Population aging is associated with an increased prevalence of atrial fibrillation (AF) and dementia. This study aimed to analyze the impact of oral anticoagulation in elderly patients with AF and moderate-severe dementia.
MethodsWe conducted a single-center retrospective study analyzing patients aged ≥ 85 years with a diagnosis of AF between 2013 and 2018. The impact of anticoagulation on mortality, embolisms, and bleeding events was assessed by multivariate Cox analysis. In patients with dementia, this analysis was complemented by propensity score matching, depending on whether the patients were prescribed anticoagulant treatment or not.
ResultsOf the 3549 patients aged ≥ 85 years with AF, 221 had moderate-severe dementia (6.1%), of whom 88 (60.2%) were anticoagulated. During a follow-up of 2.8 ±1.7 years, anticoagulation was associated with lower embolic risk and higher bleeding risk both in patients with dementia (hazard ratio [HR]embolisms, 0.36; 95%CI, 0.15-0.84; HRbleeding, 2.44; 95%CI, 1.04-5.71) and in those without dementia (HRembolisms, 0.58; 95%CI, 0.45-0.74; HRbleeding, 1.55, 95%CI, 1.21-1.98). However, anticoagulation was associated with lower mortality only in patients without dementia (HR, 0.63; 95%CI, 0.53-0.75) and not in those with dementia (adjusted HR, 1.04; 95%CI, 0.63-1.72; P=.541; HR after propensity score matching 0.91, 95%CI, 0.45-1.83; P=.785).
ConclusionsIn patients aged ≥ 85 years with moderate-severe dementia and AF, oral anticoagulation was significantly associated with a lower embolic risk and a higher bleeding risk, with no differences in total mortality.
Keywords
Population aging has increased the prevalence of chronic diseases such as atrial fibrillation (AF) and dementia.1 In patients aged ≥ 85 years, the estimated prevalence of AF is about 15%2 while that of dementia is 20%.3 Elderly patients with both AF and dementia are not infrequent. Thus, between 3% and 5% of patients with AF are estimated to have been diagnosed with some form of dementia,4 and this percentage rises to almost 10% in octogenarian patients.5 By applying these data to the Spanish population, there are currently about 40 000 octogenarians with AF and dementia in Spain, with this number expected to exceed 100 000 by 2050.1
The importance of this population subgroup—elderly people with AF and dementia—lies not only in its growing size, but also in its management.6,7 Anticoagulation has been associated with a significant reduction in the risk of embolic events in patients with AF, including elderly patients. However, patients with dementia, particularly those with advanced disease, were underrepresented in the clinical trials that evaluated anticoagulation. Thus, the aim of the present study was to analyze the prognostic impact of anticoagulant therapy in elderly patients (age ≥ 85 years) with AF and dementia.
METHODSStudy populationThe current analysis examined patients enrolled in the registry of Acute Coronary Syndrome of the University Hospital of Vigo focused on Atrial Fibrillation (CardioCHUVI-FA). This retrospective registry included all patients diagnosed with AF in the health care area of Vigo between January 1, 2014 and January 1, 2018. To create this registry, patients were first identified by using administrative databases of both inpatients and outpatients. Specific patient groups were identified using the Complex Information Analysis System of the Galician Health Service for both primary care and inpatient care and with the codes 427.31 of the International Classification of Diseases-Ninth Edition and K78 of the International Classification of Primary Care. Of a total of 16 975 patients, we selected a subgroup of patients aged ≥ 85 years. In the second phase, the medical records of all patients were reviewed to confirm the diagnosis of AF (electrocardiographically documented) and to collect data on baseline clinical variables, therapeutic strategy, and events during follow-up.
Patients with a mechanical prosthesis or moderate-to-severe mitral stenosis were excluded. Also excluded were patients receiving chronic therapy with low-molecular-weight heparin.
Because less than 2% of the values were lost for each variable analyzed (n < 70), no specific method was applied to adjust for these missing values.
The study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Autonomous Research Ethics Committee of Galicia (code HAC-ACO-2018-01, record 2018/258).
Endpoints and follow-upThe main clinical endpoint of this study was total mortality. Secondary endpoints were embolic and bleeding events. Embolic events comprised strokes (ischemic stroke and transient ischemic attack), pulmonary embolism, and peripheral embolism. Bleeding events comprised clinically relevant bleeding according to the definition of the International Society on Thrombosis and Haemostasis.8,9 Follow-up was completed for all patients at the time of their death or at the last date that they were proven to be alive. The anticoagulant decision for each patient was based on the clinical judgment of the patient's treating physician. Because the oral anticoagulation variable was time-dependent, patients who underwent treatment changes (from no anticoagulation to oral anticoagulation or vice versa) were classified according to the treatment approach at the time of their inclusion.
DefinitionsOur study specifically analyzed patients ≥ 85 years old with a diagnosis of “nonvalvular AF” according to European recommendations. Accordingly, patients with a mechanical prosthesis or mitral stenosis ≤ 1.5cm2 were not included. Patients were classified into 2 groups: with and without moderate-to-severe dementia, defined as cognitive decline between stages 5 and 7 of the Reisberg Global Deterioration Scale,10 which itself corresponds to stages of the Functional Assessment Staging scale ≥ 5.11 Accordingly, patients with mild cognitive decline or incipient/mild dementia were not included; at these stages, the patients have memory problems but are functionally autonomous for activities of daily living. The diagnosis of ischemic stroke was confirmed using concomitant imaging studies, including computed tomography and magnetic resonance. Transient ischemic attack was defined as temporary neurological dysfunction resulting from focal cerebral, spinal cord, or retinal ischemia, with no acute infarct lesion. Bleeding was defined according to the classification of the International Society of Thrombosis and Haemostasis and all clinically relevant events were included (both major and minor).8,9
Statistical analysisContinuous variables are expressed as mean±standard deviation and were compared using the Student t test. Categorical variables are expressed as percentages and were compared using the chi-square test. The impact of oral anticoagulation on mortality, embolisms, and bleeding were evaluated using Cox regression analysis with robust estimation of variance; the variable oral anticoagulation was time-dependent. These analyses were adjusted by the variables associated with events in univariate analysis () or by those whose association with clinical events has been consistently shown in previous studies. Thus, multivariate analyses were adjusted by age, sex, hypertension, diabetes mellitus, history of ischemic heart disease, heart failure, previous stroke, chronic obstructive pulmonary disease, previous hospitalization for bleeding, anemia, glomerular filtration rate measured by the CKD-EPI equation (Chronic Kidney Disease Epidemiology Collaboration), left ventricular ejection fraction ≤ 40%, CHA2DS2-VASc and HAS-BLED scores, and treatment with antiplatelet agents, beta-blockers, digoxin, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and proton pump inhibitors. The proportional hazards assumption was tested using the Schoenfeld residual test. The results are expressed as hazard ratios (HRs) with their 95% confidence intervals (95%CIs). Differences with P <.05 were considered statistically significant. The results are graphically displayed using Kaplan-Meier curves.
In addition, within the dementia patient group, the analyses were complemented with propensity score matching to balance the patients’ baseline characteristics with and without anticoagulation. This analysis used a 1:1 matching algorithm without replacement, with a 0.2 caliper for standard deviations and with the nearest neighbor matching method. The independent variables used to obtain the propensity score were age, sex, hypertension, diabetes mellitus, ischemic heart disease, previous stroke or embolism, previous heart failure or left ventricular ejection fraction ≤ 40%, history of bleeding, anemia, CKD-EPI <60mL/min/1.73 m2, CHA2DS2-VASc score, HAS-BLED score, antiplatelet therapy, and treatment with beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, digoxin, statins, and proton pump inhibitors. The statistical analyses were performed with SPSS version 25.0 and Stata MP64 version 15.0.
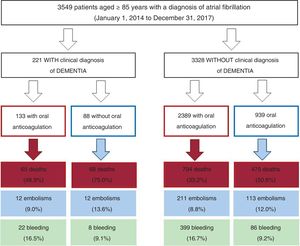
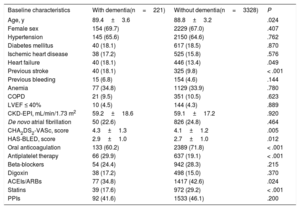
RESULTSBaseline characteristicsIn total, we identified 3595 patients aged ≥ 85 years with a confirmed diagnosis of AF. Excluded from this group were those with a mechanical prosthesis or moderate-to-severe mitral stenosis (n=10) and those receiving chronic therapy with low-molecular-weight heparin (n=36). Accordingly, the final study population comprised 3549 patients (figure 1); 2382 were women. The mean age of the overall group was 88.9±3.2 years. Of these patients, 221 had moderate-to-severe dementia (6.2%): 68.3% had Alzheimer disease, 20.8% had vascular dementia, 7.2% had mixed vascular-degenerative dementia, and 3.6% had other causes of dementia. The differences in the baseline characteristics of the patients with and without dementia are shown in table 1. Patients with dementia were older and had higher rates of heart failure history and previous ischemic stroke and higher CHA2DS2-VASc and HAS-BLED scores.
Differences in baseline, test result, echocardiographic, and medical therapy characteristics between patients aged ≥ 85 years with and without dementia.
| Baseline characteristics | With dementia(n=221) | Without dementia(n=3328) | P |
|---|---|---|---|
| Age, y | 89.4±3.6 | 88.8±3.2 | .024 |
| Female sex | 154 (69.7) | 2229 (67.0) | .407 |
| Hypertension | 145 (65.6) | 2150 (64.6) | .762 |
| Diabetes mellitus | 40 (18.1) | 617 (18.5) | .870 |
| Ischemic heart disease | 38 (17.2) | 525 (15.8) | .576 |
| Heart failure | 40 (18.1) | 446 (13.4) | .049 |
| Previous stroke | 40 (18.1) | 325 (9.8) | < .001 |
| Previous bleeding | 15 (6.8) | 154 (4.6) | .144 |
| Anemia | 77 (34.8) | 1129 (33.9) | .780 |
| COPD | 21 (9.5) | 351 (10.5) | .623 |
| LVEF ≤ 40% | 10 (4.5) | 144 (4.3) | .889 |
| CKD-EPI, mL/min/1.73 m2 | 59.2±18.6 | 59.1±17.2 | .920 |
| De novo atrial fibrillation | 50 (22.6) | 826 (24.8) | .464 |
| CHA2DS2-VASc, score | 4.3±1.3 | 4.1±1.2 | .005 |
| HAS-BLED, score | 2.9±1.0 | 2.7±1.0 | .012 |
| Oral anticoagulation | 133 (60.2) | 2389 (71.8) | < .001 |
| Antiplatelet therapy | 66 (29.9) | 637 (19.1) | < .001 |
| Beta-blockers | 54 (24.4) | 942 (28.3) | .215 |
| Digoxin | 38 (17.2) | 498 (15.0) | .370 |
| ACEIs/ARBs | 77 (34.8) | 1417 (42.6) | .024 |
| Statins | 39 (17.6) | 972 (29.2) | < .001 |
| PPIs | 92 (41.6) | 1533 (46.1) | .200 |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes mellitus, stroke (doubled), vascular disease, age 65-74 years, and sex (female); CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, age> 65 years, and concomitant use of drugs and alcohol; LVEF, left ventricular ejection fraction; PPIs, proton pump inhibitors.
Unless otherwise indicated, the data represent No. (%) or mean±standard deviation.
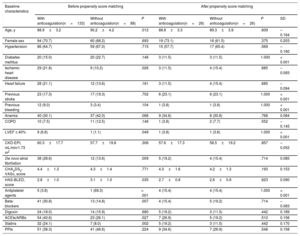
Of the 3549 patients, 2522 (71.1%) were receiving oral anticoagulant therapy (figure 1). Fewer patients with dementia were receiving oral anticoagulants than patients without dementia (60.2% vs 71.8%; P <.001). The most frequently used anticoagulants were vitamin K antagonists (VKAs) (75.9% and 79.6% in patients with and without dementia, respectively; P=.598) and the 2 groups had a similar time in therapeutic range (> 60%; 45.5% vs 46.3%; P=.883). Table 2 shows the baseline characteristics of the patients with dementia according to whether they were receiving anticoagulant therapy or not.
Baseline, test result, echocardiographic, and medical therapy characteristics of patients aged ≥ 85 years with dementia treated with and without anticoagulation. Results before and after propensity score matching
| Baseline characteristics | Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|---|
| With anticoagulation(n=133) | Without anticoagulation(n=88) | P | With anticoagulation(n=26) | Without anticoagulation(n=26) | P | SD | |
| Age, y | 88.9±3.2 | 90.2±4.2 | .012 | 88.8±3.3 | 89.3±3.9 | .609 | –0.164 |
| Female sex | 94 (70.7) | 60 (68.2) | .693 | 19 (73.1) | 16 (61.5) | .375 | 0.203 |
| Hypertension | 86 (64.7) | 59 (67.0) | .715 | 15 (57.7) | 17 (65.4) | .569 | –0.160 |
| Diabetes mellitus | 20 (15.0) | 20 (22.7) | .146 | 3 (11.5) | 3 (11.5) | 1.000 | < 0.001 |
| Ischemic heart disease | 29 (21.8) | 9 (10.2) | .026 | 3 (11.5) | 4 (15.4) | .685 | –0.093 |
| Heart failure | 28 (21.1) | 12 (13.6) | .161 | 3 (11.5) | 4 (15.4) | .685 | –0.094 |
| Previous stroke | 23 (17.3) | 17 (19.3) | .702 | 6 (23.1) | 6 (23.1) | 1.000 | < 0.001 |
| Previous bleeding | 12 (9.0) | 3 (3.4) | .104 | 1 (3.8) | 1 (3.8) | 1.000 | < 0.001 |
| Anemia | 40 (30.1) | 37 (42.0) | .068 | 9 (34.6) | 8 (30.8) | .768 | 0.084 |
| COPD | 10 (7.5) | 11 (12.5) | .146 | 1 (3.8) | 2 (7.7) | .552 | –0.145 |
| LVEF ≤ 40% | 9 (6.8) | 1 (1.1) | .049 | 1 (3.8) | 1 (3.8) | 1.000 | < 0.001 |
| CKD-EPI, mL/min/1.73 m2 | 60.3±17.7 | 57.7±19.8 | .306 | 57.6±17.3 | 58.5±19.2 | .857 | –0.052 |
| De novo atrial fibrillation | 38 (28.6) | 12 (13.6) | .009 | 5 (19.2) | 4 (15.4) | .714 | 0.085 |
| CHA2DS2-VASc, score | 4.4±1.3 | 4.3±1.4 | .771 | 4.3±1.6 | 4.2±1.3 | .193 | 0.153 |
| HAS-BLED, score | 2.8±1.0 | 3.1±1.0 | .035 | 2.7±0.8 | 2.6±0.9 | .623 | 0.090 |
| Antiplatelet agents | 5 (3.8) | 1 (69.3) | < .001 | 4 (15.4) | 4 (15.4) | 1.000 | < 0.001 |
| Beta-blockers | 41 (30.8) | 13 (14.8) | .007 | 4 (15.4) | 5 (19.2) | .714 | –0.083 |
| Digoxin | 24 (18.0) | 14 (15.9) | .680 | 5 (19.2) | 3 (11.5) | .442 | 0.189 |
| ACEIs/ARBs | 54 (40.6) | 23 (26.1) | .027 | 7 (26.9) | 5 (19.2) | .510 | 0.156 |
| Statins | 32 (24.1) | 7 (8.0) | .002 | 5 (19.2) | 3 (11.5) | .442 | 0.170 |
| PPIs | 51 (38.3) | 41 (46.6) | .224 | 9 (34.6) | 7 (26.9) | .548 | 0.158 |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes mellitus, stroke (doubled), vascular disease, age 65-74 years, and sex (female); COPD, chronic obstructive pulmonary disease; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, age> 65 years, and concomitant use of drugs and alcohol; LVEF, left ventricular ejection fraction; PPIs, proton pump inhibitors.
Unless otherwise indicated, the data represent No. (%) or mean±standard deviation (SD).
During follow-up, 29 patients with dementia (14.2%) underwent changes in antithrombotic therapy (1 started anticoagulation, 10 discontinued anticoagulation, and 18 switched from VKAs to direct oral anticoagulants [DOACs]; no patients switched from DOACs to VKAs). Analysis revealed that 41.4% of the changes were due to poor control of the international normalized ratio in patients under treatment with VKAs, 20.7% due to high perceived bleeding risk, 10.3% due to high perceived embolic risk, and 27.6% for unknown reasons. Of the patients without dementia, 471 (13.1%) changed their antithrombotic therapy (16 started anticoagulation, 73 discontinued anticoagulation, 381 switched from VKAs to DOACs, and 1 switched from DOACs to VKAs). The results indicated that 44.4% of the changes were due to poor control of the international normalized ratio in patients under treatment with VKAs, 18.9% due to high perceived bleeding risk, 10.9% due to high perceived embolic risk, and 25.9% for unknown reasons ().
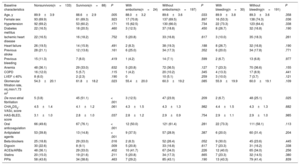
Events during follow-upThe patients were followed up for 2.8±1.7 years (2.9±1.7 years in the dementia group and 2.4±1.6 years in the group without dementia). Compared with patients without dementia, patients with dementia had higher mortality (annual incidence, 25.9% vs 13.7%; P <.001) but similar rates of embolic (annual incidence, 4.7% vs 3.6%; P=.219) and bleeding (annual incidence, 5.6% vs 5.3%; P=.765) events. Table 3 shows the baseline characteristics of the patients with dementia according to events during follow-up (mortality, embolisms, and bleeding). In addition, shows the bleeding location according to the presence of dementia and whether anticoagulant therapy was prescribed or not.
Differences in the baseline characteristics of the patients with dementia according to event (mortality, embolisms, and bleeding)
| Baseline characteristics | Nonsurvivors(n=133) | Survivors(n=88) | P | With embolisms(n=24) | Without embolisms(n=197) | P | With bleeding(n=30) | Without bleeding(n=191) | P |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 89.9±3.9 | 88.6±2.9 | .005 | 88.0±3.2 | 89.6±3.6 | .033 | 89.9±3.8 | 89.3±3.6 | .358 |
| Female sex | 93 (69.9) | 61 (69.3) | .923 | 17 (70.8) | 137 (69.5) | .897 | 16 (53.3) | 138 (74.3) | .036 |
| Hypertension | 92 (69.2) | 53 (60.2) | .171 | 15 (62.5) | 130 (66.0) | .734 | 22 (73.3) | 123 (64.4) | .338 |
| Diabetes mellitus | 22 (16.5) | 18 (20.5) | .460 | 3 (12.5) | 37 (18.8) | .450 | 8 (26.7) | 32 (16.8) | .190 |
| Ischemic heart disease | 22 (16.5) | 16 (18.2) | .752 | 5 (20.8) | 33 (16.8) | .617 | 3 (10.0) | 35 (18.3) | .261 |
| Heart failure | 26 (19.5) | 14 (15.9) | .491 | 2 (8.3) | 38 (19.3) | .188 | 8 (26.7) | 32 (16.8) | .190 |
| Previous stroke | 28 (21.1) | 12 (13.6) | .161 | 6 (25.0) | 34 (17.3) | .352 | 6 (20.0) | 34 (17.8) | .771 |
| Previous bleeding | 15 (11.3) | 7 (8.0) | .419 | 1 (4.2) | 14 (7.1) | .599 | 2 (6.7) | 13 (6.8) | .977 |
| Anemia | 48 (36.1) | 29 (33.0) | .632 | 5 (20.8) | 72 (36.5) | .127 | 7 (23.3) | 70 (36.6) | .155 |
| COPD | 16 (12.0) | 5 (5.7) | .115 | 1 (4.2) | 20 (10.2) | .345 | 4 (13.3) | 17 (8.9) | .441 |
| LVEF ≤ 40% | 8 (6.0) | 2 (2.3) | .190 | 0 | 10 (5.1) | .259 | 3 (10.0) | 7 (3.7) | .121 |
| Glomerular filtration rate, mL/min/1.73 m2 | 54.3±20.1 | 61.8±18.2 | .023 | 55.4±20.0 | 60.3±19.2 | .085 | 55.9±19.9 | 60.0±19.1 | .109 |
| De novo atrial fibrillation | 5 (3.8) | 45 (51.1) | < .001 | 3 (12.5) | 47 (23.9) | .209 | 2 (6.7) | 48 (25.1) | .025 |
| CHA2DS2-VASc, score | 4.5±1.4 | 4.1±1.2 | .061 | 4.3±1.5 | 4.3±1.3 | .982 | 4.4±1.5 | 4.3±1.3 | .682 |
| HAS-BLED, score | 3.1±1.0 | 2.8±1.0 | .037 | 2.8±1.2 | 2.9±0.9 | .754 | 2.9±1.1 | 2.9±1.0 | .985 |
| Oral anticoagulation | 66 (49.6) | 67 (76.1) | < .001 | 12 (50.0) | 121 (61.4) | .281 | 22 (73.3) | 111 (58.1) | .113 |
| Antiplatelet agents | 53 (39.8) | 13 (14.8) | < .001 | 9 (37.5) | 57 (28.9) | .387 | 6 (20.0) | 60 (31.4) | .204 |
| Beta-blockers | 25 (18.8) | 29 (33.0) | .016 | 2 (8.3) | 52 (26.4) | .052 | 9 (30.0) | 45 (23.6) | .445 |
| Digoxin | 30 (22.6) | 8 (9.1) | .009 | 5 (20.8) | 33 (16.8) | .617 | 7 (23.3) | 31 (16.2) | .338 |
| ACEIs/ARBs | 48 (36.1) | 29 (33.0) | .402 | 10 (41.7) | 67 (34.0) | .226 | 12 (40.0) | 65 (34.0) | .256 |
| Statins | 20 (15.0) | 19 (21.6) | .211 | 5 (20.8) | 34 (17.3) | .665 | 7 (23.3) | 32 (14.5) | .380 |
| PPIs | 58 (43.6) | 34 (38.6) | .463 | 7 (29.2) | 85 (43.1) | .190 | 13 (43.3) | 79 (41.4) | .839 |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes mellitus, stroke (doubled), vascular disease, age 65-74 years, and sex (female); COPD, chronic obstructive pulmonary disease; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, age> 65 years, and concomitant use of drugs and alcohol; LVEF, left ventricular ejection fraction; PPIs, proton pump inhibitors.
Unless otherwise indicated, the data represent No. (%) or mean±standard deviation.
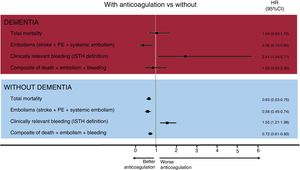
After multivariate analysis, oral anticoagulation was not associated with lower mortality in patients with dementia (HR=1.04; 95%CI, 0.63-1.72; P=.880), in contrast to patients without dementia (figure 2). However, anticoagulant therapy was associated with a significant reduction in the rate of embolic events in patients with dementia (HR, 0.36; 95%CI, 0.15-0.84; P=.018), at the expense of a significant increase in the risk of bleeding events (HR, 2.44; 95%CI, 1.04-5.71; P=.041) (). For the composite events, anticoagulant therapy was not associated with different rates of mortality, embolic events, and total bleeding in patients with dementia (adjusted HR, 0.83; 95%CI, 0.45-1.53; P=.541).
Of the 133 patients with dementia initially treated with anticoagulant therapy, 6 discontinued the anticoagulation during follow-up with no precipitating event and 4 discontinued the therapy after a bleeding event (). On the other hand, of the 88 patients with dementia not initially anticoagulated, 1 began anticoagulant therapy during follow-up with no precipitating embolic or bleeding event (). The analyses were repeated after the exclusion of the 11 patients with treatment changes during follow-up but similar results were obtained (adjusted HR for mortality, 1.09; 95%CI, 0.67-1.77; P=.731; adjusted HR for embolisms, 0.43; 95%CI, 0.21-0.95; P=.033; adjusted HR for bleeding, 2.35; 95%CI, 1.01-5.97; P=.047).
Finally, the analyses were complemented with propensity score matching, with patients with dementia paired according to anticoagulant therapy. Two groups of 26 matched patients were obtained based on the use or nonuse of anticoagulant therapy (table 2). In the patients with AF and dementia, anticoagulation was not associated with mortality (HR, 0.91; 95%CI, 0.45-1.83; P=.785) or the composite of mortality, embolisms, and bleeding (HR, 1.02; 95%CI, 0.52-2.00; P=.962).
DISCUSSIONOur study provides clinically relevant information on a subject that has thus far barely been broached by the scientific community: the usefulness of oral anticoagulation in very old patients with dementia and AF, specifically those aged 85 years or older. The main result of the study is that oral anticoagulation does not appear to be associated with lower mortality in this population. However, this is not the only relevant result of the study. The most important findings of our work are discussed below.
The first point of interest is the prevalence of dementia in patients with AF. Our study is focused on patients aged ≥ 85 years and only included those with moderate-to-severe dementia. As much as 1 in every 16 patients ≥ 85 years (6.2%) has been diagnosed with moderate-to-severe dementia. Rodríguez-Mañero et al.4 had previously documented a dementia incidence (without considering severity) of 3.6% in a population of AF patients with a mean age of 76.8 years. Taking into account the exponential increase in dementia prevalence with age and the predominance of mild dementia in patients with dementia, our prevalence of moderate-to-severe dementia in a group of patients aged ≥ 85 years with AF appears consistent. This is a major problem, given the ever advancing aging of the population. This problem is accentuated according to recent studies, which have found an association between AF and the subsequent development of dementia, particularly in patients not taking anticoagulants.12 It is unclear how to manage these patients with dementia and AF in terms of antithrombotic therapy.
A survey of American physicians revealed that dementia is the second most common reason for not prescribing anticoagulants in elderly patients.13 Patients with dementia have a higher risk of falls, bleeding, and therapeutic nonadherence, factors that can contribute to lower use of anticoagulation.14–16 In our study population, 4 out of every 10 patients ≥ 85 years with dementia were not receiving anticoagulant therapy, even though all had a CHA2DS2-VASc score ≥ 2. Of those that were anticoagulated, 8 out of every 10 were treated with VKAs (79.6%), although the use of DOACs increased during follow-up (from 20.4% at baseline to 37.2% at the end of follow-up). There were no differences in the use of DOACs between patients with and without dementia or in the time in therapeutic range between the 2 groups of patients.
In our patients with dementia, oral anticoagulation was significantly associated with lower embolic risk but also with higher bleeding risk. However, no differences were found in total mortality, in contrast to patients without dementia. This apparent neutral effect of anticoagulation on mortality in patients with dementia should be interpreted with caution, given the retrospective nature of the analysis and the potential negative impact of a more conservative therapeutic and diagnostic management on mortality in patients with dementia, which could neutralize a plausible beneficial impact of oral anticoagulants in these patients. As far as we know, only 2 studies have analyzed the topic of anticoagulation in patients with AF and dementia. In the Swedish national dementia registry (2007-2014), the authors reported lower mortality with oral anticoagulation, but only when these patients were specifically compared with patients without antithrombotic therapy17; these differences were weakened when the reference group for the comparison included single antiplatelet agents. This finding is difficult to explain, because single antiplatelet therapy has no proven prognostic benefit in patients with vascular dementia18 or in elderly patients with high cardiovascular risk.19 The Swedish national registry has startling differences from our study. While 60% of the patients with dementia and AF in our study—all ≥ 85 years old—were anticoagulated (75.9% with VKAs), only 26% of the patients with AF and dementia in the Swedish national registry—with a mean age of 82 years—were anticoagulated (100% with VKAs), indicating the influence of a major selection bias on the results. In addition, our study only included patients with moderate-to-severe dementia, whereas the Swedish registry included patients with any degree of cognitive decline, which explains why as much as 40% of their anticoagulated patients lived alone. Similarly, in a registry based on the Veterans Affairs National Healthcare System, with only 19% of the patients ≥ 85 years old, the authors found significantly reduced total mortality with continued oral anticoagulation in patients with AF who had been diagnosed with dementia.20 The strengths of our study vs the 2 described above lie both in its recency (which is why the results are applicable to the DOAC era) and in the population group studied, specifically elderly (≥ 85 years) patients with moderate-to-severe dementia (excluding mild cognitive decline), a patient group with little scientific evidence to support the highly complex decision-making required of clinical cardiologists. Clinicians managing this type of patient must always appraise the value of anticoagulant therapy, given the associated bleeding risk. In this regard, our study provides new information on this topic, in a group of patients that is usually excluded from clinical trials. We must remain conscious of the need to research the specialized care of patients with moderate-to-severe cognitive decline. Our study represents the first step in the accumulation of consistent scientific evidence that might substantiate or contradict the use of anticoagulant therapy in these patients. However, a clinical trial is required to robustly answer this question. Meanwhile, an individualized assessment of both risks (embolic and bleeding) is clearly needed to agree on the therapeutic decision concerning anticoagulation with both patients and their families.
LimitationsDespite the interest generated by our results, the following limitations of our work must be considered. First, the retrospective design of the study, despite the meticulous collection of the data and their consistency, always allows for the possibility of errors during the process of categorizing patients into different groups. The retrospective design would be associated with, on the one hand, a risk of underestimating the true percentage of patients with dementia (possibly because a certain percentage of patients with dementia was not identified by their physicians with this diagnosis in their clinical history) and, on the other hand, an inability to accurately determine why the treating clinicians decided not to anticoagulate these patients. In addition, the sample size precludes subgroup analyses that would be of considerable interest. Despite the analysis of over 3500 patients, only 221 had moderate-to-severe dementia and only 133 of these were receiving oral anticoagulation. Although it is true that the event rate in the dementia patients was high (> 50% mortality and > 10% rate of embolisms and bleeding), the power of the study is suboptimal to perform analyses according to type of anticoagulant. Additionally, we did not determine whether the DOAC dose adjustment was performed correctly and in accordance with the data sheet, which is why our cohort might exhibit an underdosing related to the feeling of frailty and bleeding risk conveyed by these patients. Nonetheless, we believe our results to be highly valuable because they provide, for the first time, evidence on anticoagulant therapy in elderly patients with dementia and AF and lay the groundwork for future studies (ideally randomized) that may confirm our findings.
CONCLUSIONSThe coexistence of AF and dementia in elderly patients is frequent (up to 1 of every 16 patients ≥ 85 years with AF has moderate-to-severe dementia). Less than two-thirds of these patients receive anticoagulation. Anticoagulant therapy in patients aged ≥ 85 years with AF and moderate-to-severe dementia is not associated with lower mortality. However, it is associated with a fall in embolic events but a marked increase in bleeding risk.
- –
In Spain, an estimated 10% of octogenarian patients with atrial fibrillation have a dementia diagnosis. However, little is known about the impact of dementia on embolic and bleeding risk according to anticoagulant therapy because patients with dementia, particularly those with moderate-to-severe disease, were not included in the clinical trials of the anticoagulant drugs.
- –
Our work provides clinically relevant information on the prevalence and impact of anticoagulant therapy in patients aged ≥ 85 years with atrial fibrillation and moderate-to-severe dementia. In this highly prevalent subgroup of patients, anticoagulation is not significantly associated with lower mortality but is linked to fewer embolic events and more bleeding events.
E. Abu Assi is an associate editor of Revista Española de Cardiología; the editorial procedure established by the journal has been followed to guarantee the impartial handling of the manuscript.
The other authors have no conflicts of interest to declare in relation to the present manuscript.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2019.10.025.