To determine whether renin-angiotensin system inhibitor (RASi) prescription is associated with better outcomes after transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR).
MethodsAll comparative studies of RASi vs no RASi prescription in patients undergoing TAVI/SAVR were gathered from PubMed, Web of Science, and Google Scholar through August, 2019. We extracted hazard ratios (HRs) with their confidence intervals (CIs) for mortality from each study and combined study-specific estimates using inverse variance-weighted averages of logarithmic HRs in the random effects model.
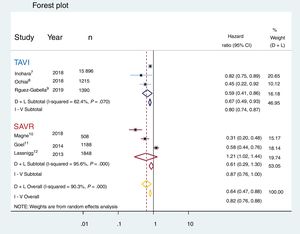
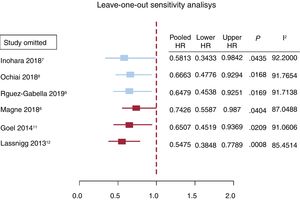
ResultsWe identified 6 eligible studies with a total of 21 390 patients (TAVI: 17 846; SAVR: 3544) and included them in the present meta-analysis. The 6 studies were observational comparative studies (including 3 propensity score matched and 3 cohort studies) of RASi vs no RASi prescription. The analysis demonstrated that RASi prescription was associated with significantly lower mortality in the whole group of patients undergoing aortic valve intervention (HR, 0.64; 95%CI, 0.47-0.88; P <.001). However, subgroup analysis suggested differences according to the selected therapy, with TAVI showing better mortality rates in the RASi group (HR, 0.67; 95%CI, 0.49-0.93) but not in the SAVR group (HR, 0.61; 95%CI, 0.29-1.30). No funnel plot asymmetry was identified, suggesting minimum publication bias. Sensitivity analyses sequentially eliminating dissimilar studies did not substantially alter the primary result favoring RASI prescription.
ConclusionsThese findings suggest a mortality benefit of RASi in patients with AS treated with aortic valve replacement that might be particularly relevant following TAVI. Future randomized studies are warranted to confirm this relevant finding.
Keywords
Renin-angiotensin system inhibitors (RASi) have demonstrated to reduce mortality not only in heart failure,1 but also in patients with hypertension,2 diabetes mellitus,3 and stable coronary artery disease.4 Conversely, their prescription before cardiovascular surgery has been associated with an increased risk of postoperative acute kidney injury and it has been reported that it may not decrease the risk of major adverse cardiac events.5
Aortic stenosis is associated with a continuous process of myocardial hypertrophy and fibrosis that influences both the symptoms and the prognosis of patients with this condition.6 Despite aortic valve replacement, reverse left ventricular remodeling is not consistently shown in the studies—specifically if late treatment of the obstruction is performed—with a residual trend to a higher mortality rate when myocardial fibrosis persists after the intervention.6
It has been suggested that the use of RASi is associated with improved survival in patients with aortic stenosis by reversing myocardial hypertrophy but it is unknown whether this prognostic benefit is similar in patients treated with surgical aortic valve replacement (SAVR) or with transcatheter aortic valve replacement.6 This is of great interest given the growing use of transcatheter aortic valve implantation (TAVI) and the current characteristics of the elderly patients treated with this technology. These patients have a higher incidence of cardiovascular risk factors, and, often, a more advanced degree of the disease and could benefit from RASi. However, this therapy could also be associated with greater risk of undesired collateral effects such as renal function decline, episodes of hypotension, or even increased mortality. Currently, there is no recommendation for the use of RASi in this population in the absence of other conditions such as diabetes mellitus or left ventricular dysfunction. Therefore, we aimed to determine whether RASi prescription at discharge is associated with better survival and major outcomes after TAVI and SAVR.
METHODSWe performed a systematic review and meta-analysis through a systematic search in Pubmed Web of Science, and Google Scholar performed by I.J. Amat-Santos and S. Santos-Martínez using the following terms: ‘Renin-Angiotensin -Inhibitor’ OR ‘-blocker’ AND ‘aortic valve replacement’ including only those articles with comparative data. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Further studies were sought by means of a manual search of secondary sources, including references from primary articles (backward snowballing) and contacts with international experts. If there were differences of opinion after discussion, a third author (J.A. San Román) gave his opinion. Potential publication bias was assessed by using a funnel plot. As a measure of the combined effect of the included studies, hazard ratios (HR) were estimated, valid for prospective and retrospective studies, along with their 95% confidence intervals (95%CI) and statistical significance. The homogeneity between studies was contrasted by the QH statistic. With regard to the low sensitivity of this test, we consider P <.10 values as significant. To overcome this limitation in some way, the I2 statistic was also estimated, which measures the proportion of the total variation of the studies explained by the heterogeneity and its 95%CI. A random effects model was used for those cases in which the I2 statistic was greater than 50% and a fixed effects model for the opposite cases. Sensitivity analyses sequentially eliminating dissimilar studies was performed. Statistical analysis was performed with the use of IBM SPSS Statistics, version 24 (IBM, United States). All tests were 2-sided at the .05 significance level.
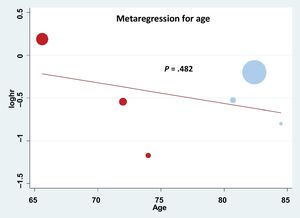
RESULTSData quality assessmentThe quality of the information included in the selected studies of the meta-analysis was assessed through a combined strategy. First, potential publication bias was assessed by using a funnel plot (). Although Egger's test suggested a lack of asymmetry, its power was limited by the small number of studies (less than 10). Second, a review protocol was followed and assessment of alternative specific bias was analyzed demonstrating limited quality in terms of cointerventions but a low risk of bias for the rest of the evaluated items (). Finally, meta-regression analysis was performed according to the study design, as shown in , demonstrating that there was a clear trend favoring the use of RASi in the matched studies; indeed, only 1 study did not favor its use and it was the only study that was not matched. However, the P value (.110) was not statistically significant due to the small number of studies that could be included in this study. Other variables such as left ventricular ejection fraction could not be assessed through meta-regression analysis due to heterogeneity in data reporting.
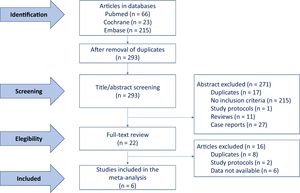
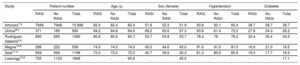
Baseline characteristicsAfter the assessment of 22 full-text articles, the final studies included in the meta-analysis were the 6 that met the inclusion criteria, as explained in methods, including 3 focused on transcatheter7–9 and 3 on SAVR patients.10–12 The flowchart summarizing study selection is depicted in figure 1. The main baseline characteristics and the total number of patients included in each study are reported in table 1. The pooled analysis was performed in 21 390 patients including 17 846 receiving TAVI, and 3544 treated with SAVR. Patients harboring transcatheter aortic valve replacement had a higher mean age (82.5 years)7–9 than those from surgical studies (74, 72, and 65.6 years, respectively).10–12 The distribution of this age difference is graphically presented in figure 2. No other baseline variable showed a clear pattern favoring any of the subgroups despite certain differences across the specific studies, namely a higher rate of hypertension reported by Inohara et al. in the transcatheter cohort,7 and a higher diabetes mellitus rate in the surgical cohort described by Goel et al.11
Baseline clinical characteristics reported in each of the studies included in this meta-analysis
| Study | Patient number | Age (y) | Sex (female) | Hypertension | Diabetes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RASi | No RASi | Total | RASi | No RASi | Total | RASi | No RASi | Total | RASi | No RASi | Total | RASi | No RASi | Total | |
| Inhorara7,a | 7948 | 7948 | 15 896 | 82.4 | 82.4 | 82.4 | 51.6 | 52.3 | 51.9 | 93.6 | 93.1 | 93.4 | 38.7 | 38.7 | 38.7 |
| Ochiai8,a | 371 | 189 | 560 | 84.2 | 84.8 | 84.6 | 68.2 | 65.6 | 67.3 | 83.8 | 61.4 | 72.0 | 27.8 | 24.3 | 26.2 |
| Rodríguez-Gabella9,a | 695 | 695 | 1390 | 80.8 | 80.6 | 80.7 | 53.7 | 53.8 | 53.7 | 78.4 | 78 | 78.2 | 33.4 | 35.4 | 34.5 |
| Magne10,b | 286 | 222 | 508 | 74.0 | 74.0 | 74.0 | 45.0 | 44.0 | 45.0 | 91.0 | 91.0 | 91.0 | 16.0 | 21.0 | 18.5 |
| Goel11,b | 594 | 594 | 1198 | 72.0 | 72.0 | 72.0 | 40.7 | 39.9 | 30.3 | 81.3 | 80.5 | 80.8 | 19.3 | 17.7 | 18.5 |
| Lassnigg12,b | 725 | 1123 | 1848 | - | - | 65.6 | - | - | 45.0 | - | - | - | - | - | 17.1 |
RASi, renin-angiotensin system inhibitors.
Values are expressed as absolute numbers or percentages.
The mortality reported by each of the 6 studies is summarized in figure 3. The overall mortality for patients treated with TAVI, SAVR, and for the global population is also reported. The pooled analysis of the patients from the 6 studies included in this meta-analysis demonstrated a 36% reduction in the risk of death at 1 year of follow up (HR, 0.64; 95%CI, 0.47-0.88; P <.001); however, the subgroup analysis could not confirm this finding for the SAVR group (HR, 0.61; 95%CI, 0.29-1.30). Only patients who underwent TAVI had a significant mortality benefit at mid-term (HR, 0.67; 95%CI, 0.49-0.93). Lack of asymmetry was demonstrated () with pooled HR, 0.779, 95%CI, 0.574-1.057; P=.11; I2=89.89% and, as shown in the Egger graph, the test did not provide evidence of the presence of small-study effects; in addition, sensitivity analyses sequentially eliminating dissimilar studies did not substantially alter the primary result favoring RASi prescription (figure 4).
Forest plot showing reduction in all-cause mortality with renin-angiotensin system inhibitors in the global population treated with aortic valve replacement (yellow) and in the subgroup analysis for the transcatheter aortic valve recipients (TAVI, in blue), but not for the surgically treated patients (SAVR, in red). 95%CI, 95% confidence interval; D+L, DerSimonian and Laird; I-V, inverse variation.
The results of this meta-analysis suggest that RASi prescription is associated with better mid-term survival after aortic valve replacement and, in particular, evidence derived from the pooled analysis and the sensitivity analysis suggests a specific beneficial effect following TAVI. Possible explanations for the failure of SAVR to demonstrate a consistent reduction in the mortality rate could be the reduced number of patients, as well as the different procedural aspects compared with TAVI. The putative beneficial effect of renin-angiotensin system blockade after valvular surgery has been explored in several studies. A retrospective study with 150 patients showed that RAS blockade reduced hospital admission and deaths.13 Of note, this effect was independent of left ventricular ejection fraction and volumes, suggesting a benefit of renin-angiotensin system blockade in patients with normal ventricular function and dimensions. Also, the landmark propensity analysis by Goel et al.11 in 1752 patients suggested a better outcome when the renin-angiotensin system was pharmacologically blocked. However, both studies have important limitations, including their retrospective nature, the lenient inclusion criteria, and the lack of information regarding type and dose of drugs given to the patients. There is only 1 prospective study in the surgical setting, with 114 patients, in which candesartan (32mg per day) compared with a control group.14 One year after valve replacement, a reduction in left ventricular mass was more pronounced in the active group, but the impact on clinical outcomes–as in the present meta-analysis–could not be confirmed.
Randomized controlled trials need to confirm these positive finding in TAVI recipients but the current evidence is reassuring regarding the safety of RASi in this scenario. The ongoing RASTAVI trial6 will help to determine the impact of RASi on outcomes following TAVI and understand whether this effect is driven from the induced left ventricular remodeling, as has been suggested.10
The effect of RASi on myocardial hypertrophy and fibrosisLeft ventricular mass regression, which seems to occur specially in patients with paravalvular regurgitation after TAVI has been independently related to RASi prescription.10 Indeed, persistent hypertrophy after SAVR has also been associated with worse clinical outcomes including mortality,15,16 whereas hypertrophy regression after SAVR following RASi prescription17 is associated with a lower incidence of myocardial infarction and stroke.16,18 However, the present meta-analysis could not confirm the association of RASi prescription after SAVR with better survival and potential left ventricular hypertrophy regression was not investigated.
RASi prescription in patients undergoing TAVI could also be associated with regression of myocardial fibrosis—probably more advanced in TAVI than in SAVR candidates given their advanced age—which may bring about better clinical outcomes including survival.8 Remarkably, in the propensity score matched study by Inohara et al., 7 RASi prescription was associated with a lower 1-year incidence of readmission for heart failure (absolute risk difference, −1.8%; 95%CI,−2.8% to−0.7%) after TAVI, but there was no difference in 1-year mortality between the RASi and no RASi groups (HR, 0.95; 95%CI, 0.81-1.12) among patients with reduced left ventricular ejection fraction (≤ 40%), despite lower 1-year mortality in the RASi group (HR, 0.78; 95%CI, 0.71-0.86) among patients with preserved left ventricular ejection fraction (> 40%), which is inconsistent with the recommendation in the current guidelines19,20 of RASi prescription only for patients with reduced left ventricular ejection fraction in the setting of heart failure. These contradictory findings, however, may be explained by a number of factors, such as smaller baseline left ventricular systolic dimensions and lower grades of postprocedural aortic and/or mitral regurgitation, which are suggested to be associated with greater left ventricular mass regression after SAVR21 and TAVI.7
Other mechanisms of RASi that might improve prognosisSympathetic modulation and antiarrhythmic effect have gained interest in the treatment of heart failure with RASi.22,23 The reduction in sympathetic activity as a mechanisms of modulating heart failure24 might explain the better outcomes of TAVI patients treated with RASi and could specifically benefit patients with residual aortic paravalvular regurgitation given the positive effect shown in the reduction of left ventricular volumes.7 On the other hand, patients with aortic stenosis often have concomitant coronary artery disease and myocardial ischemia; the electrolytic modulation induced by RASi can translate into an antiarrhythmic effect that might also improve outcomes.25
LimitationsThe present findings should be interpreted with caution because the results were drawn from nonrandomized studies (observational cohort studies), in which attrition is often worse and inadequately reported. In addition, the lower number of patients in the surgical group might explain the nonsignificant effect of RASi on mortality in that group. Additionally, prescription of RASi was defined at baseline but continuous treatment during follow up is unknown. Finally, publication bias might favor “positive” selection of those studies reporting good outcomes following RASi prescription; often, nonsignificant covariates in the univariate analysis are not entered into the multivariate analysis and are seldom reported; however, asymmetry was not detected with the present statistical assessment, suggesting minimal publication bias.
CONCLUSIONSThe findings of this meta-analysis suggest a mortality benefit of RASi in patients with aortic stenosis treated with aortic valve replacement that might be particularly relevant following TAVI. Future randomized studies are warranted to confirm this relevant finding.
FUNDINGProject granted by the Instituto de Salud Carlos III (ISCIII, Madrid, Spain). PI17/02237.
CONFLICTS OF INTERESTNone.
- -
Aortic stenosis is associated with a continuous process of myocardial hypertrophy and fibrosis that influences both the symptoms and prognosis of patients with this condition.
- -
Despite aortic valve replacement, reverse left ventricular remodeling is not consistently shown in studies, with higher mortality when myocardial fibrosis persists after the intervention.
- -
It has been suggested that the use of RASi could be associated with improved survival in patients with aortic stenosis but, currently, there is no recommendation for its systematic use after aortic valve replacement.
- -
We performed a pooled analysis of 21 390 patients including 17 846 receiving TAVI and 3544 treated with SAVR. Patients treated with SAVR were younger but no other significant differences were found in the baseline variables.
- -
RASi prescription was associated with better mid-term survival after SAVR and, in particular, sensitivity analysis suggested a specific beneficial effect following TAVI.
- -
The ongoing RASTAVI randomized controlled trial will help to determine the impact of RASi on outcomes following TAVI and to understand whether the effect is driven by induced left ventricular remodeling.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.03.004