The prognostic impact of bleeding in high bleeding risk (HBR) patients depending on the location of bleeding and prognosis in nonaccess site bleeding is unknown. We aimed to assess the impact of vascular access site on bleeding complications after percutaneous coronary interventions for HBR patients at 30-day and 2-year follow-up.
MethodsThe LEADERS FREE trial included 2432 HBR PCI patients. A Biolimus A9 drug-coated stent was superior to a bare-metal stent for safety and efficacy. This is a predefined sub-analysis of the LEADERS FREE trial.
ResultsTransradial access (TRA) was used in 1454 patients (59.8%) and transfemoral access (TFA) in 978 (40.2%), according to operator preference. The safety and benefits of drug-coated stents over bare-metal stents were independent of vascular access. At 30 days and 2 years, major bleeding had occurred in 2.4% and 7.5% of TRA patients and 4.6% and 10.9% of TFA patients (P=.003), respectively. Most of these events in both groups (2.1% and 7.0% for TRA; 3.2% and 9.4% for TFA, respectively) were nonaccess site-related. TRA was associated with a significant reduction in adjusted rates of major bleeding both at 30 days (HR, 1.98; 95%CI, 1.25-3.11; P=.003) and at 2 years of follow-up (HR, 1.51; 95%CI, 1.14-2.01; P=.003). This difference was driven by both access and nonaccess bleeding.
ConclusionsOperators preferred TRA for most HBR patients, which was associated with a significant reduction in major bleeding events. However, most of these events in this population are unrelated to vascular access.
Keywords
The vascular access site for percutaneous coronary intervention (PCI) plays a central role among procedure-related risk factors for bleeding. The adoption of the transradial access (TRA) site as the first choice of vascular access in patients undergoing PCI has spread globally and has now been shown to reduce access site-related bleeding complications in an increasing number of populations and settings,1,2 such as elderly patients,3 and those presenting with acute coronary syndrome (ACS),4 renal failure,5 or cardiogenic shock.6 In patients with ACS, the reduced vascular and major bleeding complications of TRA is strongly associated with reduced mortality.7,8
It has been estimated that about 20% of patients with coronary artery disease undergoing PCI are at high risk of bleeding.9,10 This population is mostly composed of elderly patients (≥ 75 years), those taking chronic oral anticoagulation (OAC), and those with anemia, renal insufficiency or cancer, and/or requiring major surgery.10,11 While all bleeding avoidance strategies are of particular importance for such patients, there have been no studies specifically on the potential benefits and risks of TRA vs transfemoral access (TFA).
The current study represents a prespecified subanalysis of the LEADERS FREE trial,10,11 and aimed to assess the impact of TRA and TFA on bleeding complications and outcome at 30-days and 2-years of follow-up after PCI with either a polymer-free biolimus A9 drug-coated stent or a bare-metal stent10 in patients at high bleeding risk (HBR).
METHODSPatients and study devicesWe included all patients who underwent PCI and were enrolled in the LEADERS FREE trial. The design and study results have been described previously.10,11 In summary, LEADERS FREE is a randomized, double-blind clinical trial that enrolled 2466 patients at 68 sites in 20 countries from December 2012 through May 2014. Patients were required to meet 1 or more of 13 criteria for increased bleeding risk (table 1). Those most frequently used were age 75 years or older, planned prolonged OAC, renal insufficiency, scheduled major surgery, anemia or recent transfusion, and cancer. Patients were randomly assigned 1:1 to undergo PCI with either a polymer-free BA9 drug-coated stent (BiofreedomTM DCS, Biosensors Europe, Morges, Switzerland) or a similar bare-metal stent (GazelleTM, Biosensors Interventional Technologies, Singapore). Both patients with ACS (ST-segment elevation myocardial infarction and non–ST-segment elevation myocardial infarction) and those with sable coronary artery disease were eligible for inclusion. The present study constitutes the result of a prespecified subanalysis based on the vascular access used (TRA or TFA).
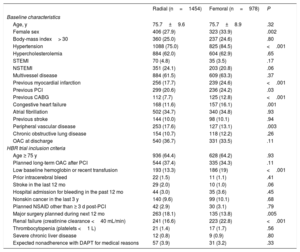
Baseline patient characteristics and inclusion criteria
| Radial (n=1454) | Femoral (n=978) | P | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, y | 75.7±9.6 | 75.7±8.9 | .32 |
| Female sex | 406 (27.9) | 323 (33.9) | .002 |
| Body-mass index> 30 | 360 (25.0) | 237 (24.6) | .80 |
| Hypertension | 1088 (75.0) | 825 (84.5) | <.001 |
| Hypercholesterolemia | 884 (62.0) | 604 (62.9) | .65 |
| STEMI | 70 (4.8) | 35 (3.5) | .17 |
| NSTEMI | 351 (24.1) | 203 (20.8) | .06 |
| Multivessel disease | 884 (61.5) | 609 (63.3) | .37 |
| Previous myocardial infarction | 256 (17.7) | 239 (24.6) | <.001 |
| Previous PCI | 299 (20.6) | 236 (24.2) | .03 |
| Previous CABG | 112 (7.7) | 125 (12.8) | <.001 |
| Congestive heart failure | 168 (11.6) | 157 (16.1) | .001 |
| Atrial fibrillation | 502 (34.7) | 340 (34.8) | .93 |
| Previous stroke | 144 (10.0) | 98 (10.1) | .94 |
| Peripheral vascular disease | 253 (17.6) | 127 (13.1) | .003 |
| Chronic obstructive lung disease | 154 (10.7) | 118 (12.2) | .26 |
| OAC at discharge | 540 (36.7) | 331 (33.5) | .11 |
| HBR trial inclusion criteria | |||
| Age ≥ 75 y | 936 (64.4) | 628 (64.2) | .93 |
| Planned long-term OAC after PCI | 544 (37.4) | 335 (34.3) | .11 |
| Low baseline hemoglobin or recent transfusion | 193 (13.3) | 186 (19) | <.001 |
| Prior intracerebral bleed | 22 (1.5) | 11 (1.1) | .41 |
| Stroke in the last 12 mo | 29 (2.0) | 10 (1.0) | .06 |
| Hospital admission for bleeding in the past 12 mo | 44 (3.0) | 35 (3.6) | .45 |
| Nonskin cancer in the last 3 y | 140 (9.6) | 99 (10.1) | .68 |
| Planned NSAID other than ≥ 3 d post-PCI | 42 (2.9) | 30 (3.1) | .79 |
| Major surgery planned during next 12 mo | 263 (18.1) | 135 (13.8) | .005 |
| Renal failure (creatinine clearance <40 mL/min) | 241 (16.6) | 223 (22.8) | <.001 |
| Thrombocytopenia (platelets <1 L) | 21 (1.4) | 17 (1.7) | .56 |
| Severe chronic liver disease | 12 (0.8) | 9 (0.9) | .80 |
| Expected nonadherence with DAPT for medical reasons | 57 (3.9) | 31 (3.2) | .33 |
CABG, coronary artery bypass grafting; DAPT, dual antiplatelet therapy; HBR, high bleeding risk; NSAID, nonsteroidal anti-inflammatory drug; NSTEMI, non–ST-segment myocardial infarction; OAC, oral anticoagulation; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data are presented as No. (%) or mean±standard deviation.
PCI was performed according to standard techniques at each participating center. Vascular access site selection, periprocedural antithrombotic regimen, and lesion preparation were left to the operator's discretion. All target lesions were treated with at least 1 study stent. Staged procedures were permitted within 1 week after the index procedure. Per protocol, all patients received 1 month of dual antiplatelet therapy with aspirin and a P2Y12 inhibitor followed by a single antiplatelet agent thereafter (aspirin preferred). Patients taking OAC at hospital discharge could either receive triple therapy or OAC plus clopidogrel (without aspirin) during the first 30 days. An on-site follow-up visit was performed at 30 days and 360 days and telephone contact at 60, 120, and 720 days.
Study endpointsIn the LEADERS FREE trial, the primary efficacy endpoint was the incidence of clinically-driven target lesion revascularization. The primary safety endpoint was the cumulative incidence of a composite of cardiac death, MI, and definite or probable ST. These endpoints were recorded up to 2 years after the index PCI. MI was defined according to the third universal definition, stent thrombosis according to the Academic Research Consortium and bleeding according to the Bleeding Academic Research Consortium (BARC) definitions, where BARC 3-5 were categorized as major bleeding events. Clinically-driven target lesion revascularization was defined as PCI or surgery for: a) restenosis of the treated lesion associated with angina or documented myocardial ischemia or, b) a core-laboratory diagnosed> 70% angiographic restenosis of the treated artery with or without myocardial ischemic manifestations. The primary study endpoints and all bleeding events were adjudicated by an independent clinical events committee.
Statistical analysisContinuous variables are presented as mean±standard deviation, and categorical data as counts and percentages. Categorical variables were compared using a chi-squared test; continuous variables were compared using a 2-sample t-test. Whenever appropriate, a Fisher exact test was used instead. For time-to-event variables, a hazard ratio or its 95% confidence interval (95%CI) was derived from an unadjusted Cox proportional hazard model. Cumulative incidence rates were calculated from the Kaplan-Meier estimator with log-rank P value to test whether the survival distributions differed over time. Proportional hazard assumptions were checked using Schoenfeld residuals, with no imputation for missing data. We formally tested the hypothesis of proportionality by adding time-dependent covariates in the model created from the interaction of the access type and the logarithm of the survival time. All available data were used in the analysis of all endpoints. Furthermore, to further investigate potential model misspecifications and in particular center effects, we included center as a random effect in a frailty model assuming a lognormal distribution for the frailty. Since patients were not randomized according to the vascular access route, all analyses comparing TRA and TFA were adjusted to correct for baseline imbalances with a propensity score model. Propensity scores were derived from a logistic regression model adjusting for 13 baseline covariates for increased bleeding risk that were necessary to enter the study (). These propensity scores were then used with the inverse probability of the treatment weight method. All data were analyzed using SAS V.9.4 (SAS Institute, Cary, NC, United States).
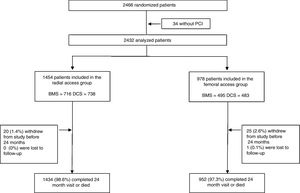
RESULTSAmong 2432 patients who underwent PCI in the LEADERS FREE trial, 1454 (59.8%) were treated ith TRA and 978 (40.2%) with TFA. Complete follow-up (excluding patients who withdrew from the study or were lost to follow-up) up to 730 days or death was achieved in 2387 (98.1%) patients (figure 1). A total of 325 patients (13.3% of the overall population) died during the study period. Cardiac death, which was part of the primary endpoint, occured in 156 (6.4%) of those patients and 169 (6.9%) were noncardiac. The breakdown of noncardiac death is given in the ( and ). The choice of vascular access site varied considerably from center to center, and TRA use was ≥ 80% in 20/68 centers and ≤ 20% in 11/68.
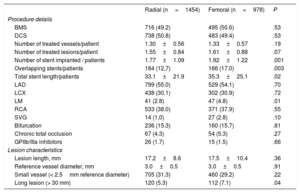
The baseline characteristics and inclusion criteria are shown in table 1. Compared with TRA patients, TFA patients were more frequently female (27.9% vs 33.9%; P=.002) and had significantly more comorbidities. Most of baseline demographic and procedural characteristics were well matched for the 2 groups (table 1). As for trial inclusion criteria, the TRA group had more patients with planned surgery requiring interruption of dual antiplatelet therapy, and the TFA group had more patients with anemia and/or recent transfusion and more patients with renal failure. Compared with the TRA group, the TFA group had more patients with a left main target lesion, or a long target lesion, and also had a greater overall number of stents implanted, overlapping stents and total stent length per patient (table 2).
Procedure details and medication
| Radial (n=1454) | Femoral (n=978) | P | |
|---|---|---|---|
| Procedure details | |||
| BMS | 716 (49.2) | 495 (50.6) | .53 |
| DCS | 738 (50.8) | 483 (49.4) | .53 |
| Number of treated vessels/patient | 1.30±0.56 | 1.33±0.57 | .19 |
| Number of treated lesions/patient | 1.55±0.84 | 1.61±0.88 | .07 |
| Number of stent implanted / patients | 1.77±1.09 | 1.92±1.22 | .001 |
| Overlapping stents/patients | 184 (12,7) | 166 (17.0) | .003 |
| Total stent length/patients | 33.1±21.9 | 35.3±25.1 | .02 |
| LAD | 799 (55.0) | 529 (54.1) | .70 |
| LCX | 438 (30.1) | 302 (30.9) | .72 |
| LM | 41 (2.8) | 47 (4.8) | .01 |
| RCA | 533 (38.0) | 371 (37.9) | .55 |
| SVG | 14 (1,0) | 27 (2.8) | .10 |
| Bifurcation | 236 (15.3) | 160 (15.7) | .81 |
| Chronic total occlusion | 67 (4.3) | 54 (5.3) | .27 |
| GPIIb/IIIa inhibitors | 26 (1.7) | 15 (1.5) | .66 |
| Lesion characteristics | |||
| Lesion length, mm | 17.2±8.6 | 17.5±10.4 | .36 |
| Reference vessel diameter, mm | 3.0±0.5 | 3.0±0.5 | .91 |
| Small vessel (< 2.5mm reference diameter) | 705 (31.3) | 460 (29.2) | .22 |
| Long lesion (> 30 mm) | 120 (5.3) | 112 (7.1) | .04 |
BMS, bare-metal stent; DCS, drug-coated stent; GPIIb/IIIa, glycoprotein IIb/IIIa; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; SVG, saphenous vein graft.
Data are presented as No. (%) or mean±standard deviation.
At discharge, 99.1% of the patients in the TRA group and 93.1% in the TFA group were on dual antiplatelet therapy (P=.001), 0.5% and 6.5%, respectively, were receiving only antiplatelet monotherapy (P=.001), and 0.3% and 0.4%, respectively, were taking no antiplatelet drug (NS). A total of 36.7% of TRA patients and 33.5% of TFA patients were on OAC (0.5% and 5.9%, respectively, on OAC+clopidogrel [P=.001], and 33.1% and 23.8%, respectively, on triple therapy [P=.001]).
Immediately after the 30-day follow-up visit, 9.3% of patients in the TRA group and 9.8% in the TFA group were on dual antiplatelet therapy, 88.9% and 86.5%, respectively, were receiving antiplatelet monotherapy, and 1.8% and 3.8%, respectively were taking no antiplatelet drug; 37.2% in the TRA group and 33.1% in the TFA group were taking OAC (2.1% and 1.3%, respectively, were on triple therapy, and 7.3% and 11.6%, respectively, were on OAC+clopidogrel [P=.001]).
At 24 months, 5.8% of the TRA group and 7.5% in the TFA group were receiving dual antiplatelet therapy (P=.11); 81.2% and 72.7%, respectively, were on antiplatelet monotherapy (P=.001), and 13.1% and 19.8%, respectively, were taking no antiplatelet drug (P=.001). A total of 39.2% in the TRA group and 35.7% in the TFA group were on OAC (P=.11).
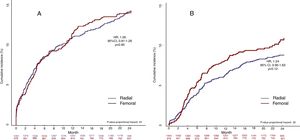
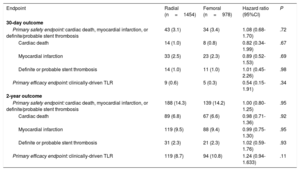
Primary endpointsAt 30 days, the primary safety endpoint (composite of cardiac death, myocardial infarction, or definite or probable stent thrombosis) had occurred in 43 patients (3.2%) in the TRA group and in 34 patients (3.4%) in the TFA group (hazard ratio [HR], 1.08; 95%CI, 0.68-1.70; P=.72) (table 3). The primary efficacy endpoint (clinically-driven target lesion revascularization) had occurred in 9 patients (0.7%) in the TRA and 5 patients (0.4%) in the TFA group (HR, 0.54; 95%CI, 0.15-1.91; P=.34) (table 3). The time-to-event curves for the primary safety and efficacy endpoints at 30 days are shown in figure 2.
Incidence of safety and efficacy endpoints at 30 days and 2 years
| Endpoint | Radial (n=1454) | Femoral (n=978) | Hazard ratio (95%CI) | P |
|---|---|---|---|---|
| 30-day outcome | ||||
| Primary safety endpoint: cardiac death, myocardial infarction, or definite/probable stent thrombosis | 43 (3.1) | 34 (3.4) | 1.08 (0.68-1.70) | .72 |
| Cardiac death | 14 (1.0) | 8 (0.8) | 0.82 (0.34-1.99) | .67 |
| Myocardial infarction | 33 (2.5) | 23 (2.3) | 0.89 (0.52-1.53) | .69 |
| Definite or probable stent thrombosis | 14 (1.0) | 11 (1.0) | 1.01 (0.45-2.26) | .98 |
| Primary efficacy endpoint: clinically-driven TLR | 9 (0.6) | 5 (0.3) | 0.54 (0.15-1.91) | .34 |
| 2-year outcome | ||||
| Primary safety endpoint: cardiac death, myocardial infarction, or definite/probable stent thrombosis | 188 (14.3) | 139 (14.2) | 1.00 (0.80-1.25) | .95 |
| Cardiac death | 89 (6.8) | 67 (6.6) | 0.98 (0.71-1.36) | .92 |
| Myocardial infarction | 119 (9.5) | 88 (9.4) | 0.99 (0.75-1.30) | .95 |
| Definite or probable stent thrombosis | 31 (2.3) | 21 (2.3) | 1.02 (0.59-1.76) | .93 |
| Primary efficacy endpoint: clinically-driven TLR | 119 (8.7) | 94 (10.8) | 1.24 (0.94-1.633) | .11 |
95%CI, 95% confidence interval; TLR, target lesion revascularization.
Unless otherwise specified, data are presented as No. (%).
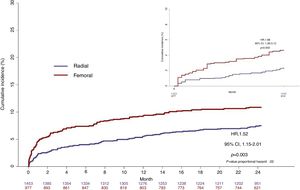
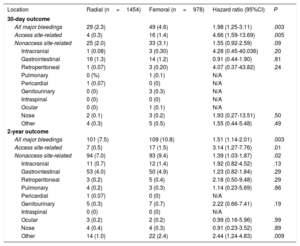
At 30 days, a major bleeding (BARC 3-5) had occurred in 78 patients: 29 (2.4%) in the TRA group and 49 (4.6%) in the TFA group (HR, 1.98; 95%CI, 1.25–3.11; P=.003). Among these, access site-related bleeding complications accounted for 20 (26%) patients, 4 in the TRA and 16 in the TFA (HR, 4.66; 95%CI, 1.59-13.69; P=.005) (figure 3). The remaining 58 (74%) major bleeding events were nonaccess site-related bleedings: 25 (2.1%) in the TRA group and 33 (3.2%) in the TFA group), and were mostly gastrointestinal bleedings in both groups (figure 3, figure 4, and table 4).
Incidence and location of major bleeding events (BARC 3-5) at 30 days and 2 years
| Location | Radial (n=1454) | Femoral (n=978) | Hazard ratio (95%CI) | P |
|---|---|---|---|---|
| 30-day outcome | ||||
| All major bleedings | 29 (2.3) | 49 (4.6) | 1.98 (1.25-3.11) | .003 |
| Access site-related | 4 (0.3) | 16 (1.4) | 4.66 (1.59-13.69) | .005 |
| Nonaccess site-related | 25 (2.0) | 33 (3.1) | 1.55 (0.92-2.59) | .09 |
| Intracranial | 1 (0.08) | 3 (0.30) | 4.28 (0.45-40.036) | .20 |
| Gastrointestinal | 16 (1.3) | 14 (1.2) | 0.91 (0.44-1.90) | .81 |
| Retroperitoneal | 1 (0.07) | 3 (0.20) | 4.07 (0.37-43.82) | .24 |
| Pulmonary | 0 (%) | 1 (0.1) | N/A | |
| Pericardial | 1 (0.07) | 0 (0) | N/A | |
| Genitourinary | 0 (0) | 3 (0.3) | N/A | |
| Intraspinal | 0 (0) | 0 (0) | N/A | |
| Ocular | 0 (0) | 1 (0.1) | N/A | |
| Nose | 2 (0.1) | 3 (0.2) | 1.93 (0.27-13.51) | .50 |
| Other | 4 (0.3) | 5 (0.5) | 1.55 (0.44-5.48) | .49 |
| 2-year outcome | ||||
| All major bleedings | 101 (7.5) | 109 (10.8) | 1.51 (1.14-2.01) | .003 |
| Access site-related | 7 (0.5) | 17 (1.5) | 3.14 (1.27-7.76) | .01 |
| Nonaccess site-related | 94 (7.0) | 93 (9.4) | 1.39 (1.03-1.87) | .02 |
| Intracranial | 11 (0.7) | 12 (1.4) | 1.92 (0.82-4.52) | .13 |
| Gastrointestinal | 53 (4.0) | 50 (4.9) | 1.23 (0.82-1.84) | .29 |
| Retroperitoneal | 3 (0.2) | 5 (0.4) | 2.18 (0.50-9.48) | .29 |
| Pulmonary | 4 (0.2) | 3 (0.3) | 1.14 (0.23-5.69) | .86 |
| Pericardial | 1 (0.07) | 0 (0) | N/A | |
| Genitourinary | 5 (0.3) | 7 (0.7) | 2.22 (0.66-7.41) | .19 |
| Intraspinal | 0 (0) | 0 (0) | N/A | |
| Ocular | 3 (0.2) | 2 (0.2) | 0.99 (0.16-5.96) | .99 |
| Nose | 4 (0.4) | 4 (0.3) | 0.91 (0.23-3.52) | .89 |
| Other | 14 (1.0) | 22 (2.4) | 2.44 (1.24-4.83) | .009 |
95%CI, 95% confidence interval; N/A, not applicable
Data are presented as No. (%) or mean±standard deviation.
At 2 years, the primary safety endpoint had occurred in 327 (14.3%) patients: 188 (14.3%) in the TRA and 139 (14.3%) in the TFA (HR, 1.00; 95%CI, 0.80-1.25; P=.95) (table 3). The primary efficacy endpoint had occurred in 213 (9.6%) patients: 119 (8.8%) in the TRA group and 94 (10.9%) in the TFA group (HR, 1.24; 95%CI, 0.94-1.63; P=.11). The time-to-event curves for the primary safety and efficacy endpoints at 2 years are shown in figure 2.
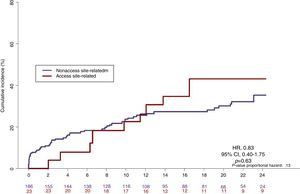
At 2 years, a major bleeding had occurred in 101 patients (7.5%) in the TRA group and 109 patients (10.9%) in the TFA group (HR, 1.51; 95%CI, 1.14-2.01; P=.003). Among these, access site-related bleeding complications accounted for 24 (11%) patients, 7 in the TRA group and 17 in the TFA group (HR, 3.14; 95%CI, 1.27-7.76; P=.01). The remaining 187 (89%) major bleeding events were nonaccess site-related bleedings (94 [7.0%] for the TRA group and 93 [9.4%] for the TFA group; P=.02), and more than half were gastrointestinal bleedings in both groups (figure 3, figure 4, table 4). The associated mortality rate during the 2-year follow-up was similar for access- and nonaccess-related major bleeding events (figure 5). The results of the frailty model are provided in the () and were in line with the standard analysis.
Study device analysesWhen analyzing whether vascular access impacted the previously described benefit of drug-coated stents over bare-metal stents, the P value for interaction was nonsignificant for both safety (P=.51) and efficacy (P=.39). The primary efficacy endpoint was maintained over the 2-year follow-up in both the TRA group (HR, 0.49; 95%CI, 0.33-0.72; P <.001) and the TFA group (HR, 0.63; 95%CI, 0.41-0.95; P=.03). The primary safety endpoint was maintained over the 2-year follow-up in the TRA group (HR, 0.75; 95%CI, 0.56-0.99; P=.046), but with only a nonsignificant trend in the smaller TFA group (HR, 0.87; 95%CI, 0.62-1.23; P=.43).
DISCUSSIONThese data are the first to report on the impact of vascular access site on outcomes for HBR patients undergoing PCI. Two main findings emerged: a) the use of TRA was associated with a significantly reduced occurrence of major bleeding complications compared with TFA at 30 days and 2 years of follow-up, and was driven by a decrease in both access and nonaccess site bleeding; b) the safety and efficacy benefit of drug-coated stents over bare metal stents that have been previously reported11,12 are independent of vascular acces site, and are of similar magnitude following either radial or femoral access PCI.
The European Society of Cardiology guidelines for the management of ACS patients presenting with non–ST-segment elevation myocardial infarction recommends the use of TRA with a class I, level of evidence A, when it can be provided by experienced operators.13 Guidelines for the management of ST-segment elevation myocardial infarction patients recommend the use of TRA over TFA with a class IIa level of evidence B.14 In our study, including patients with ACS and stable coronary artery disease between 2012 and 2014, the use of TRA was 60%. This was perhaps less than could have been expected for an HBR population and reflects major variations between participating operators and centers: some centers used ≥ 80% TRA and other ≤ 20%. Patients treated with TFA tended to have more severe comorbidities, and while this may represent a clinically justified decision in some cases (contralateral internal mammary grafts are generally easier to cannulate using TFA, and arteriovenous shunts in the forearm of dialysis patients may contraindicate TRA), it could also represent a selection process by those less experienced operators who mostly use TRA for less comorbid patients and with favorable anatomy.
While access site-related bleeding is considered to comprise about half of all periprocedural bleeds in all-comer patients, the reported incidence of periprocedural bleeding complications varies greatly between trials, ranging from 1% to 12%, and is associated with a marked increase in 30-day mortality for ACS patients.15,16 The variation among trials is a function of the studied population as well as the definition used to classify bleeds.17,18 In our study, use of the radial route was associated with a significant decrease in the incidence of access site bleeding at 30 days and this benefit was maintained over the 2-year follow-up for HBR patients (figure 4A).
In a pooled patient-level analysis of 7 RCT,7 1-year mortality in patients with access site-related bleeding was significantly higher compared with patients without periprocedural bleeds (4.5% and 2.5%, respectively; OR, 2.03; 95%CI, 1.49-2.77). The mortality benefit associated with TRA vs TFA for ACS patients has also been confirmed by another meta-analysis19 and several mechanisms have been suggested to explain the association between bleeding events and mortality from ischemic causes; these include anemia, discontinuation of dual antiplatelet therapy, a prothrombotic state induced by bleeding, and the effects of blood transfusions.20 The fact that we observed no benefit of TRA vs TFA in terms of cardiac mortality, MI or ST in our trial, both at 30 days and at 2 years, may reflect several factors: only a minority of patients presented with ACS in LEADERS FREE, HBR patients may behave differently than all-comers, and the protocol-driven short dual antiplatelet therapy course for all patients could be expected to minimize the need for unplanned antiplatelet therapy adjustements beyond 30 days.
Importantly, HBR patients had an incidence of both access and nonaccess site bleeding events that was markedly higher than that observed for all-comer patients. At 30 days, we observed an overall major bleeding rate of 2.4% for the TRA group and 4.6% for the TFA group. In the RIVAL trial,1 which enrolled only ACS patients, non–CABG-related major bleeds at 30 days occurred in 0.7% of patients in the TRA group compared with 0.9% of patients in the TFA group. In the MATRIX trial,4 the overall incidence of major BARC 3 or 5 bleeding at 30 days was 1.6% in the radial group vs 2.3% in the femoral group. When considering long-term events in the present trial, 8.9% of patients had had a major bleeding event at 2 years, 7.5% in the TRA group and 10.9% in the TFA group. In the PARIS registry,21 which analyzed 4190 patients discharged after successful PCI with a guideline-based dual antiplatelet regimen, out-of-hospital major BARC 3 or 5 bleeding occurred in 3.3% over 2 years, but the choice of vascular access was not reported.
Nonaccess site-related major bleeding in our series accounted for more than three-quarters of all major bleeds in the TRA group and for more than two-thirds in the TFA group. This is more than the reported incidence in several other studies4,22 and clearly relates to the advanced age, high rate of comorbid conditions and need for OAC that define the HBR population studied in the LEADERS FREE trial. In a pooled analysis of 3 randomized clinical trials including more than 17 000 PCI patients,15 nonaccess site-related bleeding also represented the majority of all major and minor TIMI bleeding events, with a 4-fold increase in 1-year mortality, and the hazard ratio for 1-year mortality of a nonaccess site-related bleeding was approximately twice as high as that of access site bleed. The latter finding differs from the present series, in which the associated mortality rate during the 2-year follow-up was high and was similar for access- and nonaccess- related major bleeding events (HR, 0.83; 95%CI, 0.40-1.75, P=.63) (figure 5). This apparent discrepancy may be explained both by the definitions used for bleeding (TIMI vs BARC 3-5) and by the patient populations that were studied (all-comers vs HBR).
In our series, the location of major bleeding events was similar to previous reports of all-comer patients,15,23 with gastrointestinal, urinary, and intracranial events representing most nonaccess site-related major bleeding. The fact that nonaccess bleeding was significantly less frequent with TRA than TFA (table 4) most probably reflects the baseline inequalities between the 2 groups of patients rather than any specific benefit of TRA in this respect. Overall, the incidence of access-related bleeding between 30 days and 2 years was low in both groups, and all were associated with repeated invasive coronary, vascular or valvular procedures. Early studies reported an increased incidence of cerebrovascular embolization with TRA compared with TFA1. No impact of vascular access site was found in our population with respect to rates of stroke following PCI, and this is in line with a recently published meta-analysis.24
LimitationsThe comparison of outcomes following PCI with either a TRA or a TFA is limited by the fact that choice of access site was not randomized but operator-defined. In addition, the experience of each center/operator in both vascular access routes was not previously assessed and may have had an impact on the results. We attempted to correct for several baseline inequalites using a propensity score model, but some differences may have remained unaccounted for. This is the most likely explanation for a lower nonaccess site bleeding rate in the TRA group that persisted for 4 months after PCI (figure 4).
Differences in antiplatelet therapy regimens are apparent between the TRA and TFA groups at different time points, and again reflect the nonrandomized nature of the comparison.Ssimilar to the choice of access site, these differences are probably mostly a consequence of individual center preferences in their interpretation of the guidelines. For instance, the same centers that showed a preference for TFA also more often used the WOEST regimen during the first 30 days or interrupted all antiplatelet therapy beyond 1 year for patients on long-term OAC.
As a predefined substudy, the present analysis was not powered to fully assess drug-coated stents vs bare-metal stents in the TRA and TFA groups separately, and therefore the results should be considered as hypothesis-generating only. Finally, we recorded only the final vascular access site that was used, and no information is therefore available on possible access site crossover.
CONCLUSIONSThe use of TRA compared with TFA, was associated with a significantly reduced rate of both access site and nonaccess site major bleeding over a 2-year follow-up period after PCI in HBR patients, with similar outcomes in terms of clinically-driven target lesion revascularization and a composite safety endpoint including cardiac death, myocardial infarction, and stent thrombosis. The safety and efficacy benefits of drug-coated stents over drug-coated stent were independent of vascular access route.
FUNDINGLEADERS FREE trial was funded by Biosensors Europe.
CONFLICTS OF INTERESTP. Urban is a paid consultant to Biosensors, D. Walters has received research funding from Biosensors and both S. Copt and H.-P. Stoll are employees of Biosensors. None of the other coauthors have any conflict of interest to declare.
- -
The adoption of the TRA site as the preferred vascular approach in patients undergoing PCI has been shown to reduce access site-related bleeding complications in an increasing number of populations and settings.
- -
However, there is a lack of compelling evidence on the prognostic impact of bleeding events in HBR patients depending on the location of bleeding and prognosis in nonaccess site bleeding at short- and mid-term follow-up.
- -
The use of TRA was associated with a significant reduction in adjusted rates of major bleeding both at 30 days and at 2 years of follow-up in the HBR population. This difference was driven by both access and nonaccess bleeding.
- -
The study demonstrated that the safety and benefits of drug-coated stents over bare-metal stents were independent of the vascular access route. Therefore, the benefit of TRA is even greater in the HBR population and its use should be prioritized.