To analyze the percutaneous revascularization strategy for severe lesions in the secondary branches (SB) (diameter ≥ 2mm) of major epicardial arteries compared with conservative treatment.
MethodsThis study analyzed patients with severe SB lesions who underwent percutaneous revascularization treatment compared with patients who received pharmacological treatment. The study examined the percentage of branch-related events (cardiovascular death, myocardial infarction attributable to SB, or the need for revascularization of the SB).
ResultsWe analyzed 679 SB lesions (662 patients). After a mean follow-up of 22.2±10.5 months, there were no significant differences between the 2 treatment groups regarding the percentage of death from cardiovascular causes (1.7% vs 0.4%; P=.14), nonfatal acute myocardial infarction (AMI) (1.7% vs 1.7%; P=.96), the need for SB revascularization (4.1% vs 5.4%; P=.45) or in the total percentage of events (5.1% vs 6.3%; P=.54). The variables showing an association with event occurrence on multivariate analysis were diabetes (SHR, 2.87; 95%CI, 1.37-5.47; P=.004), prior AMI (SHR, 3.54; 95%CI, 1.77-7.30; P<.0001), SB reference diameter (SHR, 0.16; 95%CI, 0.03-0.97; P=.047), and lesion length (SHR, 3.77; 95%CI, 1.03-1.13; P<.0001). These results remained the same after the propensity score analysis.
ConclusionsThe percentage of SB-related events during follow-up is low, with no significant differences between the 2 treatment strategies. The variables associated with event occurrence in the multivariate analysis were the presence of diabetes mellitus, prior AMI, and greater lesion length.
Keywords
Secondary branch (SB) vessels are the generally less developed, smaller caliber branches of main coronary arteries that supply blood to a smaller area of the myocardium. The treatment criterion for an SB tends to focus on diameter, but there are other factors that should also be considered such as the length and development of the SB, the size of the main vessel, and the amount of myocardium irrigated.1
The few studies that focus on the treatment of SB lesions are based on a post hoc analysis of clinical trials, and on the revascularization of small caliber vessels, whether SB or not. The studies published on SB lesion interventions focus on an analysis of the treatment of bifurcation lesions with simple or complex techniques; however, the effectiveness of SB treatment in this context is still the subject of debate.2–7 Focusing on the context of angiographically severe lesions located in SB vessels, this study aimed to examine a treatment strategy based on percutaneous coronary revascularization compared with pharmacological treatment in routine clinical practice.
METHODSA retrospective cohort study was designed to evaluate patients with severe lesions in the SB of epicardial arteries undergoing percutaneous revascularization treatment and compare them with patients with severe lesions in SB who received pharmacological treatment exclusively. The choice of treatment type was at the operator's criterion in all cases.
Inclusion CriteriaPatients included had severe coronary lesions (stenosis ≥ 70% based on visual estimation) in secondary coronary branches ≥ 2mm diameter, and underwent coronary angiography for stable angina or non–ST-segment elevation acute coronary syndrome. Secondary branch included diagonal branches, marginal branches (or the distal circumflex if it was of smaller caliber and less developed than the marginal branch), the ramus intermedius, and the posterior descending and posterolateral artery.
Exclusion CriteriaPatients with the following were excluded from the study: indication for coronary angiography for ST-segment elevation acute coronary syndrome, severe nonrevascularized lesions in main coronary arteries, prior surgical coronary revascularization, restenosis of a previously implanted stent in the SB, contraindication for dual antiplatelet therapy, severe valvulopathies or patients with valve prosthesis, indication for surgical treatment, and any lesion in SB whose treatment would require a bifurcation technique affecting the main vessel (patients with SB lesions that could affect the main artery, and patients with lesions in main arteries in which treatment could involve the SB) ie, only independent lesions were taken into account.
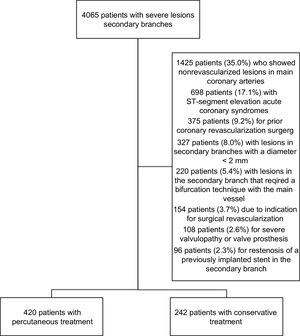
Study PopulationThis study included a total 4065 patients who had severe lesions in SBs, underwent angiography, and were attended over a 3-year period (January 2013 to December 2015) at 2 high-volume hospitals. After a review of the medical history and the angiogram, we included 662 patients in the study (Figure 1).
Definitions and Clinical EventsThe included patients had severe coronary lesions (stenosis ≥ 70% estimated visually, based on angiography) in secondary coronary branches with a diameter ≥ 2mm. The choice of treatment was made by the interventional cardiologist performing the procedure, and the pharmacological treatment prescribed on discharge was chosen by the clinical cardiologist who treated the patient.
The clinical follow-up of each patient was conducted by reviewing the medical history and in outpatient clinic. Clinical events related to the SB were considered to be: a) the need for revascularization of the SB vessel (defined as repeat revascularization of the lesion under study or of the adjacent 5mm); b) nonfatal myocardial infarction attributable to the target lesion (defined as a coronary event considered to be a myocardial infarction that was associated with the destabilization, or the occurrence of a complication of the lesion under study); c) death from cardiovascular causes (defined as death due to heart failure, ischemia, arrhythmia, or sudden death).
The definition of myocardial infarction was as follows: detection of an increase in cardiac biomarker values (troponin) with at least a value over the 99th percentile, in accordance with the laboratory ranges of each center and at least 1 of the following: symptoms of ischemia, new or supposedly new significant changes in the ST-segment or a new left bundle branch block, onset of a pathologic Q wave on the electrocardiogram, images showing new loss of viable myocardium or new regional anomalies in movement of the wall, and angiographic identification of an intracoronary thrombus of the lesion under study.
Angiographic VariablesDigital quantification was conducted with quantitative coronary analysis measurement software (Siemens Artis Syngo X Workplace VB21).
After review of the coronary angiogram, the baseline and residual SYNTAX scores were calculated for all patients by using the online calculator.8 In each patient, the SYNTAX score was calculated by 2 interventional cardiologists who had been trained in the use of the tool. The SYNTAX score value for each patient was determined by the mean score calculated by each observer. The interobserver intraclass correlation coefficient was 0.88 (95% confidence interval [95%CI], 0.83-0.93; P < .0001) and the intraobserver intraclass correlation coefficient was 0.90 (95%CI, 0.86-0.94; P < .0001).
EthicsTo access data from the medical histories for research purposes, we followed the established protocols of each center. This study complies with the Declaration of Helsinki (1975) of the World Medical Association on ethical principles for medical research involving human participants.
Statistical AnalysisCalculation of the sample size was performed based on demonstration of superiority and for comparison of proportions. The frequency of combined events was considered as 8% for the medical treatment group, and 4% for the percutaneous coronary intervention (PCI) group. With an alpha error of 0.05 and a power of 80%, it was estimated that the number of patients to include was a total 458 (229 patients in each group). The inclusion process ended when at least the minimum number of patients was reached for each group.
Qualitative variables are expressed as absolute number and percentage of the total. Quantitative variables are expressed as mean and standard deviation as long as distribution of the values was symmetrical. When distribution of the quantitative variables was asymmetric, they are represented as mean±[interquartile range]. To determine the existence of significant differences we used the chi-square test for qualitative variables and the ANOVA test for quantitative variables.
A survival study was performed using Fine and Gray competing risk proportional hazard regression,9 estimating the cumulative incidence function and the subdistribution hazard ratio (SHR) with a 95%CI. The cumulative incidence curves were compared using Gray's P value test. A value of P < .05 was considered to indicate statistical difference in all tests. The primary variable of combined events during follow-up was calculated for each patient according to presentation of the following: a) need for revascularization of a SB vessel included in the study; b) nonfatal myocardial infarction attributed to 1 of the studied lesions (target lesions); and c) death due to cardiovascular cause (defined as death due to heart failure, ischemia, arrhythmia or sudden death).
After the initial analysis, a sensitivity analysis was performed using the propensity score method and logistic regression to adjust for possible confounding variables. This analysis was performed using pair matching (nearest neighbor matching) by patient. The included variables were hospital of origin, hypertension, smoking, a history of prior myocardial infarction, the number of epicardial arteries involved, location of the lesion under study, and treatment with aspirin and clopidogrel. The value of the C-statistic was 0.87.
All analyses were conducted using SPSS 21.0 software (SSPS Inc, Chicago, Illinois, United States), R (Foundation for Statistical Computing, Vienna, Austria), and STATA (STATA Data Analysis and Statistical Software, Texas, United States).
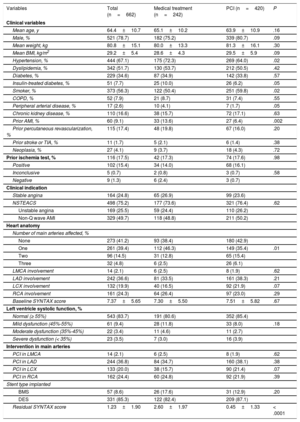
RESULTSBaseline PopulationClinical Characteristics and Baseline AngiographyWe analyzed 679 SB vessel lesions in 662 patients. Of the 679 lesions, 430 (63.3%) received PCI (420 patients, 63.4%), whereas 249 lesions (36.7%) received conservative treatment (242 patients, 36.6%). There were no significant differences in the clinical indication for catheterization. Table 1 summarizes the patients’ clinical characteristics.
Clinical and Angiographic Characteristics of the Included Patients
| Variables | Total (n=662) | Medical treatment (n=242) | PCI (n=420) | P |
|---|---|---|---|---|
| Clinical variables | ||||
| Mean age, y | 64.4±10.7 | 65.1±10.2 | 63.9±10.9 | .16 |
| Male, % | 521 (78.7) | 182 (75.2) | 339 (80.7) | .09 |
| Mean weight, kg | 80.8±15.1 | 80.0±13.3 | 81.3±16.1 | .30 |
| Mean BMI, kg/m2 | 29.2±5.4 | 28.6±4.3 | 29.5±5.9 | .09 |
| Hypertension, % | 444 (67.1) | 175 (72.3) | 269 (64.0) | .02 |
| Dyslipidemia, % | 342 (51.7) | 130 (53.7) | 212 (50.5) | .42 |
| Diabetes, % | 229 (34.6) | 87 (34.9) | 142 (33.8) | .57 |
| Insulin-treated diabetes, % | 51 (7.7) | 25 (10.0) | 26 (6.2) | .05 |
| Smoker, % | 373 (56.3) | 122 (50.4) | 251 (59.8) | .02 |
| COPD, % | 52 (7.9) | 21 (8.7) | 31 (7.4) | .55 |
| Peripheral arterial disease, % | 17 (2.6) | 10 (4.1) | 7 (1.7) | .05 |
| Chronic kidney disease, % | 110 (16.6) | 38 (15.7) | 72 (17.1) | .63 |
| Prior AMI, % | 60 (9.1) | 33 (13.6) | 27 (6.4) | .002 |
| Prior percutaneous revascularization, % | 115 (17.4) | 48 (19.8) | 67 (16.0) | .20 |
| Prior stroke or TIA, % | 11 (1.7) | 5 (2.1) | 6 (1.4) | .38 |
| Neoplasia, % | 27 (4.1) | 9 (3.7) | 18 (4.3) | .72 |
| Prior ischemia test, % | 116 (17.5) | 42 (17.3) | 74 (17.6) | .98 |
| Positive | 102 (15.4) | 34 (14.0) | 68 (16.1) | |
| Inconclusive | 5 (0.7) | 2 (0.8) | 3 (0.7) | .58 |
| Negative | 9 (1.3) | 6 (2.4) | 3 (0.7) | |
| Clinical indication | ||||
| Stable angina | 164 (24.8) | 65 (26.9) | 99 (23.6) | |
| NSTEACS | 498 (75.2) | 177 (73.6) | 321 (76.4) | .62 |
| Unstable angina | 169 (25.5) | 59 (24.4) | 110 (26.2) | |
| Non-Q wave AMI | 329 (49.7) | 118 (48.8) | 211 (50.2) | |
| Heart anatomy | ||||
| Number of main arteries affected, % | ||||
| None | 273 (41.2) | 93 (38.4) | 180 (42.9) | |
| One | 261 (39.4) | 112 (46.3) | 149 (35.4) | .01 |
| Two | 96 (14.5) | 31 (12.8) | 65 (15.4) | |
| Three | 32 (4.8) | 6 (2.5) | 26 (6.1) | |
| LMCA involvement | 14 (2.1) | 6 (2.5) | 8 (1.9) | .62 |
| LAD involvement | 242 (36.6) | 81 (33.5) | 161 (38.3) | .21 |
| LCX involvement | 132 (19.9) | 40 (16.5) | 92 (21.9) | .07 |
| RCA involvement | 161 (24.3) | 64 (26.4) | 97 (23.0) | .29 |
| Baseline SYNTAX score | 7.37±5.65 | 7.30±5.50 | 7.51±5.82 | .67 |
| Left ventricle systolic function, % | ||||
| Normal (≥ 55%) | 543 (83.7) | 191 (80.6) | 352 (85.4) | |
| Mild dysfunction (45%-55%) | 61 (9.4) | 28 (11.8) | 33 (8.0) | .18 |
| Moderate dysfunction (35%-45%) | 22 (3.4) | 11 (4.6) | 11 (2.7) | |
| Severe dysfunction (< 35%) | 23 (3.5) | 7 (3.0) | 16 (3.9) | |
| Intervention in main arteries | ||||
| PCI in LMCA | 14 (2.1) | 6 (2.5) | 8 (1.9) | .62 |
| PCI in LAD | 244 (36.8) | 84 (34.7) | 160 (38.1) | .38 |
| PCI in LCX | 133 (20.0) | 38 (15.7) | 90 (21.4) | .07 |
| PCI in RCA | 162 (24.4) | 60 (24.8) | 92 (21.9) | .39 |
| Stent type implanted | ||||
| BMS | 57 (8.6) | 26 (17.6) | 31 (12.9) | .20 |
| DES | 331 (85.3) | 122 (82.4) | 209 (87.1) | |
| Residual SYNTAX score | 1.23±1.90 | 2.60±1.97 | 0.45±1.33 | < .0001 |
AMI, acute myocardial infarction; BMS, bare metal stent; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DES, drug-eluting stent; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; NSTEACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIA, transient ischemic attack.
The data are expressed as mean±or No. (%).
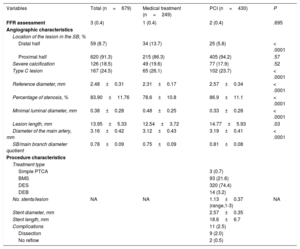
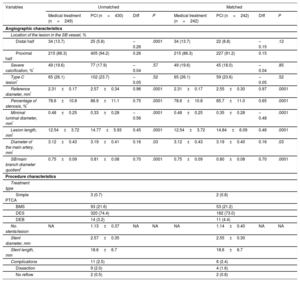
There were 679 severe coronary lesions located in SB. The angiographic characteristics of these lesions are summarized in Table 2. The lesions where PCI was performed were located predominantly in the proximal half of the SB (94.2% vs 86.3%; P < .0001) and showed a larger reference diameter (2.57±0.34mm vs 2.31±0.17mm; P < .0001), a greater lesion length (14.77±5.93mm vs 12.54±3.72; P < .0001), greater severity of stenosis (86.9±11.1% vs 78.6±10.8%; P < .0001), and a smaller minimal luminal diameter (0.33±0.28 vs 0.48±0.25mm; P < .0001). In addition, the caliber of the main vessel that the SB depended on was significantly higher in the PCI group (3.19±0.41mm vs 3.12±0.43mm; P=.03) as was the quotient between the diameter of the SB and the main branch (0.81±0.08 vs 0.75±0.09; P < .0001). Only 3 lesions (0.4%) underwent a functional assessment of the lesion with pressure wire quantification of fractional flow reserve (FFR), and percutaneous treatment was performed on those whose measured FFR was lower than 0.80. provides a summary of the interventional procedures in major epicardial arteries.
Angiographic Characteristics of the Severe Lesions Located in the Secondary Branch and Procedure-related Variables
| Variables | Total (n=679) | Medical treatment (n=249) | PCI (n=430) | P |
|---|---|---|---|---|
| FFR assessment | 3 (0.4) | 1 (0.4) | 2 (0.4) | .695 |
| Angiographic characteristics | ||||
| Location of the lesion in the SB, % | ||||
| Distal half | 59 (8.7) | 34 (13.7) | 25 (5.8) | < .0001 |
| Proximal half | 620 (91.3) | 215 (86.3) | 405 (94.2) | .57 |
| Severe calcification | 126 (18.5) | 49 (19.6) | 77 (17.9) | .52 |
| Type C lesion | 167 (24.5) | 65 (26.1) | 102 (23.7) | < .0001 |
| Reference diameter, mm | 2.48±0.31 | 2.31±0.17 | 2.57±0.34 | < .0001 |
| Percentage of stenosis, % | 83.90±11.76 | 78.6±10.8 | 86.9±11.1 | < .0001 |
| Minimal luminal diameter, mm | 0.38±0.28 | 0.48±0.25 | 0.33±0.28 | < .0001 |
| Lesion length, mm | 13.95±5.33 | 12.54±3.72 | 14.77±5.93 | .03 |
| Diameter of the main artery, mm | 3.16±0.42 | 3.12±0.43 | 3.19±0.41 | < .0001 |
| SB/main branch diameter quotient | 0.78±0.09 | 0.75±0.09 | 0.81±0.08 | |
| Procedure characteristics | ||||
| Treatment type | ||||
| Simple PTCA | 3 (0.7) | |||
| BMS | 93 (21.6) | |||
| DES | 320 (74.4) | |||
| DEB | 14 (3.2) | |||
| No. stents/lesion | NA | NA | 1.13±0.37 (range,1-3) | NA |
| Stent diameter, mm | 2.57±0.35 | |||
| Stent length, mm | 18.6±6.7 | |||
| Complications | 11 (2.5) | |||
| Dissection | 9 (2.0) | |||
| No reflow | 2 (0.5) | |||
BMS, bare metal stent; DEB, drug-eluting balloon; DES, drug-eluting stent; FFR, fractional flow reserve; NA, not applicable; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplast; SB, secondary branch.
The data are expressed as mean±or No. (%).
In terms of pharmacological treatment at discharge (), a higher percentage of the percutaneous treatment group of patients received aspirin treatment at discharge compared with the conservative treatment group (99.4% vs 93.9% respectively; P < .0001). Likewise, clopidogrel, the second most used antiplatelet drug, was prescribed for a higher number of patients in the percutaneous treatment group (66.8% vs 54.2%; P=.003). There were no significant differences in the use of ticagrelor or prasugrel.
In the PCI group, there was a higher percentage of patients with beta-blockers (83.0% vs 75.9%; P=.04) and statins (94.7% vs 88.2%; P=.005) prescribed at discharge, whereas in the medical treatment group, the use of nitrates (14.0% vs 32.5%; P < .0001) and ranolazine (1.7% vs 7.5%; P < .0001) was more frequent. There were significant differences in the total number of anti-ischemic drugs prescribed at discharge, with a higher number of patients in the PCI group with 1 or no anti-ischemic drug, whereas the percentage of patients with 2, 3, 4 or more drugs was higher in the conservative treatment group.
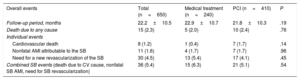
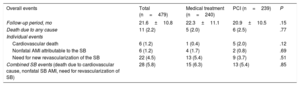
Events per Treatment GroupIt was possible to conduct follow-up of 650 of the 662 patients included in the study sample. After a mean follow-up of 22.2±10.5 months (21.8±10.3 months vs 22.9±10.7 months; P=.19), mortality due to any cause occurred in 15 patients (2.3%) and mortality due to a cardiovascular cause occurred in 8 patients (1.2%). The percentage of nonfatal acute myocardial infarction (AMI) attributable to the target lesion in the SB was 1.6% (11 cases) and the need for revascularization of the SB was 4.5% (30 cases). The percentage of overall events related to the SB (death from cardiovascular cause, nonfatal AMI attributable to the SB, the need for revascularization of the SB) was 5.4% (36 events).
There were no significant differences between the 2 treatment groups regarding the percentage of mortality due to cardiovascular causes, nonfatal AMI, the need for SB revascularization, or in the total percentage of combined events related to the SB (Table 3).
Events During Follow-up
| Overall events | Total (n=650) | Medical treatment (n=240) | PCI (n=410) | P |
|---|---|---|---|---|
| Follow-up period, months | 22.2±10.5 | 22.9±10.7 | 21.8±10.3 | .19 |
| Death due to any cause | 15 (2.3) | 5 (2.0) | 10 (2.4) | .76 |
| Individual events | ||||
| Cardiovascular death | 8 (1.2) | 1 (0.4) | 7 (1.7) | .14 |
| Nonfatal AMI attributable to the SB | 11 (1.6) | 4 (1.7) | 7 (1.7) | .96 |
| Need for a new revascularization of the SB | 30 (4.5) | 13 (5.4) | 17 (4.1) | .45 |
| Combined SB events (death due to CV cause, nonfatal SB AMI, need for SB revascularization) | 36 (5.4) | 15 (6.3) | 21 (5.1) | .54 |
AMI, acute myocardial infarction; CV, cardiovascular; PCI, percutaneous coronary intervention; SB, secondary branch.
The data are expressed as mean±or No. (%).
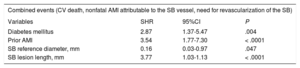
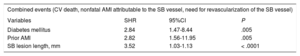
The multivariate analysis conducted using the competing risks model (Table 4) showed the most significant variables related to the occurrence of combined events to be the presence of diabetes mellitus (SHR, 2.87; 95%CI, 1.37-5.47; P=.004), prior AMI (SHR, 3.54; 95%CI, 1.77-7.30; P < .0001), SB vessel reference diameter (SHR, 0.16; 95%CI, 0.03-0.97; P=.047), and lesion length (SHR, 3.77; 95%CI, 1.03-1.13; P < .0001).
Multivariate Analysis: Variables Associated With the Rate of Combined Events in Follow-up
| Combined events (CV death, nonfatal AMI attributable to the SB vessel, need for revascularization of the SB) | |||
|---|---|---|---|
| Variables | SHR | 95%CI | P |
| Diabetes mellitus | 2.87 | 1.37-5.47 | .004 |
| Prior AMI | 3.54 | 1.77-7.30 | < .0001 |
| SB reference diameter, mm | 0.16 | 0.03-0.97 | .047 |
| SB lesion length, mm | 3.77 | 1.03-1.13 | < .0001 |
95%CI, 95% confidence interval; AMI, acute myocardial infarction; CV, cardiovascular; SB, secondary branch; SHR, subdistribution hazard ratio.
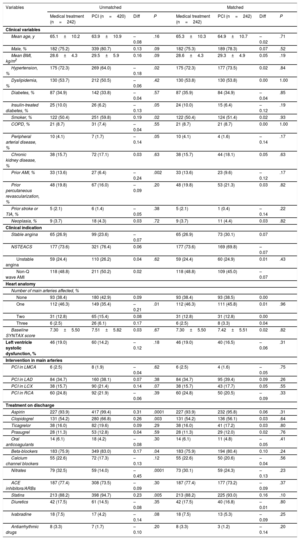
For each group, a propensity score analysis was computed using logistic regression to adjust for possible confounding variables. The included variables were hospital of origin, hypertension, smoking, a history of prior myocardial infarction, the number of epicardial arteries involved, location of the lesion under study, and treatment with aspirin and clopidogrel. This resulted in a selection of 484 patients (242 patients for each treatment group). The distribution of the different variables is illustrated in Table 5 and Table 6.
Clinical, Angiographic, and Pharmacological Treatment Characteristics for Both Treatment Groups (With Propensity Score Adjustment)
| Variables | Unmatched | Matched | ||||||
|---|---|---|---|---|---|---|---|---|
| Medical treatment (n=242) | PCI (n=420) | Diff | P | Medical treatment (n=242) | PCI (n=242) | Diff | P | |
| Clinical variables | ||||||||
| Mean age, y | 65.1±10.2 | 63.9±10.9 | –0.08 | .16 | 65.3±10.3 | 64.9±10.7 | –0.02 | .71 |
| Male, % | 182 (75.2) | 339 (80.7) | 0.13 | .09 | 182 (75.3) | 189 (78.3) | 0.07 | .52 |
| Mean BMI, kg/m2 | 28.6±4.3 | 29.5±5.9 | 0.16 | .09 | 28.6±4.3 | 29.3±4.9 | 0.05 | .19 |
| Hypertension, % | 175 (72.3) | 269 (64.0) | –0.18 | .02 | 175 (72.3) | 177 (73.5) | 0.02 | .84 |
| Dyslipidemia, % | 130 (53.7) | 212 (50.5) | –0.06 | .42 | 130 (53.8) | 130 (53.8) | 0.00 | 1.00 |
| Diabetes, % | 87 (34.9) | 142 (33.8) | –0.04 | .57 | 87 (35.9) | 84 (34.9) | –0.04 | .85 |
| Insulin-treated diabetes, % | 25 (10.0) | 26 (6.2) | –0.13 | .05 | 24 (10.0) | 15 (6.4) | –0.12 | .19 |
| Smoker, % | 122 (50.4) | 251 (59.8) | 0.19 | .02 | 122 (50.4) | 124 (51.4) | 0.02 | .93 |
| COPD, % | 21 (8.7) | 31 (7.4) | –0.04 | .55 | 21 (8.7) | 21 (8.7) | 0.00 | 1.00 |
| Peripheral arterial disease, % | 10 (4.1) | 7 (1.7) | –0.14 | .05 | 10 (4.1) | 4 (1.6) | –0.14 | .17 |
| Chronic kidney disease, % | 38 (15.7) | 72 (17.1) | 0.03 | .63 | 38 (15.7) | 44 (18.1) | 0.05 | .63 |
| Prior AMI, % | 33 (13.6) | 27 (6.4) | –0.24 | .002 | 33 (13.6) | 23 (9.6) | –0.12 | .17 |
| Prior percutaneous revascularization, % | 48 (19.8) | 67 (16.0) | –0.09 | .20 | 48 (19.8) | 53 (21.3) | 0.03 | .82 |
| Prior stroke or TIA, % | 5 (2.1) | 6 (1.4) | –0.05 | .38 | 5 (2.1) | 1 (0.4) | –0.14 | .22 |
| Neoplasia, % | 9 (3.7) | 18 (4.3) | 0.03 | .72 | 9 (3.7) | 11 (4.4) | 0.03 | .82 |
| Clinical indication | ||||||||
| Stable angina | 65 (26.9) | 99 (23.6) | –0.07 | 65 (26.9) | 73 (30.1) | 0.07 | ||
| NSTEACS | 177 (73.6) | 321 (76.4) | 0.06 | 177 (73.6) | 169 (69.8) | –0.07 | ||
| Unstable angina | 59 (24.4) | 110 (26.2) | 0.04 | .62 | 59 (24.4) | 60 (24.9) | 0.01 | .43 |
| Non-Q wave AMI | 118 (48.8) | 211 (50.2) | 0.02 | 118 (48.8) | 109 (45.0) | –0.07 | ||
| Heart anatomy | ||||||||
| Number of main arteries affected, % | ||||||||
| None | 93 (38.4) | 180 (42.9) | 0.09 | 93 (38.4) | 93 (38.5) | 0.00 | ||
| One | 112 (46.3) | 149 (35.4) | –0.21 | .01 | 112 (46.3) | 111 (45.8) | 0.01 | .96 |
| Two | 31 (12.8) | 65 (15.4) | 0.08 | 31 (12.8) | 31 (12.8) | 0.00 | ||
| Three | 6 (2.5) | 26 (6.1) | 0.17 | 6 (2.5) | 8 (3.3) | 0.04 | ||
| Baseline SYNTAX score | 7.30±5.50 | 7.51±5.82 | 0.03 | .67 | 7.30±5.50 | 7.42±5.51 | 0.02 | .82 |
| Left ventricle systolic dysfunction, % | 46 (19.0) | 60 (14.2) | –0.12 | .18 | 46 (19.0) | 40 (16.5) | –0.06 | .31 |
| Intervention in main arteries | ||||||||
| PCI in LMCA | 6 (2.5) | 8 (1.9) | –0.04 | .62 | 6 (2.5) | 4 (1.6) | –0.05 | .75 |
| PCI in LAD | 84 (34.7) | 160 (38.1) | 0.07 | .38 | 84 (34.7) | 95 (39.4) | 0.09 | .26 |
| PCI in LCX | 38 (15.7) | 90 (21.4) | 0.14 | .07 | 38 (15.7) | 43 (17.7) | 0.05 | .55 |
| PCI in RCA | 60 (24.8) | 92 (21.9) | –0.06 | .39 | 60 (24.8) | 50 (20.5) | –0.09 | .33 |
| Treatment on discharge | ||||||||
| Aspirin | 227 (93.9) | 417 (99.4) | 0.31 | .0001 | 227 (93.9) | 232 (95.8) | 0.06 | .31 |
| Clopidogrel | 131 (54.2) | 280 (66.8) | 0.26 | .003 | 131 (54.2) | 136 (56.1) | 0.03 | .64 |
| Ticagrelor | 38 (16.0) | 82 (19.6) | 0.09 | .29 | 38 (16.0) | 41 (17.2) | 0.03 | .80 |
| Prasugrel | 28 (11.3) | 53 (12.8) | 0.04 | .59 | 28 (11.3) | 29 (12.0) | 0.02 | .76 |
| Oral anticoagulants | 14 (6.1) | 18 (4.2) | –0.08 | .30 | 14 (6.1) | 11 (4.8) | –0.05 | .41 |
| Beta-blockers | 183 (75.9) | 349 (83.0) | 0.17 | .04 | 183 (75.9) | 194 (80.4) | 0.10 | .24 |
| Calcium channel blockers | 55 (22.6) | 72 (17.3) | –0.13 | .12 | 55 (22.6) | 50 (20.6) | –0.04 | .56 |
| Nitrates | 79 (32.5) | 59 (14.0) | –0.45 | .0001 | 73 (30.1) | 59 (24.3) | –0.13 | .23 |
| ACE inhibitors/ARBs | 187 (77.4) | 308 (73.5) | –0.09 | .30 | 187 (77.4) | 177 (73.2) | –0.09 | .37 |
| Statins | 213 (88.2) | 398 (94.7) | 0.23 | .005 | 213 (88.2) | 225 (93.0) | 0.16 | .10 |
| Diuretics | 42 (17.5) | 61 (14.5) | –0.08 | .35 | 42 (17.5) | 40 (16.8) | –0.01 | .80 |
| Ivabradine | 18 (7.5) | 17 (4.2) | –0.14 | .08 | 18 (7.5) | 13 (5.3) | –0.09 | .25 |
| Antiarrhythmic drugs | 8 (3.3) | 7 (1.7) | –0.10 | .20 | 8 (3.3) | 3 (1.2) | –0.14 | .20 |
ACE, angiotensin-converting enzyme; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; NSTEACS, non–ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; RCA, right coronary artery; TIA, transient ischemic attack.
The data are expressed as mean±or No. (%).
Angiographic Characteristics of the Severe Lesions Located in the Secondary Branch and Variables Related to the Procedure (With Propensity Score Adjustment)
| Variables | Unmatched | Matched | ||||||
|---|---|---|---|---|---|---|---|---|
| Medical treatment (n=249) | PCI (n=430) | Diff | P | Medical treatment (n=242) | PCI (n=242) | Diff | P | |
| Angiographic characteristics | ||||||||
| Location of the lesion in the SB vessel, % | ||||||||
| Distal half | 34 (13.7) | 25 (5.8) | –0.26 | .0001 | 34 (13.7) | 22 (8.8) | –0.15 | .12 |
| Proximal half | 215 (86.3) | 405 (94.2) | 0.26 | 215 (86.3) | 227 (91.2) | 0.15 | ||
| Severe calcification, %* | 49 (19.6) | 77 (17.9) | –0.04 | .57 | 49 (19.6) | 45 (18.0) | –0.04 | .85 |
| Type C lesion* | 65 (26.1) | 102 (23.7) | –0.05 | .52 | 65 (26.1) | 59 (23.6) | –0.05 | .52 |
| Reference diameter, mm* | 2.31±0.17 | 2.57±0.34 | 0.96 | .0001 | 2.31±0.17 | 2.55±0.30 | 0.97 | .0001 |
| Percentage of stenosis, %* | 78.6±10.8 | 86.9±11.1 | 0.75 | .0001 | 78.6±10.8 | 85.7±11.0 | 0.65 | .0001 |
| Minimal luminal diameter, mm* | 0.48±0.25 | 0.33±0.28 | –0.56 | .0001 | 0.48±0.25 | 0.35±0.28 | –0.48 | .0001 |
| Lesion length, mm* | 12.54±3.72 | 14.77±5.93 | 0.45 | .0001 | 12.54±3.72 | 14.84±6.09 | 0.46 | .0001 |
| Diameter of the main artery, mm* | 3.12±0.43 | 3.19±0.41 | 0.16 | .03 | 3.12±0.43 | 3.19±0.40 | 0.16 | .03 |
| SB/main branch diameter quotient* | 0.75±0.09 | 0.81±0.08 | 0.70 | .0001 | 0.75±0.09 | 0.80±0.08 | 0.70 | .0001 |
| Procedure characteristics | ||||||||
| Treatment type | ||||||||
| Simple PTCA | 3 (0.7) | 2 (0.8) | ||||||
| BMS | 93 (21.6) | 53 (21.2) | ||||||
| DES | 320 (74.4) | 182 (73.0) | ||||||
| DEB | 14 (3.2) | 11 (4.4) | ||||||
| No. stents/lesion | NA | 1.13±0.37 | NA | NA | NA | 1.14±0.40 | NA | NA |
| Stent diameter, mm | 2.57±0.35 | 2.55±0.30 | ||||||
| Stent length, mm | 18.6±6.7 | 18.6±6.7 | ||||||
| Complications | 11 (2.5) | 6 (2.4) | ||||||
| Dissection | 9 (2.0) | 4 (1.6) | ||||||
| No reflow | 2 (0.5) | 2 (0.8) | ||||||
BMS, bare metal stent; DEB, drug-eluting balloon; DES, drug-eluting stent; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; SB, secondary branch.
The data are expressed as mean±or No. (%).
Follow-up was possible for 479 of the 484 patients included in the propensity score analysis. After a mean follow-up of 21.6±10.8 months, the percentage of combined overall events related to the SB was 5.8% (28 cases), without differences between the treatment groups. The events observed during follow-up are shown in Table 7.
Events During Follow-up by Adjusted Group
| Overall events | Total (n=479) | Medical treatment (n=240) | PCI (n=239) | P |
|---|---|---|---|---|
| Follow-up period, mo | 21.6±10.8 | 22.3±11.1 | 20.9±10.5 | .15 |
| Death due to any cause | 11 (2.2) | 5 (2.0) | 6 (2.5) | .77 |
| Individual events | ||||
| Cardiovascular death | 6 (1.2) | 1 (0.4) | 5 (2.0) | .12 |
| Nonfatal AMI attributable to the SB | 6 (1.2) | 4 (1.7) | 2 (0.8) | .69 |
| Need for new revascularization of the SB | 22 (4.5) | 13 (5.4) | 9 (3.7) | .51 |
| Combined SB events (death due to cardiovascular cause, nonfatal SB AMI, need for revascularization of SB) | 28 (5.8) | 15 (6.3) | 13 (5.4) | .85 |
AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; SB, secondary branch.
The data are expressed as mean±or No. (%).
The multivariate analysis conducted using the competing risks method showed the most significant variables related to the occurrence of events to be the presence of diabetes mellitus (SHR, 2.84; 95%CI, 1.47-8.44; P=.005), prior AMI (SHR, 2.82; 95%CI, 1.56-11.95; P=.005), and lesion length (SHR, 3.52; 95%CI, 1.03-1.13; P < .0001, Table 8).
Multivariate Analysis: Variables Associated With the Rate of Combined Events in Follow-up
| Combined events (CV death, nonfatal AMI attributable to the SB vessel, need for revascularization of the SB vessel) | |||
|---|---|---|---|
| Variables | SHR | 95%CI | P |
| Diabetes mellitus | 2.84 | 1.47-8.44 | .005 |
| Prior AMI | 2.82 | 1.56-11.95 | .005 |
| SB lesion length, mm | 3.52 | 1.03-1.13 | < .0001 |
95%CI, 95% confidence interval; AMI, acute myocardial infarction; CV, cardiovascular; SB, secondary branch; SHR, subdistribution hazard ratio.
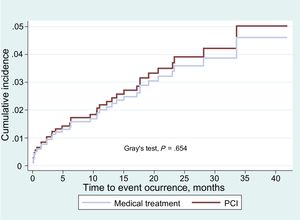
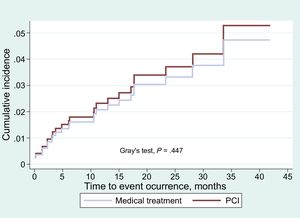
The cumulative incidence functions for combined events (death due to cardiovascular cause, nonfatal AMI of the SB and need for revascularization of the SB) showed no differences in the overall patient sample (Figure 2) or in the propensity score adjusted sample (Figure 3). The proportional assumption was met for treatment in both (total sample P=.975, propensity score sample P=.871).
The main findings of our study were as follows: a) in our series, most angiographically severe lesions observed in SB received percutaneous treatment; b) percutaneous treatment was chosen in SB of a larger diameter, longer lesion length, higher percentage of stenosis and a size similar to that of the main vessel; c) the percentage of events during follow-up was low in the overall patient group, with 5.4% of combined events related to SB vessels, principally due to the need for a new revascularization; d) no significant differences were observed in combined event-free survival between patients undergoing conservative treatment and those who had percutaneous treatment; e) in the multivariate analysis, the variables that correlated with the rate of events in follow-up were the presence of diabetes mellitus, prior AMI, a smaller diameter of the SB, and a longer lesion length; and f) after propensity score adjustment, there were no significant differences in the results obtained.
Although there have been a great many comparative studies on the subject of treatment using percutaneous revascularization for severe heart lesions vs medical treatment, to date no study has focused on comparing percutaneous treatment vs medical treatment of severe stenosis in the SB of major epicardial arteries. The studies published on interventional procedures in SB focus on the analysis of bifurcation lesion treatment with a simple technique or a double stent technique. Nevertheless, in daily clinical practice we often encounter patients undergoing catheterization for stable angina or acute coronary syndrome where severe coronary lesions are observed in the SB, the smaller caliber, less developed vessels that supply blood to a smaller area of the myocardium, with or without the involvement of other main coronary arteries. The prognostic value of these lesions with the possible improvements contributed by percutaneous and pharmacological treatment has yet to be clarified.
The presence of inducible ischemia related to a coronary stenosis is significant when taking the decision whether to revascularize stenosis. Whereas reducing a patient's myocardial ischemia with revascularization seems to improve functional classification, the revascularization of nonischemic lesions has always been debatable.10,11 Within the context of multivessel disease, it is frequently difficult to determine the culprit lesions of the ischemia, as in many cases we do not have prior ischemia tests available to guide the procedure. In the context of lesions located in SB, operators need to ask themselves whether the revascularization of a given lesion has clinical relevance, whether a given stenosis has caused the ischemia or if the revascularization can improve the patient's progress. Using FFR, Ahn et al.12 evaluated a total 230 bifurcation lesions in which a stent had been implanted in the main vessel, leaving a jailed SB; notably, they found that for ostial side branch lesions shown by angiography to have an involvement higher than 50%, the frequency of positive FFR was only 28.4%. Likewise, Koh et al.13 observed that there was no correlation between FFR and the percentage of stenosis in the ostial lesions of SBs (r=–0.067; P=.635) and a weak correlation between the FFR and the minimum luminal area estimated by intravascular ultrasound (r=0.30; P=.026). These studies illustrate the difficulties that arise when assessing severe coronary lesions located in SB based exclusively on angiographic criteria. Nevertheless, although FFR is currently the gold standard for assessing the functional repercussion of coronary lesions, it remains an underused technique.14 Moreover, in DKCRUSH-VI, the randomized clinical trial that compared angio-guided and FFR-guided treatment performed on SB bifurcation lesions, although conducting FFR gave a lower need for a stent implant in the SB, no differences between the 2 strategies were observed in the percentage of events at 1 year.15
The prognostic significance of severe lesions located in SB undergoing percutaneous interventional procedures or medical treatment has not been established, but there are studies available on the mid-term prognosis for SB in bifurcation lesions. In their meta-analysis based the results of a group of 5 randomized studies, Zhang et al.16 reported a lower percentage of myocardial infarction during follow-up in the conservative treatment SB vessel group compared with the complex technique group (5.0% vs 9.4%; P=.0001), with a similar percentage of SB restenosis (14.1% vs 12.6%; P=.140). Likewise, there are studies that focus on the prognostic value of percutaneous revascularization performed in small caliber coronary arteries, which represent between 35% and 50% of interventional procedures. It is known that there is an inverse relationship between the caliber of the vessel and the risk of restenosis and a worse result after PCI. In the ISAR-SMART study, Kastrati et al.17 reported the need for a new revascularization of 20.1% after 6 to 7 months of follow-up in the group treated with simple angioplasty, which was very similar to the group treated with a conventional stent implant. In our series, the percentage of events related to the SB was 5.4%, lower than in the aforementioned studies. That is to say, once the main coronary arteries had been treated, both courses of action (revascularization or nonrevascularization of an SB) were related to a low number of events, mainly with the need for a new revascularization, which highlights the debate about which treatment to perform on coronary arteries that will have a lower clinical repercussion on patients who have stable angina or non-ST-segment elevation acute coronary syndrome.
LimitationsThis research has several limitations. It is a nonrandomized, retrospective, 2-center study, and these circumstances make it difficult to control data and to analyze and subsequently extrapolate results. Nevertheless, it is a “real life” registry of patients with whom the interventional cardiologist has contact in habitual clinical practice and about whom the operator must decide to treat or maintain a conservative treatment strategy. Secondly, there was an imbalance in the number of patients included in the 2 groups. This was because in most SB lesions the operator opted for percutaneous revascularization. However, this study did include the minimum number of patients previously calculated in the sample size estimation. To adjust this factor and any possible confounding variables, our analysis was completed using the propensity score method. However, given that this is an observational study, residual confounding may have affected the results, by the noninclusion of variables collected but not included in the propensity score and/or unmeasured covariates. Finally, the low percentage of FFR-guided functional assessment of the target lesions is worthy of note and shows that this technique is currently underused.
CONCLUSIONSThe percentage of SB-related events during follow-up was low in the overall group of patients, with no significant differences between the 2 treatment strategies. In the multivariate analysis, the variables associated with event occurrence were the presence of diabetes mellitus, prior AMI, and greater lesion length.
FUNDINGThis work was supported by grant from Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CB16/11/00360), Instituto de Salud Carlos III co-founded by Fondo Europeo de Desarrollo Regional.
This article received funding support for the statistical analysis from Malaga University's Cátedra de Terapias Avanzadas en Patología Cardiovascular (Chair of Advanced Therapies in Cardiovascular Pathologies. CIF Q-2918001-E).
CONFLICTS OF INTERESTNone declared.
- –
Secondary branch vessels are the less developed, smaller caliber branches of the main coronary arteries and supply blood to a smaller area of the myocardium. Published studies on interventional procedures in SB lesions focus on analyzing the treatment of bifurcation lesions. However, in other contexts there are no available studies about the prognostic value of this type of lesion.
- –
The percentage of SB-related events during follow-up was low in the overall patient group, with no significant differences between the 2 treatment strategies (medical treatment or PCI). Variables that were associated with events were the presence of diabetes mellitus, prior AMI, and greater lesion length.
The authors would like to thank Dr Mario Gutiérrez Bedmar, professor in the Department of Preventive Medicine and Public Health at Malaga University, Spain, for his invaluable help.