Renal impairment and fluctuations in renal function are common in patients recently hospitalized for acute heart failure and in those with atrial fibrillation. The aim of the present study was to evaluate the hypothetical need for dosage adjustment (based on fluctuations in kidney function) of dabigatran, rivaroxaban and apixaban during the first 6 months after hospital discharge in patients with concomitant atrial fibrillation and heart failure.
MethodsAn observational study was conducted in 162 patients with nonvalvular atrial fibrillation after hospitalization for acute decompensated heart failure who underwent creatinine determinations during follow-up. The hypothetical recommended dosage of dabigatran, rivaroxaban and apixaban according to renal function was determined at discharge. Variations in serum creatinine and creatinine clearance and consequent changes in the recommended dosage of these drugs were identified during 6 months of follow-up.
ResultsAmong the overall study population, 44% of patients would have needed dabigatran dosage adjustment during follow-up, 35% would have needed rivaroxaban adjustment, and 29% would have needed apixaban dosage adjustment. A higher proportion of patients with creatinine clearance < 60mL/min or with advanced age (≥ 75 years) would have needed dosage adjustment during follow-up.
ConclusionsThe need for dosage adjustment of nonvitamin K oral anticoagulants during follow-up is frequent in patients with atrial fibrillation after acute decompensated heart failure, especially among older patients and those with renal impairment. Further studies are needed to clarify the clinical importance of these needs for drug dosing adjustment and the ideal renal function monitoring regime in heart failure and other subgroups of patients with atrial fibrillation.
Keywords
Renal impairment and fluctuations in renal function are common in patients with recent acute decompensated heart failure (ADHF) hospitalization,1,2 and in those with atrial fibrillation (AF).3 However, there have been no studies of the effect of these fluctuations occurring at different time points after hospital discharge on the need for dosage adjustment of nonvitamin K oral anticoagulants (NOACs). The aim of the present study was to evaluate the hypothetical need for dosage adjustment (based exclusively on fluctuations in kidney function) of dabigatran, rivaroxaban and apixaban during the first 6 months after hospital discharge in patients with concomitant AF and ADHF.
METHODSStudy Population and DesignWe identified a cohort of 253 consecutive patients discharged from the Hospital Clínico Universitario Virgen de la Arrixaca (Murcia, Spain) with a concomitant diagnosis of AF and ADHF. Patients with contraindications for NOACs and those without serum creatinine measurement within 6 months of hospital discharge were excluded (). Given that kidney function may improve during follow-up, patients with a contraindication to NOACs due to renal dysfunction and without other contraindications were included in the analyses. The final study population consisted of 162 patients and their baseline clinical characteristics were all recorded in detail. During the study period, clinical management decisions about each patient were made by the responsible cardiologist. The study was approved by the local ethics committee.
The CHA2DS2-VASc (congestive heart failure/left ventricular systolic dysfunction, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled]-vascular disease, age 65-74 and female sex) and HAS-BLED (noncontrolled hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly [age>65 years], drugs/ alcohol concomitantly) scores were calculated as assessment of stroke and bleeding risk.
To evaluate the impact of variations of kidney function on NOACs dosing adjustment, we calculated the hypothetical recommended dosing of NOACs based exclusively on kidney function estimate according to the recommendations of the European Heart Rhythm Association Practical Guide.4 The last serum creatinine measured during the index hospitalization was used to define baseline renal function status. All serum creatinine measurements during the first 6 months following hospital discharge were collected to assess the fluctuations in renal function. Creatinine clearance (CrCl) was estimated using the Cockroft-Gault equation ([140 – age] × weight [Kg])/(serum creatinine [mg/dL] × 72) (× 0.85 for women). We identified a hypothetical need for dosage adjustment when the recommended dose of NOACs based on 1 kidney function estimation (or serum creatinine in the case of apixaban) differed from the previous one. We used Rosendaal's method to estimate the time in therapeutic range (TTR) of patients taking vitamin K antagonists. This method assumes a linear increase or decrease between 2 consecutive international normalized ratio (INR) determinations in order to estimate the time (as a proportion of the time of follow-up) in which the INR would have be in range (between 2.0 and 3.0).
Statistical MethodsContinuous variables are presented as the mean ± standard deviation or median [interquartile range], as appropriate, and categorical variables as a percentage. Differences in baseline characteristics were compared using the Student t test or Man Whitney U test for continuous variables, and the chi-square test for categorical variables. The McNemar test was used to compare paired proportions. All P values < .05 were accepted as statistically significant. Statistical analysis was performed using SPSS version 15.0 (SPSS, Inc.; Chicago, Illinois, United States).
RESULTSThe demographic and clinical characteristics of the study population are listed in Table 1. The median CHA2DS2-VASc score was 5 [4-6] and 158 (98%) patients had a CHA2DS2-VASc score ≥ 2. The mean estimated CrCl was 60mL/min ± 27mL/min, and 93 (57%) patients had a CrCl < 60mL/min.
Study Population Clinical Characteristics
| Patients, No. | 162 |
| Age, y | 74±10 |
| Age ≥ 75 years | 82 (51) |
| Male sex | 84 (52) |
| Weight, Kg | 78±15 |
| Body mass index, Kg/m2 | 29 [26-33] |
| Hypertension | 139 (86) |
| Diabetes mellitus | 60 (37) |
| LVEF ≤ 40% | 72 (44) |
| Previous stroke or TIA | 27 (17) |
| Coronary artery disease | 58 (36) |
| Peripheral artery disease | 12 (7.4) |
| Abnormal liver function | 5 (3.1) |
| COPD | 28 (17) |
| Current alcoholic consumption (> 8 drinks/wk) | 6 (4) |
| Previous major bleeding episode | 24 (15) |
| CrCl at discharge, mL/min | 60±27 |
| Chronic kidney disease (< 60 mL/min) | 93 (57) |
| CHA2DS2-VASc score | 5 [4-6] |
| HAS-BLED score | 2 [1-3] |
| Previous TTR, % (n=62) | 54±32 |
| Treatment at discharge | |
| Acenocoumarol | 133 (82) |
| NOACs | 1 (0.6) |
| Antiplatelet therapy | 73 (45) |
| Beta-blockers | 127 (79) |
| ACE inhibitors/ARB | 143 (88) |
| Aldosterone antagonists | 53 (33) |
| Loop diuretic | 149 (92) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CHA2DS2-VASc, congestive heart failure/left ventricular systolic dysfunction, hypertension, age ≥ 75 (doubled), diabetes, stroke (doubled)-vascular disease, age 65-74 and female sex; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; HHAS-BLED, noncontrolled hypertension, abnormal renal/ liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age > 65 years), drugs/ alcohol concomitantly; LVEF, left ventricular ejection fraction; NOACs, nonvitamin K oral anticoagulants; TIA, transient ischemic attack; TTR, time in therapeutic range.
Data are expressed as mean ± standard deviation, median [interquartile range] and No. (%).
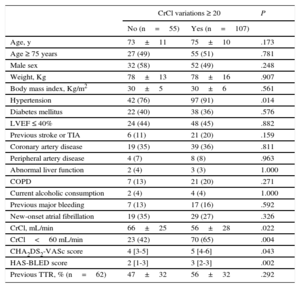
Over the study period, 3 [2-6] serum creatinine measurements per patient were analyzed. The maximum absolute and relative variations of CrCl from the baseline values were 15 [9-25] mL/min and 28% [17%-46%], respectively. A total of 107 patients (66%) had ≥ 20% of variation in estimated CrCl from the baseline values; patients with ≥ 20% CrCl variations had more prevalent hypertension and poorer baseline kidney function (Table 2).
Clinical Characteristics Associated With ≥ 20% Variation in Creatinine Clearance
| CrCl variations ≥ 20 | P | ||
|---|---|---|---|
| No (n=55) | Yes (n=107) | ||
| Age, y | 73±11 | 75±10 | .173 |
| Age ≥ 75 years | 27 (49) | 55 (51) | .781 |
| Male sex | 32 (58) | 52 (49) | .248 |
| Weight, Kg | 78±13 | 78±16 | .907 |
| Body mass index, Kg/m2 | 30±5 | 30±6 | .561 |
| Hypertension | 42 (76) | 97 (91) | .014 |
| Diabetes mellitus | 22 (40) | 38 (36) | .576 |
| LVEF ≤ 40% | 24 (44) | 48 (45) | .882 |
| Previous stroke or TIA | 6 (11) | 21 (20) | .159 |
| Coronary artery disease | 19 (35) | 39 (36) | .811 |
| Peripheral artery disease | 4 (7) | 8 (8) | .963 |
| Abnormal liver function | 2 (4) | 3 (3) | 1.000 |
| COPD | 7 (13) | 21 (20) | .271 |
| Current alcoholic consumption | 2 (4) | 4 (4) | 1.000 |
| Previous major bleeding | 7 (13) | 17 (16) | .592 |
| New-onset atrial fibrillation | 19 (35) | 29 (27) | .326 |
| CrCl, mL/min | 66±25 | 56±28 | .022 |
| CrCl<60 mL/min | 23 (42) | 70 (65) | .004 |
| CHA2DS2-VASc score | 4 [3-5] | 5 [4-6] | .043 |
| HAS-BLED score | 2 [1-3] | 3 [2-3] | .002 |
| Previous TTR, % (n=62) | 47±32 | 56±32 | .292 |
CHA2DS2-VASc, congestive heart failure/left ventricular systolic dysfunction, hypertension, age ≥ 75 (doubled), diabetes, stroke (doubled)-vascular disease, age 65-74 and female sex; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; HAS-BLED, noncontrolled hypertension, abnormal renal/ liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly (age > 65 years), drugs/ alcohol concomitantly; LVEF, left ventricular ejection fraction; TIA, transient ischemic attack; TTR, time in therapeutic range.
Data are expressed as mean ± standard deviation, median [interquartile range] and No. (%).
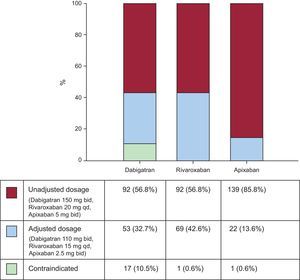
At hospital discharge, the recommended dosages of each NOAC according to baseline estimated CrCl would have been widely variable. As shown in Figure 1, most patients (85.8%) would have continued with a nonadjusted dose for apixaban (5mg twice daily). The proportion of patients who would have required reduced dosages for dabigatran (110mg twice daily) and rivaroxaban (15mg once daily) was significantly higher than for apixaban (2.5mg twice daily): 33%, 43%, and 14%, respectively. The proportion of patients with a contraindication for dabigatran would have been higher than for rivaroxaban and apixaban: 11.0%, 0.6%, and 0.6%, respectively.
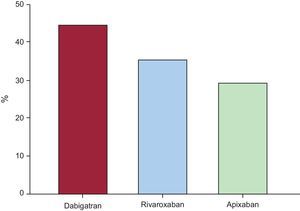
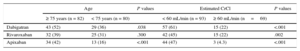
During the 6 months following hospital discharge, 72 (44%), 57 (35%), and 47 (29%) would have required at least 1 dosage adjustment for dabigatran, rivaroxaban and apixaban, respectively (Figure 2). As expected, the variations in CrCl from the baseline values were significantly higher among patients who would have required dosage adjustment regardless of NOACs (). Moreover, the proportion of patients who would have required hypothetical NOACs dosage adjustment was higher among older patients (≥ 75 years) and those with CrCl<60mL/min for all 3 NOACs (Table 3). Specifically, among elderly patients, dabigatran showed the highest hypothetical need for dosage adjustment (P=.013 for dabigatran vs rivaroxaban; P=.039 for dabigatran vs apixaban and P=.855 for rivaroxaban vs apixaban), while in younger patients, apixaban showed the lowest (P=.017 for apixaban vs rivaroxaban; P<.001 for apixaban vs dabigatran and P=.219 for dabigatran vs rivaroxaban). In patients with CrCl<60mL/min, dabigatran also showed the highest hypothetical need for dosage adjustment (P=.003 for dabigatran vs rivaroxaban; P=.026 for dabigatran vs apixaban and P=.874 for rivaroxaban vs apixaban), while among patients with normal or mildly depressed renal function (CrCl ≥ 60mL/min), apixaban showed an even lower need for adjustment (3 [4%] for apixaban vs 15 [22%] for dabigatran and rivaroxaban, both P values <.001). Additionally, the percentage of patients that would have needed at least 1 hypothetical dosage adjustment of each NOAC according to CrCl intervals are detailed in .
Proportion of Patients Who Would Have Needed at Least 1 Hypothetical Dosage Adjustment According to Age and Kidney Function
| Age | P values | Estimated CrCl | P values | |||
|---|---|---|---|---|---|---|
| ≥ 75 years (n = 82) | < 75 years (n = 80) | < 60 mL/min (n = 93) | ≥ 60 mL/min (n=69) | |||
| Dabigatran | 43 (52) | 29 (36) | .038 | 57 (61) | 15 (22) | <.001 |
| Rivaroxaban | 32 (39) | 25 (31) | .300 | 42 (45) | 15 (22) | .002 |
| Apixaban | 34 (42) | 13 (16) | <.001 | 44 (47) | 3 (4.3) | <.001 |
CrCl, creatinine clearance.
Data are expressed as No. (%).
Lastly, among patients with available follow-up INR values (n=108), the average estimated TTR was 48% and two-thirds of them (n=71) had poor TTR control (TTR<60%).
DISCUSSIONThe major findings of this study are as follows. Firstly, fluctuations in renal function are common in the mid.-term after hospitalization for ADHF. Secondly, these changes in renal function should be taken into account for dosage adjustment of NOACs. Thirdly, the need for dosage adjustment was more likely in the elderly and in patients with renal dysfunction at baseline. Fourthly, in younger patients and in those with normal or only mildly depressed renal function, the need for dosage adjustment can vary widely across NOACs, tending to be the lower with apixaban.
The early post-discharge period is sometimes referred to as the “vulnerable phase” when morbidity and mortality is highest and is therefore a critical time-period to closely monitor patients.5,6 Indeed, a strategy of early visits led to better outcomes in a recent analysis of the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized) and GWTG-HF (Get With The Guidelines-Heart Failure) registries.7 Although few data exist on fluctuations in renal function after hospitalization for ADHF, a subanalysis of the COACH study2 showed that the magnitude of these changes are predominant in the short- and mid-term. In our study, up to two-thirds of patients experienced ≥ 20% variation in CrCl over 6 months, confirming the absence of stable renal function in a significant proportion of patients. This period is influenced by changes in renal perfusion and venous congestion, neurohormonal activation, and inflammation.
Moreover, initiation of adequate therapy may affect renal function in opposite ways. While in some patients it can prevent further worsening of renal function and may eventually improve renal function, in others—and especially in patients with comorbidities and with high diuretic requirements—it can impair renal function. This complex scenario is the “real world” clinical practice scenario as reflected in our baseline clinical demographics.
Heart failure and AF frequently coexist8,9 as they share common risk factors.10,11 The presence of AF in heart failure is associated with increased morbidity, hospitalizations, and poorer functional status.12,13 Moreover, AF in heart failure patients is an independent risk factor for ischemic stroke and thromboembolism14 and has been incorporated in validated risk stratification scores.15,16 Classically, adjusted-dose vitamin K antagonists have been the oral anticoagulants most frequently used in the prevention of thromboembolic events in these patients,17,18 but the effectiveness and safety of these drugs are strongly associated with its stability, reflected by the TTR of the INR.19
Recently studies have shown that AF patients with heart failure tended to present significantly poorer TTR control than those without heart failure.20–22 Given that these patients are less likely to keep within the target INR range with vitamin K antagonists, some authors suggest that NOACs could be an attractive alternative.23 In fact, in large phase III randomized trials of NOACs, heart failure patients were highly represented (ranging from 32.0% to 62.5%), and in subgroup analyses, no statistically significant heterogeneity of treatment efficacy or safety was observed in these patients.24–26 However, all NOACs are partially eliminated by renal clearance and hence require dose adjustment depending on renal function. This is a critical point, as evidence shows that the risk of major bleeding and ischemic stroke may be highly correlated to plasma concentrations of these drugs.27
The 3 currently available NOACs differ in terms of renal elimination. Apixaban is less dependent on renal clearance than the other 2 NOACs, with about 27% of renal excretion compared with 80% for dabigatran and 35% for rivaroxaban.28–30 The different recommendations about dosage adjustment of these drugs based in these features explain our study finding that 44%, 35%, and 29% of patients required at least 1 hypothetical dosage adjustment for dabigatran, rivaroxaban, and apixaban respectively in a real world population of ADHF patients with AF soon after discharge. We found that this is especially true for older patients (age ≥ 75 years) and/or in those with poor renal function (CrCl<60mL/min) who are otherwise at high risk of bleeding and thromboembolic complications, as demonstrated previously.31–35 Therefore, they would need a careful risk-benefit balance, avoiding either over- or underexposure to these drugs. Of the different NOACs, apixaban was theoretically associated with less need for dose adjustments in our population, especially in younger patients (< 75 years) and in those with normal or mildly depressed renal function (CrCl ≥ 60mL/min) at hospital discharge. Nevertheless, it is important to note that renal function is not the only clinical parameter in NOACs dosing adjustment in all patients. Moreover, we recently showed that differences between equations for estimating kidney function and drug dosing are frequent in AF patients.36 Therefore, regardless of renal function, clinicians must always choose the NOACs regimen that optimizes the risk-benefit ratio, given the patient-specific clinical scenario.
Frequent monitoring of renal function is advised in AF patients starting treatment with NOACs. Current European Society of Cardiology guidelines recommend annual assessment of renal function in patients with CrCl ≥ 50mL/min, and 2 to 3 times per year in patients with CrCl from 30mL/min to 50mL/min.37 However, there are no specific recommendations in patients with heart failure or other clinical conditions that coexist in our population. Our results suggest that the recommendation for annual assessment of renal function in these patients could be inadequate. Indeed, renal function testing 2-3 times per year could be safer in patients in this context, or even more frequently in those with renal impairment or in older patients (≥ 75 years).
LimitationsThe limitations of our study are similar to those of any single-center prospective observational study. The small sample size also makes it difficult to draw firm conclusions. Thus, larger studies are necessary prior to generalizing our results. Other limitations include the lack of prespecified renal function assessments during follow-up. Indeed, the conclusions of the study were obtained from serum creatinine determinations as part of routine management. Thus, patients without analytical determinations during follow-up were excluded from the study. The lack of creatinine determinations during follow-up was primarily related to premature death and these patients tended to be older and had worse renal function than patients included in the study. Given that these factors were associated with wider renal function variations and need for dosage adjustment in our population, the exclusion of these patients could have led to underestimation the real needs of renal adjustment. Moreover, we used changes in drug dosage recommendations as an outcome, rather than actual observed drug dosage changes in clinical practice. In actual clinical practice, those patients taking NOACs may have had more frequent renal assessment, resulting in higher needs for real dosage adjustment. Although the use of the Cockroft-Gault equation can result in lower estimated values of CrCl than other algorithms of renal function estimation, especially in elderly patients, which could have resulted in different recommended doses of NOACs in our population.36 However, in the present study, we used this equation because the European Medicines Agency recommends it for NOACs dose adjustment in daily clinical practice.24–26 Finally, to facilitate interpretation of the results, we analyzed the dose recommendations exclusively based on changes in estimated renal function or serum creatinine, without taking into account other considerations for dosage adjustment.
CONCLUSIONSFluctuations in renal function and consequently the need for dosage adjustment of NOACs are frequent in the mid-term after ADHF, especially among the elderly or in patients with poor renal function. Further studies are needed to clarify the clinical importance of these needs for dose/drug adjustment and the ideal renal function monitoring regime in heart failure and other subgroups of patients with AF.
CONFLICTS OF INTERESTV. Roldán has received funding for consultancy and lecturing from Bristol-Myers Squibb, Bayer, and Boehringer Ingelheim. G.Y.H. Lip has received funding for research, consultancy, and lecturing from different manufacturers of drugs used for the treatment of atrial fibrillation, including AstraZeneca, Bayer, Boehringer Ingelheim, Astellas, Sanofi-Aventis, and Daiichi Sankyo.
We would like to thank Dr. F. Marín for his cooperation and comments.