Evidence for the role of intravascular ultrasound (IVUS)-guided percutaneous coronary intervention (PCI) in patients at high ischemic risk of acute myocardial infarction (AMI) is lacking. This study aimed to investigate the long-term clinical impact of IVUS-guided PCI in patients at high ischemic risk of AMI.
MethodsAmong 13 104 patients with AMI enrolled in the Korea Acute Myocardial Infarction Registry-National Institutes of Health, we selected 8890 patients who underwent successful PCI with second-generation drug-eluting stent implantation and classified them into 2 groups based on whether or not they were at high ischemic risk or not, defined as any of the following: number of stents implanted ≥ 3, 3 vessels treated, ≥ 3 lesions treated, total stent length> 60mm, left main PCI, diabetes mellitus, and chronic kidney disease. The primary outcome was target lesion failure including cardiac death, target vessel myocardial infarction, and ischemia-driven target lesion revascularization at 3 years.
ResultsIn 4070 AMI patients at high ischemic risk, IVUS-guided PCI (21.6%) was associated with a significantly lower risk of target lesion failure at 3 years (6.7% vs 12.0%; HR, 0.54; 95%CI, 0.41-0.72; P <.001) than angiography-guided PCI. The results were consistent after confounder adjustment, inversed probability weighting, and propensity score matching.
ConclusionsIn patients at high ischemic risk of AMI who underwent PCI with second-generation drug-eluting stent implantation, use of IVUS guidance was associated with a significant reduction in 3-year target lesion failure.
iCreaT study No. C110016.
Keywords
Intravascular ultrasound (IVUS)-guided percutaneous coronary intervention (PCI) with a drug-eluting stent (DES) has shown better clinical outcomes than angiography-guided PCI in several clinical trials due to guidance of preinterventional lesion characterization, vessel size, and optimal balloon or stent size and postinterventional evaluation of the complication, minimal stent area, and stent optimization.1–5 Recently, IVUS-guided PCI reduced long-term major adverse cardiovascular events even in patients with acute myocardial infarction (AMI).6,7
Along with the development of newer generation devices, PCI has been attempted more frequently in patients with severe lesion complexity.8,9 Additionally, its use has increased in patients with a clinically poor prognosis due to high ischemic risk.10,11 In complex and high ischemic risk patients, IVUS-guided PCI showed better clinical outcomes than angiography-guided PCI in 2 observational studies in which not all patients had an AMI.12,13 Therefore, these 2 studies were insufficient to confirm the benefit of IVUS in the setting of AMI with the additionally high ischemic condition. Although the proportion of AMI patients at high ischemic risk is increasing, there is still a lack of data on the role of IVUS in patients undergoing PCI in the current second-generation DES era. Therefore, this study aimed to evaluate the long-term clinical impact of IVUS guidance for second-generation DES implantation in AMI patients at high ischemic risk.
METHODSStudy populationWe collected clinical data from a nationwide, multicenter, prospective Korea Acute Myocardial Infarction Registry-National Institutes of Health (KAMIR-NIH) registry. Twenty major cardiovascular centers recruited patients with AMI from November 2011 to December 2015. The detailed clinical parameters of all patients have been described previously.14 Trained study coordinators at each center collected the information using a web-based report form on the Internet-based Clinical Research and Trial management system. The follow-up of patients’ clinical outcomes was performed at 1, 6, 12, 24, and 36 months by attending physicians using the web-based case report forms. The study was supported by a grant from the Korea Centers for Disease Control and Prevention since November 2011 (iCreaT study No. C110016). The study protocols were approved by the ethics committees of each participating center, all complying with the principles of the revised Declaration of Helsinki (Institutional Review Board approval number: CNUH-2011-172). Informed consent was gained from all enrolled patients in the KAMIR-NIH.
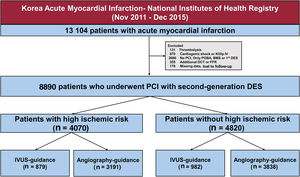
Among 13 104 patients with AMI enrolled in the KAMIR-NIH registry, we selected 8890 patients who underwent PCI with second-generation DES implantation. The exclusion criteria were thrombolysis before PCI, cardiogenic shock or Killip IV, no PCI or PCI without stenting, PCI with a bare-metal stent or first-generation DES, optical coherence tomography or fractional flow reserve use, missing data, and patients lost to follow-up (ie, when the patient was safely discharged but did not visit the hospital again). Finally, we divided patients into 2 groups: those at high ischemic risk (figure 1), which was defined as any of the following: number of stents implanted ≥ 3, 3 vessels treated, ≥ 3 lesions treated, total stent length> 60mm, left main PCI, presence of diabetes mellitus and chronic kidney disease (CKD), and those not at high ischemic risk.15–17
Study flowchart. The data used in this study were drawn from the nationwide, multicenter, prospective Korea Acute Myocardial Infarction Registry-National Institutes of Health Registry. BMS, bare-metal stent; DES, drug-eluting stent; FFR, fractional flow reserve; IVUS, intravascular ultrasound; OCT, optical coherence tomography; POBA, plain old balloon angioplasty; PCI, percutaneous coronary intervention.
Patients with AMI who underwent second-generation DES implantation were managed according to the current AMI guidelines.18,19 Antiplatelet agents (300mg of aspirin and a P2Y12 inhibitor [clopidogrel, 300-600mg; prasugrel, 60mg; or ticagrelor, 180mg]) before the procedure were routinely administered to the patients, followed by daily aspirin (100mg) and P2Y12 inhibitors (clopidogrel, 75mg once; prasugrel, 10mg once; ticagrelor, 90mg twice daily). All procedures were performed by each operator using standard interventional techniques. The selection of angiography- or IVUS-guided PCI optimization was made by each operator. Similarly, the choice of the preoperative balloon size or stent size and type, interventional strategy (eg, use of thrombus aspiration), and therapeutics (eg, the use of glycoprotein IIb/IIIa inhibitors, heparin dose) was left to each physician. Successful PCI was defined as postthrombolysis in myocardial infarction flow ≥ 2 and residual stenosis <30%.
Study outcomesThe primary outcome was target lesion failure (TLF) at 3 years after the index procedure, defined as the composite of cardiac death, target vessel myocardial infarction (TV-MI), and ischemia-driven target lesion revascularization (ID-TLR). Death was regarded as cardiac death unless a definite noncardiac cause of death could be identified. TV-MI was defined as an MI with evidence of myocardial necrosis in the territory of a previously treated target vessel according to the third universal definition of MI.20 ID-TLR was considered as any revascularization of the target lesion by PCI due to the presence of ≥ 50% angiographic diameter stenosis associated with symptoms of angina or a positive functional study, or ≥ 70% angiographic diameter stenosis without symptoms of angina or a positive functional study. Secondary outcomes included individual components of TLF with definite or probable stent thrombosis, which was defined according to the Academic Research Consortium definitions,21 and major adverse cardiovascular events, which were defined as a composite of all-cause death, any MI, and any revascularization.
Statistical analysisCategorical variables are expressed as frequencies and percentages. Depending on the number of each variable, the chi-square or Fisher exact test was performed. Continuous variables were analyzed with descriptive methods depending on their distribution, and variables with a normal distribution are presented as means and standard deviations. The cumulative incidences of clinical events at 3 years were calculated based on a Kaplan-Meier curve, and comparisons of clinical outcomes between the IVUS and angiography-guided PCI groups were analyzed using the log-rank test.22
As differences in baseline characteristics could affect clinical outcomes, sensitivity analyses were performed to adjust for confounding factors as much as possible. First, a multivariable Cox regression model was used for each to assess clinical outcomes. Variables that were significant on univariate analysis (P <.1) were included in the multivariate analysis with the following covariates: age, sex, Killip class III as acute pulmonary edema, ST-elevation myocardial infarction, hypertension, diabetes mellitus, prior PCI, history of stroke, left ventricular ejection fraction ≤ 50% as left ventricle dysfunction, estimated glomerular filtration rate ≤ 60mL/min/1.73 m2 as CKD, statin use, multivessel disease, left main PCI, glycoprotein IIb/IIIa inhibitors, and procedural factors (transradial approach, stent length ≥ 35mm, and stent number ≥ 2). Second, we performed inverse probability weighting (IPW) and propensity score matching (PSM) between the groups for the numerical difference between the 2 PCI strategies in patients with and without a high ischemic risk, respectively. The IPW and PSM of all variables was assessed using the proportional hazard regression model. The values after PSM and IPW adjustment were within±10% across all matched covariates, demonstrating a successful balance between the comparative groups in AMI patients at high ischemic risk (, ). Third, comparisons of the primary outcome between IVUS and angiography-guided strategies in patients at high ischemic risk according to the exploratory subgroups of interest were followed, and the interaction between the IVUS effect and these covariates was assessed using a Cox regression model. To evaluate the difference in 3-year TLF by quartiles of the proportion of institutional IVUS guidance when second-generation DES implantation was performed in patients with AMI, Kaplan-Meier curves and a multivariable Cox regression model of TLF at 3 years by quartiles of the institutional volume of IVUS use were used.
All statistical analyses were performed using survival, MatchIt, and WeightIt packages R, version 3.6.3 software (R Foundation for Statistical Computing, Austria).
RESULTSStudy populationThe selection of the enrolled patients is shown in figure 1. In total, 8890 patients with AMI underwent PCI with second-generation DES implantation; 4070 (45.8%) and 4820 (54.2%) were identified as being and not being at high ischemic risk, respectively (). Among 4070 AMI patients at high ischemic risk, 879 (21.6%) underwent IVUS-guided PCI and 3191 (78.4%) underwent angiography-guided PCI. Among 4820 AMI patients not at high ischemic risk, 982 (20.4%) underwent IVUS guidance and 3838 (79.6%) underwent angiography-guidance, respectively.
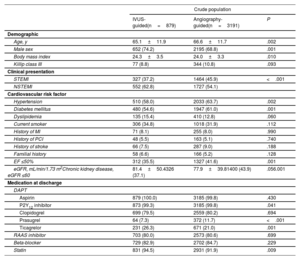
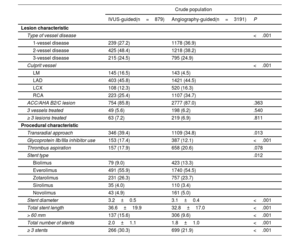
Patients’ baseline characteristicsOverall, the patients’ mean age was 63.3±12.4 years, and 6722 patients (75.6%) were male. The baseline clinical, lesion, and procedural characteristics of AMI patients at high ischemic risk are summarized in table 1 and table 2. Patients undergoing IVUS-guided PCI were younger and more likely to be male than those undergoing angiography-guided PCI. The IVUS-guided PCI group had a lower prevalence of patients with ST-elevation myocardial infarction, histories of hypertension, diabetes mellitus, and CKD than the angiography-guided PCI group. Ticagrelor and statins were more frequently prescribed on discharge in the IVUS-guided PCI group than in the angiography-guided PCI group. The IVUS-guided PCI group had higher rates of multivessel disease, left main disease, transradial approach use, and glycoprotein IIb/IIIa inhibitor use than the angiography-guided PCI group. A significantly larger stent diameter, longer stent length, and a greater number of stent implantations (≥ 3 stents) were observed in the IVUS-guided PCI group than in the angiography-guided PCI group. The baseline characteristics of AMI patients not at high ischemic risk are shown in table 3 and .
Baseline clinical characteristics of AMI patients at high ischemic risk
| Crude population | |||
|---|---|---|---|
| IVUS-guided(n=879) | Angiography-guided(n=3191) | P | |
| Demographic | |||
| Age, y | 65.1±11.9 | 66.6±11.7 | .002 |
| Male sex | 652 (74.2) | 2195 (68.8) | .001 |
| Body mass index | 24.3±3.5 | 24.0±3.3 | .010 |
| Killip class III | 77 (8.8) | 344 (10.8) | .093 |
| Clinical presentation | |||
| STEMI | 327 (37.2) | 1464 (45.9) | <.001 |
| NSTEMI | 552 (62.8) | 1727 (54.1) | |
| Cardiovascular risk factor | |||
| Hypertension | 510 (58.0) | 2033 (63.7) | .002 |
| Diabetes mellitus | 480 (54.6) | 1947 (61.0) | .001 |
| Dyslipidemia | 135 (15.4) | 410 (12.8) | .060 |
| Current smoker | 306 (34.8) | 1018 (31.9) | .112 |
| History of MI | 71 (8.1) | 255 (8.0) | .990 |
| History of PCI | 48 (5.5) | 163 (5.1) | .740 |
| History of stroke | 66 (7.5) | 287 (9.0) | .188 |
| Familial history | 58 (6.6) | 166 (5.2) | .128 |
| EF ≤50% | 312 (35.5) | 1327 (41.6) | .001 |
| eGFR, mL/min/1.73 m2Chronic kidney disease, eGFR ≤60 | 81.4±50.4326 (37.1) | 77.9±39.81400 (43.9) | .056.001 |
| Medication at discharge | |||
| DAPT | |||
| Aspirin | 879 (100.0) | 3185 (99.8) | .430 |
| P2Y12 inhibitor | 873 (99.3) | 3185 (99.8) | .041 |
| Clopidogrel | 699 (79.5) | 2559 (80.2) | .694 |
| Prasugrel | 64 (7.3) | 372 (11.7) | <.001 |
| Ticagrelor | 231 (26.3) | 671 (21.0) | .001 |
| RAAS inhibitor | 703 (80.0) | 2573 (80.6) | .699 |
| Beta-blocker | 729 (82.9) | 2702 (84.7) | .229 |
| Statin | 831 (94.5) | 2931 (91.9) | .009 |
Values are presented as mean±standard deviation or No. (%).
DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; IVUS, intravascular ultrasound; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; RAAS, renin-angiotensin-aldosterone system; STEMI, ST-elevation myocardial infarction.
Lesion and procedural characteristics of acute myocardial infarction patients at high ischemic risk
| Crude population | |||
|---|---|---|---|
| IVUS-guided(n=879) | Angiography-guided(n=3191) | P | |
| Lesion characteristic | |||
| Type of vessel disease | <.001 | ||
| 1-vessel disease | 239 (27.2) | 1178 (36.9) | |
| 2-vessel disease | 425 (48.4) | 1218 (38.2) | |
| 3-vessel disease | 215 (24.5) | 795 (24.9) | |
| Culprit vessel | <.001 | ||
| LM | 145 (16.5) | 143 (4.5) | |
| LAD | 403 (45.8) | 1421 (44.5) | |
| LCX | 108 (12.3) | 520 (16.3) | |
| RCA | 223 (25.4) | 1107 (34.7) | |
| ACC/AHA B2/C lesion | 754 (85.8) | 2777 (87.0) | .363 |
| 3 vessels treated | 49 (5.6) | 198 (6.2) | .540 |
| ≥ 3 lesions treated | 63 (7.2) | 219 (6.9) | .811 |
| Procedural characteristic | |||
| Transradial approach | 346 (39.4) | 1109 (34.8) | .013 |
| Glycoprotein IIb/IIIa inhibitor use | 153 (17.4) | 387 (12.1) | <.001 |
| Thrombus aspiration | 157 (17.9) | 658 (20.6) | .078 |
| Stent type | .012 | ||
| Biolimus | 79 (9.0) | 423 (13.3) | |
| Everolimus | 491 (55.9) | 1740 (54.5) | |
| Zotarolimus | 231 (26.3) | 757 (23.7) | |
| Sirolimus | 35 (4.0) | 110 (3.4) | |
| Novolimus | 43 (4.9) | 161 (5.0) | |
| Stent diameter | 3.2±0.5 | 3.1±0.4 | <.001 |
| Total stent length | 36.6±19.9 | 32.8±17.0 | <.001 |
| > 60 mm | 137 (15.6) | 306 (9.6) | <.001 |
| Total number of stents | 2.0±1.1 | 1.8±1.0 | <.001 |
| ≥ 3 stents | 266 (30.3) | 699 (21.9) | <.001 |
Values are presented as mean±standard deviation or No. (%).
ACC, American College of Cardiology; AHA, American Heart Association; DES, drug-eluting stent; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main; RCA, right coronary artery.
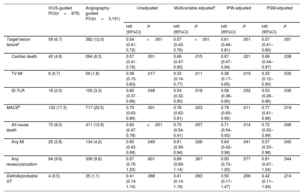
Comparison of the 3-year clinical outcome in acute myocardial infarction patients at high ischemic risk
| IVUS-guided PCI(n=879) | Angiography-guided PCI(n=3,191) | Unadjusted | Multivariable adjustedc | IPW-adjusted | PSM-adjusted | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |||
| Target lesion failurea | 59 (6.7) | 382 (12.0) | 0.54 (0.41-0.72) | <.001 | 0.57 (0.43-0.76) | <.001 | 0.61 (0.46–0.81) | .001 | 0.57 (0.41–0.80) | .001 |
| Cardiac death | 42 (4.8) | 264 (8.3) | 0.57 (0.41-0.78) | .001 | 0.66 (0.47-0.92) | .015 | 0.67 (0.47–0.94) | .021 | 0.66 (0.44–0.97) | .038 |
| TV-MI | 6 (0.7) | 59 (1.8) | 0.36 (0.15-0.83) | .017 | 0.33 (0.14-0.77) | .011 | 0.38 (0.17–0.85) | .019 | 0.33 (0.12–0.90) | .030 |
| ID-TLR | 18 (2.0) | 105 (3.3) | 0.60 (0.37-0.99) | .048 | 0.54 (0.32-0.90) | .018 | 0.58 (0.36–0.95) | .032 | 0.53 (0.28–0.96) | .036 |
| MACEb | 152 (17.3) | 717 (22.5) | 0.75 (0.63-0.89) | .001 | 0.76 (0.63-0.91) | .003 | 0.79 (0.65–0.95) | .011 | 0.77 (0.61–0.96) | .019 |
| All-cause death | 70 (8.0) | 411 (12.9) | 0.60 (0.47-0.78) | <.001 | 0.70 (0.54-0.91) | .007 | 0.71 (0.54–0.93) | .014 | 0.72 (0.52–0.99) | .046 |
| Any MI | 25 (2.8) | 134 (4.2) | 0.65 (0.43-0.99) | .049 | 0.61 (0.39-0.94) | .026 | 0.64 (0.42–0.98) | .041 | 0.57 (0.33–0.98) | .045 |
| Any revascularization | 84 (9.6) | 306 (9.6) | 0.97 (0.76-1.23) | .801 | 0.89 (0.69-1.14) | .367 | 0.93 (0.72–1.20) | .577 | 0.91 (0.67–1.24) | .544 |
| Definite/probable ST | 4 (0.5) | 35 (1.1) | 0.41 (0.14-1.14) | .088 | 0.41 (0.14-1.16) | .093 | 0.50 (0.17–1.47) | .206 | 0.42 (0.11–1.64) | .214 |
Unless otherwise indicated, values are presented as No. (%).
95%CI, 95% confidence interval; CKD, chronic kidney disease; HR, hazard ratio; ID-TLR, ischemia-driven target lesion revascularization; IPW, inverse probability weighting; LM, left main; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular event; MI, myocardial infarction; PSM, propensity score matching; ST, stent thrombosis; STEMI, ST-elevation myocardial infarction TLR, target lesion revascularization; TV-MI, target vessel myocardial infarction.
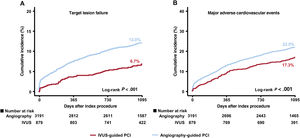
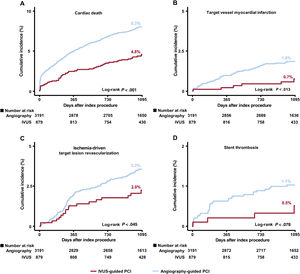
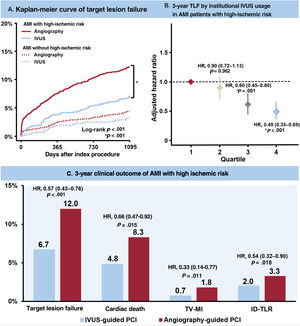
The median follow-up duration was 3 years (interquartile range: 2.88-3.23 years). Figure 2, figure 3, and table 3 present a comparison of clinical outcomes between the IVUS and angiography-guided PCI groups in AMI patients at high ischemic risk. The risk of 3-year TLF was significantly lower in the IVUS-guided group than in the angiography-guided group (6.7% and 12.0%, respectively; HR, 0.54; 95%CI, 0.41-0.72; P <.001) (figure 2A). The results were consistent after multivariable Cox regression analysis (multivariable-adjusted HR, 0.57; 95%CI, 0.43-0.76; P <.001), IPW adjustment (IPW-adjusted HR, 0.61; 95%CI, 0.46-0.81; P=.001), and PSM adjustment (PSM-adjusted HR, 0.57; 95%CI, 0.41-0.80; P=.001) (table 3). IVUS-guided PCI was significantly associated with a reduction in major adverse cardiovascular events, cardiac death, TV-MI, ID-TLR, all-cause death, and all MIs after multiple adjustments for various confounding factors (figure 2, figure 3, and table 3). However, there was no significant difference in the risk of any revascularization and stent thrombosis between the groups (figure 3 and table 3). In AMI patients not at high ischemic risk, IVUS-guided PCI did not reduce the 3-year clinical outcomes compared with angiography-guided PCI ().
Cumulative incidence of clinical outcomes according to IVUS use. Kaplan-Meier curves are shown for comparison of the rates of target lesion failure (A) and major adverse cardiovascular events (B) between IVUS- vs angiography-guided PCI in acute myocardial infarction patients at high ischemic risk. IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention.
Cumulative incidence of target lesion failure components and stent thrombosis. Kaplan-Meier curves are shown for comparison of the rates of cardiac death (A) target vessel myocardial infarction (B) ischemia-driven target lesion revascularization (C) and stent thrombosis (D) between IVUS- vs angiography-guided PCI in acute myocardial infarction patients at high ischemic risk. IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention.
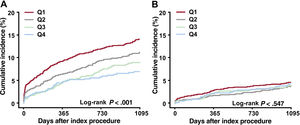
The 20 enrolled centers were divided into quartiles categorized by the proportion of institutional IVUS guidance among patients with AMI who underwent PCI with second-generation DES implantation (quartiles 1-4). In AMI patients at high ischemic risk, the adjusted 3-year TLF gradually decreased from quartiles 1 to 4 (figure 4A). Quartile 4 was significantly associated with a reduction in adjusted TLF at 3 years compared with quartile 1 (multivariable-adjusted HR, 0.49; 95%CI, 0.35-0.69; P <.001) (figure 5). There was no reduction in the 3-year TLF in AMI patients not at high ischemic risk (figure 4B).
Central illustration. The clinical outcomes between IVUS-guided and angiography-guided PCI in AMI patients at high ischemic risk. In AMI patients with high ischemic risk, IVUS-guided PCI was associated with a significantly lower risk of 3-year TLF compared with angiography-guided PCI although the benefit of IVUS guidance was not observed in AMI patients without high ischemic risk (A). Quartile analysis by the proportion of institutional IVUS-guided PCI showed quartile 4 significantly associated with a reduction in adjusted TLF at 3 years compared with quartile 1 (B). Compared with angiography-guided PCI, IVUS guidance was significantly associated with a lower risk of TLF at 3 years, driven by cardiac death, TV-MI, and ID-TLR, in AMI patients with high ischemic risk (C).
AMI, acute myocardial infarction; HR, hazard ratio (all hazard ratio of this figure represent multivariable adjusted hazard ratio); ID-TLR, ischemic driven target lesion revascularization; IVUS, intravascular ultrasound; TLF, target lesion failure; TV-MI, target vessel myocardial infarction.
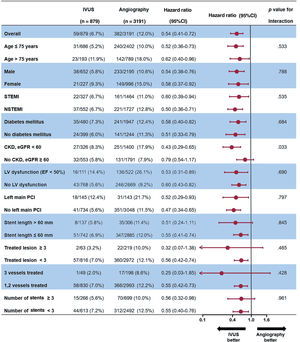
Figure 6 presents a forest plot showing the prognostic impact of IVUS-guided PCI on TLF among the various ischemic factors in AMI patients at high ischemic risk. The favorable impact of IVUS-guided PCI on 3-year TLF was consistent across all subgroups. In particular, the impact of IVUS guidance was more dominant among patients with CKD (HR, 0.43; 95%CI, 0.29-0.65), than among those without CKD (HR, 0.79; 95%CI, 0.54-1.17; P=.033 for interaction).
Exploratory subgroup analysis of the 3-year target lesion failure by IVUS use in acute myocardial infarction patients with high ischemic risk. CKD, chronic kidney disease; EF, ejection fraction; IVUS, intravascular ultrasound; LV, left ventricle; NSTEMI, non–ST elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
In the present study, we compared the 3-year clinical outcomes between IVUS- and angiography-guided PCI in second-generation DES implantations in AMI patients with or without high ischemic risk using data from a nationwide, multicenter, prospective dedicated AMI registry. The main findings of the current study are as follows: a) compared with angiography-guided PCI, IVUS-guidance was significantly associated with a lower risk of TLF at 3 years, driven by cardiac death, TV-MI, and ID-TLR, in AMI patients at high ischemic risk, but not in patients not at high ischemic risk (figure 5); and b) in the quartile analysis by the proportion of institutional IVUS guidance, higher usage of IVUS guidance was associated with a reduction in the 3-year TLF in AMI patients at high ischemic risk.
The extended results of a previous 1-year randomized study reporting that the clinical improvement of IVUS-guided PCI lasts for 3 to 5 years have been recently published, and the outcomes of IVUS-guided PCI were also improved even in acute coronary syndrome in a subgroup analysis.23,24 More recently, registry data showed that IVUS-guided DES implantation was associated with a reduction in clinical outcomes including hard endpoints, such as death, MI in the setting of AMI, but a subgroup analysis did not show a significant benefit in high ischemic risk PCI, defined as PCI for bifurcation lesions, chronic total occlusion, left main lesion, multivessel disease, restenosis, diffuse long lesion (≥ 60mm), and number of implanted stents ≥ 3.6
With the development of the stent profile, intravascular imaging modalities and medications including potent antiplatelet agents, the ischemic event rates have decreased and use of PCI for patients with complex lesions and high ischemic risk has gradually increased.25,26 Although the definition of high ischemic risk differed slightly in each study and there is no randomized trial on the topic, IVUS-guided PCI for complex lesions includes bifurcation, chronic total occlusion, left main, stent length ≥ 38mm, multivessel PCI, ≥ 3 implanted stents, in-stent restenosis, and severely calcified lesion associated the lower long-term adverse events including death, MI, and TLR.13 Furthermore, the benefits of IVUS guidance in patients with AMI who underwent second-generation DES, which commonly has high ischemic risks, also showed better clinical outcomes than angiography-guided PCI.7 Our study of dedicated AMI patients with second-generation DES implantation and high ischemic risk included additional procedural and patient factors with reference to other studies on high ischemic risks.15–17 In the present study, the high ischemic risk of patients with AMI who underwent second-generation DES implantation by IVUS guidance showed better clinical outcomes in all-cause death, any MI, and all individual components of TLF. However, this clinical benefit was not seen in AMI patients not at high ischemic risk.
There are several potential explanations for the outcome in this study that IVUS-guided PCI improved hard endpoints including death and MI in only AMI patients at high ischemic risk. Between 2012 and 2015, the use of IVUS in the AMI setting increased from 17.5% to 23.5% in these registries,27 more than twice that in the United States,28 such that the operators had increased levels of experience with IVUS-guided PCI, and the improved stent profile of the second-generation DES allowed them to perform more complex PCI with more stent optimization than in the first-generation DES era. Additionally, similar to a previous study,6,7,13 the present study also showed that patients undergoing IVUS-guided PCI had a larger number and diameter and longer length of stents than those undergoing angiography-guided PCI. It is thought that IVUS-guided PCI was helpful in full lesion coverage, stent optimization with postdilation with noncompliant balloon, and correction of procedural complications even in high ischemic risk AMI settings. However, this benefit of IVUS was not seen in AMI patients not at high ischemic risk. The development of the stent profile and medications could have led to the low number of ischemic events with or without IVUS-guided PCI, despite AMI settings without high ischemic risk. This finding should be confirmed by randomized trials of AMI patients with and without high ischemic risk who underwent second-generation DES implantation.
A quartile analysis by the proportion of institutional IVUS guidance showed that the center with the highest proportion showed better clinical outcomes in high ischemic risk patients. A previous study showed that therapeutic variability between regions led to significant differences in clinical outcomes in AMI patients.29,30 It is also thought that the more familiar operators are with IVUS-guided PCI in high ischemic risk patients, the better they can implant DESs with more full lesion coverage and stent optimization and administer proper personalized antiplatelet agents based on IVUS findings.
In the subgroup analysis, the benefit of IVUS guidance was consistent across various ischemic factors. Interestingly, the clinical benefit of IVUS use was shown to be more predominant in AMI patients with CKD than in those without. The recent AMI registry data also showed that the benefits of IVUS guidance was greatest in patients with CKD.6 Patients with CKD are more likely to have a higher risk for long lesions, multivessel disease, and calcification associated with periprocedural and long-term higher mortality.31 A subgroup study of the Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions trial showed the benefit of IVUS use on target vessel failure in the setting of CKD compared with angiography-guided PCI use.10 IVUS guidance might have led to larger stent/balloon size selection and more frequent postdilation achievement with noncompliant balloon dilatation, and it could have contributed to the larger minimal stent area with stent optimization; these factors are thought to have a greater benefit in CKD patients with complex and high ischemic risk features.32–34 Therefore, our subgroup analysis suggested that the use of IVUS for stent optimization during PCI for AMI patients with renal impairment should be considered more strongly, although further randomized studies are needed to determine the benefits of IVUS-guided PCI in AMI patients with CKD.
LimitationsThis study has some limitations; first, it has the inherent limitations of nonrandomized, observational registry data, which inevitably have selection and information biases. However, various sensitivity analyses were conducted to adjust for the measured or unmeasured confounders of different confounding factors as much as possible. Second, the current study excluded chronic total occlusion or bifurcation PCI or severely calcified lesions, because those lesions are rare in patients with AMI and those factors could not be identified in the registry data. Third, as the selection and use of IVUS was made at the operator's discretion, the exact reason for IVUS use was not obtained in this registry. Therefore, individual operators’ experience might have affected IVUS use and clinical outcomes. Fourth, there were no detailed procedural data including postdilatation with noncompliant balloon after stenting. Furthermore, the pre- or posttiming of IVUS use and detailed IVUS parameters were not collected in this registry during PCI. Fifth, without dedicated criteria for IVUS-guided PCI, stent optimization is likely to have underestimated the beneficial effects observed with IVUS use in this registry. Sixth, it is possible that the benefit of IVUS use in CKD patients was due to lesion complexity rather than renal function itself, and it is also possible that the use of lower contrast use in CKD patients affected the benefit of IVUS; however, information on amount of contrast is not available. To overcome these limitations, randomized clinical trials for proving beneficial of IVUS in high-risk AMI patients will be necessary.
CONCLUSIONSIn this nationwide multicenter registry, IVUS guidance for AMI patients at high ischemic risk undergoing second-generation DES implantation was associated with a lower risk of TLF at 3 years, driven by cardiac death, TV-MI, and ID-TLR, although IVUS guidance did not show benefits in AMI patients not at high ischemic risk.
FUNDINGThis work was funded by the Research of Korea Centers for Disease Control and Prevention (2016-ER6304-02).
AUTHORS’ CONTRIBUTIONSConception of design of the work: Y. Kim, M.H. Jeong, J.W. Roh. Acquisition: Y. Kim, M.H. Jeong, J.W. Roh, S. Bae. Analysis: Y. Kim, M.H. Jeong, J.W. Roh, S. Bae. Interpretation, drafting and manuscript revising, and final approval: Y. Kim, M.H. Jeong, J.W.Roh, S. Bae, T.W. Johnson, D.K. Cho, J.S. Kim, B.K. Kim, D. Choi, M.K. Hong, Y. Jang.
CONFLICTS OF INTERESTT.W. Johnson has received consultancy and speaker fees from Boston Scientific. The remaining authors have no conflicts of interest. Y. Kim, M.H. Jeong, J.W.Roh, S. Bae, T.W. Johnson, D.K. Cho, J.S. Kim, B.K. Kim, D. Choi, M.K. Hong, and Y. Jang agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Although there is no randomized clinical trial on the subject, IVUS-guided PCI improves clinical outcome in patients with AMI.
WHAT DOES THIS STUDY ADD?The use of PCI in patients at high ischemic risk has been increasing, and IVUS-guided PCI in these patients with AMI has shown the clinical benefit of reducing TLF including cardiac death, target vessel myocardial infarction, and ischemia-driven target lesion revascularization. This trend seems to be more beneficial in institutional centers with a high volume of IVUS and patients with CKD.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.10.006