5.9) | 39 (8.0) | .74 |
| Warfarin | 165 (13.5) | 16 (10.0) | 40 (13.0) | 31 (11.4) | 78 (16.1) | .14 |
| DOAC | 357 (29.1) | 34 (21.3) | 103 (33.4) | 58 (21.3) | 162 (33.4) | <.001 |
| LMWH | 138 (11.3) | 15 (9.4) | 49 (15.9) | 17 (6.3) | 57 (11.8) | .003 |
| eGFR, mL/min/1.73 m2 | 65.3 (46.5-93.7) | 60.1 (44.6-91.7) | 68.0 (48.4-97.5) | 65.3 (46.8-93.1) | 64.7 (46.8-93.1) | .38 |
| Dialysis | 65 (6.0) | 4 (3.7) | 14 (4.7) | 11 (5.4) | 36 (7.7) | .22 |
| LVEF, % | 55.4±10.3 | 54.9±11.5 | 55.0±11.0 | 54.0±11.1 | 56.6±9.0 | .01 |
AF, atrial fibrillation; AT, antithrombotic therapy; CAD, coronary artery disease; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtrated rate; ENT, ear, nose, throat; CHADS-VASc score, congestive heart failure, hypertension, age ≥ 75 years, age 65 to 74 years, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, vascular disease, sex female; LMWH, low molecular weight heparin; LVEF, left ventricular ejection fraction.
Values are expressed as No. (%) or mean±standard deviation.
Central illustration. Incidence and predictors of device-related thrombus after left atrial appendage closure in patients with an suboptimal or optimal implant receiving nonintensive or intensive AT. Left panel: main baseline and procedural characteristics and outcomes of the study population. Mid panel: incidence of device-related thrombus in patients with an optimal or suboptimal implant who were prescribed intensive or a nonintensive AT. Right panel: predictors of device-related thrombus in the study population. Exploratory analysis in patients with an optimal implant, showing no impact of AT intensity or device type. AT, antithrombotic treatment; BARC, Bleeding Academic Research Consortium; DRT, device-related thrombus; ICH, intracranial hemorrhage; LAAC, left atrial appendage closure; SE, systemic embolic events.
Study flowchart. A total of 1225 patients with successful LAAC were stratified by implant features into the suboptimal LAAC device implant group (distal implant and/or moderate or greater peridevice leak) or optimal LAAC device implant group (proximal implant and/or less than moderate peridevice leak) and nonintensive antithrombotic therapy (none or aspirin) or intensive antithrombotic therapy (other than previously described) at discharge. AT, antithrombotic treatment; LAAC, left atrial appendage closure; PDL, peridevice leak.
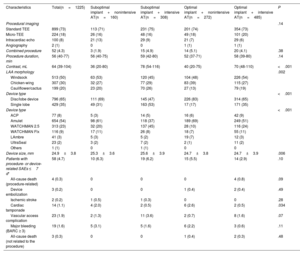
Table 2 shows the procedural characteristics in the 4 predefined groups. Compared with single-lobe devices, disc/lobe devices were more frequently associated with an optimal implant with either an intensive or nonintensive AT (17% vs 83% and 35% vs 65%, P<.001), which was mainly due to higher rates of deep single-lobe device implants. Procedural outcomes were similar between the groups, but a higher rate of tamponade was observed in patients with nonintensive AT. The rate of procedure-related serious adverse events was 4.7%. Four deaths related to the procedure were observed in the optimal implant and intensive AT group (vs none in the other groups, P=.09). Three device embolizations were reported in the optimal implant group (vs none in the suboptimal implant group, P=.49). The overall reported rate of significant bleeding (BARC ≥3) was 1.6% with a slight but nonstatistical increase in patients with nonintensive AT in both groups.
Procedural characteristics and outcomes
| Characteristics | Total(n=1225) | Suboptimal implant+nonintensive AT(n=160) | Suboptimal implant+intensive AT(n=308) | Optimal implant+nonintensive AT(n=272) | Optimal implant+intensive AT(n=485) | P |
|---|---|---|---|---|---|---|
| Procedural imaging | .14 | |||||
| Standard TEE | 899 (73) | 113 (71) | 231 (75) | 201 (74) | 354 (73) | |
| Micro-TEE | 224 (18) | 26 (16) | 48 (16) | 49 (18) | 101 (20) | |
| Intracardiac echo | 100 (8) | 21 (13) | 29 (9) | 21 (7) | 29 (6) | |
| Angiography | 2 (1) | 0 | 0 | 1 (1) | 1 (1) | |
| Combined procedure | 52 (4.3) | 3 (1.9) | 15 (4.9) | 14 (5.1) | 20 (4.1) | .38 |
| Procedure duration, min | 56 (40-77) | 56 (40-75) | 59 (42-80) | 52 (37-71) | 58 (39-80) | .14 |
| Contrast, mL | 64 (39-104) | 36 (20-80) | 78 (54-116) | 40 (20-75) | 70 (48-110) | <.001 |
| LAA morphology | .002 | |||||
| Windsock | 513 (50) | 63 (53) | 120 (45) | 104 (48) | 226 (54) | |
| Chicken-wing | 307 (30) | 32 (27) | 77 (29) | 83 (39) | 115 (27) | |
| Cauliflower/cactus | 199 (20) | 23 (20) | 70 (26) | 27 (13) | 79 (19) | |
| Device type | <.001 | |||||
| Disc/lobe device | 796 (65) | 111 (69) | 145 (47) | 226 (83) | 314 (65) | |
| Single lobe | 429 (35) | 49 (31) | 163 (53) | 17 (17) | 171 (35) | |
| Device type | <.001 | |||||
| ACP | 77 (8) | 5 (3) | 14 (5) | 16 (6) | 42 (9) | |
| Amulet | 654 (54) | 98 (61) | 118 (37) | 189 (69) | 249 (51) | |
| WATCHMAN 2.5 | 313 (23) | 32 (20) | 137 (45) | 28 (10) | 116 (24) | |
| WATCHMAN Flx | 116 (9) | 17 (11) | 26 (8) | 18 (7) | 55 (11) | |
| LAmbre | 41 (3) | 5 (3) | 5 (2) | 19 (7) | 12 (3) | |
| UltraSeal | 23 (2) | 3 (2) | 7 (2) | 2 (1) | 11 (2) | |
| Others | 1 (1) | 0 | 1 (1) | 0 | 0 | |
| Device size, mm | 24.9±3.8 | 25.3±3.6 | 25.6±3.9 | 24.7±3.8 | 24.7±3.9 | .006 |
| Patients with procedure- or device-related SAEs ≤7 d* | 58 (4.7) | 10 (6.3) | 19 (6.2) | 15 (5.5) | 14 (2.9) | .10 |
| All-cause death (procedure-related) | 4 (0.3) | 0 | 0 | 0 | 4 (0.8) | .09 |
| Device embolization | 3 (0.2) | 0 | 0 | 1 (0.4) | 2 (0.4) | .49 |
| Ischemic stroke | 2 (0.2) | 1 (0.5) | 1 (0.3) | 0 | 0 | .28 |
| Cardiac tamponade | 14 (1.1) | 4 (2.0) | 2 (0.5) | 6 (2.6) | 2 (0.5) | .034 |
| Vascular access complication | 23 (1.9) | 2 (1.3) | 11 (3.6) | 2 (0.7) | 8 (1.6) | .07 |
| Major bleeding (BARC ≥ 3) | 19 (1.6) | 5 (3.1) | 5 (1.6) | 6 (2.2) | 3 (0.6) | .11 |
| All-cause death (not related to the procedure) | 3 (0.3) | 0 | 0 | 1 (0.4) | 2 (0.3) | .48 |
BARC, Bleeding Academic Research Consortium; LAA, left atrial appendage; SAE, serious adverse event; TEE, transesophageal echocardiography.
Values are expressed as No. (%) or mean±standard deviation.
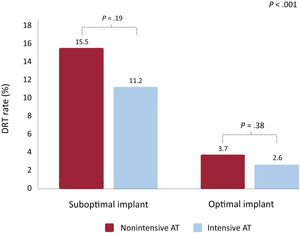
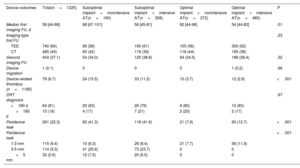
As shown in table 3, DRT was detected in 79 patients (6.7%). Overall, 81% of DRTs were detected <180 days post-LAAO and 19%>180 days after the procedure. Compared with patients with a suboptimal device implant, patients with an optimal implant showed a lower rate of DRT (22/757, 2.9% vs 57/468, 12.1%, respectively, P<.001). Moreover, compared with patients with nonintensive AT, the incidence of DRT was slightly but not significantly decreased with intensive AT in both groups with optimal (2.6% vs 3.7% for patients with nonintensive AT, P=.38) and suboptimal implants (11.2% vs 15.5%, respectively, P=.19), as shown in table 3, figure 1, and figure 3. Of note, the incidence of DRT was higher among patients with a suboptimal device implant than among those with an optimal implant, irrespective of the AT intensity. This association was independent of the type of implanted device ().
Device outcomes at maximum follow-up
| Device outcomes | Total(n=1225) | Suboptimal implant+nonintensive AT(n=160) | Suboptimal implant+intensive AT(n=308) | Optimal implant+nonintensive AT(n=272) | Optimal implant+intensive AT(n=485) | P |
|---|---|---|---|---|---|---|
| Median first imaging FU, d | 58 [44-88] | 68 [47-101] | 56 [45-81] | 62 [44-96] | 54 [44-82] | .01 |
| Imaging type first FU | .23 | |||||
| TEE | 740 (60) | 95 (58) | 190 (61) | 155 (56) | 300 (62) | |
| CT | 485 (40) | 65 (42) | 118 (39) | 118 (44) | 185 (38) | |
| Second imaging FU | 454 (37.1) | 54 (34.0) | 120 (38.8) | 94 (34.5) | 186 (38.4) | .32 |
| Device migration | 1 (0.1) | 0 | 0 | 0 | 1 (0.2) | .68 |
| Device-related thrombus (n=1185) | 79 (6.7) | 24 (15.5) | 33 (11.2) | 10 (3.7) | 12 (2.6) | <.001 |
| DRT diagnosis | .97 | |||||
| <180 d | 64 (81) | 20 (83) | 26 (79) | 8 (80) | 10 (83) | |
| >180 d | 15 (19) | 4 (17) | 7 (21) | 2 (20) | 2 (17) | |
| Peridevice leak | 261 (22.3) | 62 (41.3) | 118 (41.6) | 21 (7.9) | 60 (12.7) | <.001 |
| Peridevice leak | <.001 | |||||
| 1-3 mm | 115 (9.4) | 10 (6.3) | 26 (8.4) | 21 (7.7) | 58 (11.9) | |
| 3-5 mm | 114 (9.3) | 41 (25.6) | 73 (23.7) | 0 | 0 | |
| >5 mm | 32 (2.6) | 12 (7.5) | 20 (6.5) | 0 | 0 |
AT, antithrombotic treatment; CT, computed tomography; DRT, device-related thrombus; FU, follow-up; ICH, intracranial hemorrhage; TEE, transesophageal echocardiography.
Values are expressed as No. (%), or median [interquartile range].
Device-related thrombus rates according to implant features and antithrombotic therapy at discharge. The proportion of patients with a DRT was not significantly different with intensive or nonintensive AT after a suboptimal (11.2% vs 15.5%, P=.19, respectively) or optimal device implant (2.6% vs 3.7%, P=.38, respectively). AT, antithrombotic treatment; DRT, device-related thrombus.
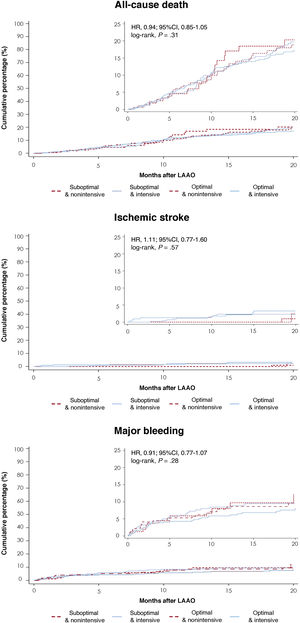
Four strokes were observed at 3 months in the suboptimal implant and intensive AT group (vs none in the other groups, P=.014). At 12 months of follow-up, clinical outcomes, including stroke, systemic embolism, major bleeding, and all-cause mortality, did not differ between the groups (). The cumulative incidence of all-cause death, ischemic stroke and major bleeding at maximum follow-up showed no difference between the groups (figure 4).
Clinical outcomes at follow-up. Clinical outcomes stratified by implant features and AT at discharge. A: all-cause death; B: ischemic stroke, and C: major bleeding events. 95%CI, 95% confidence interval; AT, antithrombotic therapy; HR, hazard ratio; LAAO, left atrial appendage occlusion.
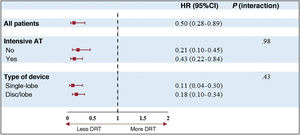
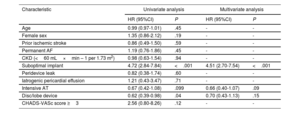
Table 4 and figure 1 show the independent predictors of DRT. After multivariate analysis, suboptimal implantation was identified as the sole independent predictor of DRT. Importantly, device type and intensive AT did not emerge as independent DRT predictors. Optimal implantation was significantly associated with lower DRT, with no interaction of AT or device type as shown in figure 1 and figure 5.
Predictors of device-related thrombus
| Characteristic | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 0.99 (0.97-1.01) | .45 | - | - |
| Female sex | 1.35 (0.86-2.12) | .19 | - | - |
| Prior ischemic stroke | 0.86 (0.49-1.50) | .59 | - | - |
| Permanent AF | 1.19 (0.76-1.86) | .45 | - | - |
| CKD (<60 mL×min – 1 per 1.73 m2) | 0.98 (0.63-1.54) | .94 | - | - |
| Suboptimal implant | 4.72 (2.84-7.84) | <.001 | 4.51 (2.70-7.54) | <.001 |
| Peridevice leak | 0.82 (0.38-1.74) | .60 | - | - |
| Iatrogenic pericardial effusion | 1.21 (0.43-3.47) | .71 | - | - |
| Intensive AT | 0.67 (0.42-1.08) | .099 | 0.66 (0.40-1.07) | .09 |
| Disc/lobe device | 0.62 (0.39-0.98) | .04 | 0.70 (0.43-1.13) | .15 |
| CHADS-VASc score ≥3 | 2.56 (0.80-8.26) | .12 | - | - |
95%CI, 95% confidence interval; AF, atrial fibrillation; AT, antithrombotic treatment; CKD, chronic kidney disease; HR, hazard ratio; OR, odds ratio.
Variables identified following univariable logistic regression of P values <.10 included in the final multivariable logistic regression model. Multivariable analysis data are presented as ORs with corresponding 95%CIs.
The main findings of the present study assessing the impact of intensive vs nonintensive AT in patients with optimal and suboptimal implants after transcatheter LAAC were as follows: a) suboptimal implants were associated with a higher incidence of DRT after LAAC, which was not mitigated by intensive AT; b) in patients with optimal implants, the use of intensive AT might not be necessary, as the incidence of DRT was low and similar between the intensive and nonintensive AT groups; and c) suboptimal device implantation, but not intensive AT, emerged as an independent predictor of DRT.
DRT remains one of the main concerns after LAAC, as it is associated with a significant increase in ischemic stroke and systemic embolization.25 Considering the growing procedural volumes and the expansion of LAAC to lower-risk patients, predicting and preventing DRT is of the utmost importance. Risk factors for DRT are mostly nonmodifiable and related to patient characteristics, such as prior transient ischemic attack and stroke, permanent AF, advanced age, heart failure, and renal insufficiency.19 In contrast to these conditions, the depth of device implantation seems to be the only modifiable factor associated with varying DRT rates.15,16,18–22
Although the rationale for AT after LAAC is to prevent DRT by facilitating adequate device endothelialization, the association between post-LAAC AT and DRT remains contentious. Some studies showed a strong correlation between the type of AT after the procedure and the incidence of DRT,26 while others have found no significant association.7,19,22,27 These conflicting data are reflected in the significant heterogeneity of post-LAAC AT in current practice and result from a lack of evidence supporting a specific AT intensity after LAAC.28
Using data from the largest cohort to date, including both disc/lobe and single- lobe occluders, we classified patients according to achieved implant as either optimal (proximal implant and the absence of significant PDL) or suboptimal (distal implant and/or the presence of significant PDL). According to the latest evidence, different cutoffs were selected to define distal implants: ≥1mm for disc/lobe devices and>10mm for single lobe devices.15,22,24,27 In addition, the cutoff value for a large PDL was primarily considered to be>5mm, but recent data indicate that PDL 3 to 5mm may be associated with an increased rate of thromboembolic events after LAAC.28 Accordingly, PDL>3mm was considered significant for both devices and entered the definition of a suboptimal implant.
Our study reinforces the lack of additional benefits from intensive AT in preventing DRT, since both intensive and nonintensive AT were associated with comparable incidences of DRT in patients with either suboptimal or optimal implants. In other words, the present study suggests that in patients with an optimal device implant, the use of intensive therapies after LAAC might not be justified and would only translate into a potential higher risk of bleeding. Moreover, the intensity of AT did not seem to offset the deleterious effects of a distal implant. This finding is clinically relevant as several studies have previously reported a higher risk of DRT after deep implants.15,19,22 Thus, at hospital discharge, physicians were tempted to prescribe intensive AT in patients with a suboptimal device implant to decrease the incidence of DRT.
The incidence of PDL and DRT varies considerably among published studies due to the variability of imaging tools and the lack of standardized post-LAAC surveillance imaging. Because of higher spatial resolution, CT has been shown to be more sensitive than TEE in detecting PDL.24 However, while PDL detected by TEE has been associated with adverse events, residual leaks identified by coronary computed tomography angiography (CCTA) are more frequent but lack prognostic significance.21 These findings underscore the need for further research to characterize and identify high-risk PDLs using CCTA.
Regarding DRT, TEE and CCTA offer similar diagnostic capabilities, although CCTA provides higher sensitivity in detecting hypoattenuated thickening (HAT), a commonly observed imaging finding in all transcatheter and surgical devices.24 Although the presence of DRT has largely been considered a binary finding in the literature, HAT is now increasingly recognized on post-LAAC CT. The question of whether this subclinical finding should be considered a precursor to DRT remains open.
In the present study, initial imaging follow-up was performed using either TEE (60%) or CT (40%), and DRT was defined according to classical criteria as a thrombus adherent to the left atrial surface of the device. This definition excluded low grade HAT (grades 0 and 1). The first follow-up imaging study was performed between the 6th and 12th weeks after the procedure, at a median of 58 [44-88] days, when most patients were still receiving the AT prescribed at discharge. Consequently, most teams consider a DRT-free examination to be a prerequisite for AT de-escalation. As proposed by expert recommendations,17 a second follow-up imaging study was performed in 37% of patients between the 6th and 12th months after the index procedure, and it is notable that DRT were mostly diagnosed (81%) during the initial examination. This is not in accordance with previous reports showing a balanced detection between early and late timings.
For instance, in the PROTECT-AF and PREVAIL trials, 51% of DRTs detected during scheduled TEE were observed at the 1-year examination, and 62% of those detected during unscheduled examinations were observed at>1 year.10 Interestingly, in the AMULET IDE trial, most DRTs associated with the AMULET device (61%) occurred ≤45 days, whereas most DRTs associated with the WATCHMAN device (74%) were identified>45 days.29 This discrepancy has raised concerns about possible device-specific mechanisms underlying the timing of DRT formation, although our data showed consistency for both devices. Irrespective of our findings, the nature and duration of the optimal AT still remain an open and challenging question.
LimitationsThe first limitation of this study is its observational design, which carries the risk of inherent bias for several analyzed variables; thus, our results should be considered hypothesis-generating. In particular, AT intensity was left to the physician's discretion, which implies several selection biases. For example, patients deemed at higher risk for bleeding were more likely to receive nonintensive AT, while those with a risk balance favoring ischemic events were likely prescribed more intensive AT for longer durations. Further studies are needed to confirm our findings. Second, the clinical and imaging results were site-reported, with no independent adjudication or centralized core lab involved. Third, we used TEE and CT for imaging follow-up, although these 2 techniques may have different capabilities for detecting PDL and/or DRT. Additionally, there is no standardized definition of DRT with TEE, and even less so with CT. Fourth, although all patients underwent imaging follow-up between 6 and 12 weeks after the procedure, only 37% had a scheduled repeat examination. Fifth, while the cutoff values selected have clinical significance, they are arbitrary and require further exploration and external validation for use in clinical practice. Sixth, the study was not designed to identify factors associated with endpoints other than DRT (eg, ischemic stroke). Finally, an independent event adjudication committee was not involved in this study, and the data were based on clinical reports from each center.
CONCLUSIONSThe incidence of DRT after LAAC was higher in patients with suboptimal implants, which was not mitigated by intensive AT. Compared with nonintensive AT, intensive AT was not associated with a lower incidence of DRT after either an optimal or suboptimal implant. Suboptimal implants, but not intensive AT, emerged as an independent predictor of DRT. Further studies are warranted to confirm these results.
FUNDINGThis study received no funding.
ETHICAL CONSIDERATIONSThe study was conducted in accordance with the institutional ethics committee of each participating center, and all patients provided signed informed consent for the procedures. The study conformed to the guiding principles of the Declaration of Helsinki. In accordance with the SAGER guidelines, the information was disaggregated in table 1 with no difference between groups.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEThe authors declare that they have not used any type of generative artificial intelligence for the preparation of this manuscript.
AUTHORS’ CONTRIBUTIONSEach author contributed significantly to the submitted work. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: P. Garot, P. Cepas-Guillén, X. Freixa. Drafting the work or revising it critically for important intellectual content: E. Flores-Umanzor, N. Leduc, V. Bajoras, N. Perrin, A. McInerney, A. Lafond, J. Farjat-Pasos, X. Millán, G. ÓHara, S. Zandjebil, R. Ibrahim, P. Salinas, O. de Backer, J.E Nielsen-Kudsk, I. Cruz-González, D. Arzamendi, L. Sanchis, L. Nombela-Franco, A. Aminian, J. Rodés-Cabau. All authors approved the final version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICTS OF INTERESTP. Garot reports receiving speaker/advisory/proctor fees from Abbott, Biosensors, Boston Scientific, Edwards Lifesciences, General Electric HealthCare, and Terumo. He is medical director and shareholder of the Cardiovascular European Research Center in Massy, France. O. de Backer received institutional research grants and consulting fees from Abbott and Boston Scientific. R. Ibrahim reports receiving speaker/advisory/proctor fees from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. L. Sanchis is proctor for Abbott Medical and is associate editor of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed. A. Aminian is a proctor and consultant for Abbott and Boston Scientific. J.E. Nielsen-Kudsk is a proctor and consultant for Abbott and Boston Scientific. X. Freixa is a proctor for Abbott and Boston Scientific. The remaining authors report no potential conflicts of interest with respect to the content of this article.
- -
The selection of antithrombotic therapy to prevent thromboembolism in patients with atrial fibrillation after left atrial appendage closure (LAAC) must consider several clinical and procedure-related factors. After LAAC, different antithrombotic regimens, including oral direct thrombin and factor Xa inhibitors (direct oral anticoagulants) or single antiplatelet therapy/dual antiplatelet therapy, are prescribed to mitigate the risk of DRT and associated thromboembolic events. Direct oral anticoagulants and dual antiplatelet therapy are currently approved for clinical use in the United States and in Europe after LAAC but there is currently no validated strategy as these regimens have not been compared in a randomized clinical trial.
- -
Optimal device implantation is associated with a lower risk of DRT, independently of AT intensity or device type. Intensive AT (direct oral anticoagulants or dual antiplatelet therapy) fails to mitigate the incidence of DRT after both optimal and suboptimal LAAC device implantation. Additional research is needed to determine the optimal antithrombotic regimen and its duration after LAAC. Nonintensive AT might be recommended, given its clinical impact, in patients with optimal implants.
Supplementary data associated with this article can be found in the online version, available at https://doi.org/10.1016/j.rec.2024.11.006