Diagnosis of non–ST-segment elevation acute coronary syndromes (NSTEACS) is based on 3 cornerstones: clinical presentation, 12-lead electrocardiogram, and cardiac troponin measurement. Advances in the development of high-sensitivity cardiac troponin (hs-cTn) assays have substantially improved the detection of cardiomyocyte injury in a shorter time period, and hs-cTn has consequently been established as the gold-standard biomarker for the assessment of patients with suspected NSTEACS. The implementation of these assays in clinical practice allows a faster “rule-out”, especially among low-risk patients, as well as a safer and more rapid “rule-in”, with its therapeutic consequences. Current guidelines for the diagnosis of NSTEACS recommend the use of hs-cTn applied in rapid diagnostic algorithms based on serial hs-cTn sampling within the first few hours. The current work provides an overview of the use of hs-cTn for the early detection of NSTEACS.
Keywords
More than 15 million patients present to the emergency department (ED) with symptoms suggestive of myocardial infarction (MI) annually worldwide.1 Early diagnosis of MI is critical for the immediate initiation of highly effective and evidence-based therapy.
The term NSTEACS includes non–ST-segment elevation myocardial infarction (NSTEMI) and unstable angina. In patients with symptoms suggestive of acute coronary syndrome (ACS), an electrocardiogram should be performed within the first 10minutes. After exclusion of electrocardiographic signs of acute vessel obstruction (ie, ST-segment elevation MI), the use of cardiac troponin (cTn) will differentiate between NSTEMI and unstable angina.2,3
Therefore, the assessment of patients with acute chest pain is based on the following 3 pillars: patient history and physical examination, the 12-lead electrocardiogram, and cTn T and I.2,4
In MI, cTn T and I are released from necrotic myocardium both as intact proteins and degradation products and hence can be detected in blood.5 Cardiac troponins T and I are more sensitive and specific markers of cardiomyocyte injury than other markers such as creatine kinase, its myocardial band isoenzyme, and myoglobin.6 Advances in cTn assay technology have led to a refinement in the clinical ability to detect and quantify myocardial injury. These novel assays, called high-sensitivity cardiac troponin (hs-cTn) assays, can accurately detect cTn at lower levels than older-generation assays, giving them higher sensitivity for the detection of smaller MI and earlier identification of MI, which means that the time interval to the second measurement of hs-cTn can be significantly shortened, thereby reducing the time to diagnosis and improving efficiency in the ED. For these reasons, in current guidelines, serial hs-cTn measurements have become the biomarker of choice in the assessment of patients with acute chest pain.2,7
ALGORITHMS USING HIGH-SENSITIVITY CARDIAC TROPONINThe most important clinical advantage of the new, more sensitive cTn assays is their ability to substantially reduce the initial “troponin-blind” interval in the first few hours after MI onset. This has enabled the creation and extensive validation of early hs-cTn-based diagnostic algorithms, which rely on serial hs-cTn testing. Some of these algorithms are the current recommendation in the European Society of Cardiology (ESC) guidelines for the assessment of patients with suspected ACS and therefore deserve in-depth discussion.
ESC 0h/3h hs-cTn algorithmsThe ESC introduced the 0h/3h ESC algorithm for the first time in 2011.8 With this strategy, MI can be ruled out with a negative predictive value> 98% if hs-cTn concentrations remain within the normal range (below the respective 99th percentile) in the blood samples drawn at presentation and 3hours after presentation, and if the patient fulfils 2 additional requirements: being pain-free and at low-risk of in-hospital mortality as quantified by a Global Registry of Acute Coronary Events (GRACE) score below 140.2,7 The current ESC guidelines recommend this strategy only as an alternative to the ESC 0h/1h and 0h/2h hs-cTn algorithms, and it therefore has a class IIa recommendation.2
ESC 0h/1h hs-cTn algorithmsThe concept of these strategies is based on serial hs-cTn measurement at presentation (0h) and after 1h. The creation of these algorithms aimed to achieve predefined very high safety (quantified by the negative predictive value and sensitivity for NSTEMI) and efficacy (percentage of patients triaged early) for rule-out, as well as to improve the positive predictive value and specificity for NSTEMI.1,2
The 0h/1h strategy exclusively uses hs-cTn data to triage patients without the use of a specific clinical risk score and thereby achieves a comparable negative predictive value and sensitivity for rule-out by also taking into account absolute concentration changes within 1 hour. The lack of a relevant absolute change from presentation to 1 hour, combined with the fact that both concentrations need to be normal, obviates the need for a predefined score to safely rule-out MI.
Accordingly, the use of these strategies is highly effective and allows an accurate early triage in approximately 75% of patients: 60% toward rule-out and 15% toward rule-in of MI.1
ESC 0h/2h hs-cTn ALGORITHMThe concept of the ESC 0h/2h algorithm is identical to that of the ESC 0h/1h algorithm, in this case the second blood draw is be performed after 2hours (instead of 1 hour) and different cutoff values apply. This algorithm is recommended as an alternative strategy to the ESC 0h/1h algorithm and also has a class I recommendation.2
ESC 0h/1h hs-cTn ALGORITHM FOR SUSPECTED MYOCARDIAL INFARCTIONInitially, 4 studies showed that the safety and timing of the 0h/3h algorithm could be improved with a 0h/1h hs-cTn algorithm.9,10 The safety and efficacy of these novel 0h/1h hs-cTn algorithms were verified in 3 further implementation studies (one of them randomized).4,10,11 Hence, the ESC 0h/1h hs-cTn algorithm is the preferred strategy recommended in the ESC guidelines (class I recommendation, level of evidence A).2
The concept of the ESC 0h/1h algorithm is based on 2 points:
- -
hs-cTn are released from necrotic myocardium due to the interruption of oxygen supply. Therefore, the higher the hs-cTn concentrations in blood, the higher the probability of an MI.
- -
Absolute changes in hs-cTn concentrations within 1 hour can be used as the surrogate for absolute changes within 3 and 6hours. 12–14 For example, an absolute change of 6 ng/L within 3hours suggests a change of 4 ng/L after 2hours or 2 ng/L after 1 hour. The higher the dynamic changes after 1 hour (an increase or decrease in concentrations), the higher the probability of an MI.
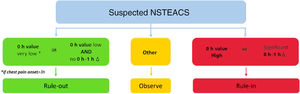
According to the hs-cTn concentrations at presentation, as well as the dynamic within 1 hour, patients can be triaged in 3 different groups: rule-out, rule-in, and observe zone (figure 1). The proposed cutoff values (figure 1 and table 1) are assay-specific and were derived and validated in large studies.12,13,15–36
Central illustration. 0h/1h rule-out and rule-in algorithm using hs-cTn assays in hemodynamically stable patients presenting with non–ST-segment elevation acute coronary syndrome (NSTEACS) to the emergency department. Non–ST-segment elevation myocardial infarction (NSTEMI) can be ruled out at presentation if the high-sensitivity cardiac troponin (hs-cTn) concentration is very low. NSTEMI can also be ruled out by the combination of low baseline levels and the lack of a relevant increase within 1 hour. Patients have a high likelihood of NSTEMI if the hs-cTn concentration at presentation is at least moderately elevated or hs-cTn concentrations show a clear rise within the first hour. Cutoffs are assay specific (see table 1) and derived to meet predefined criteria for sensitivity and specificity for NSTEMI. Adapted with permission from Collet et al.2.
Assay-specific cutoff levels in ng/L within the 0h/1h and 0h/2h algorithm
| 0 h/1 h algorithm | Very low | Low | No 1 h Δ | High | Very high |
|---|---|---|---|---|---|
| hs-cTn T (Elecsys; Roche)13 | <5 | <12 | <3 | ≥ 52 | ≥ 5 |
| hs-cTn I (Architect; Abbott)12 | <4 | <5 | <2 | ≥ 64 | ≥ 6 |
| hs-cTn I (Centaur; Siemens)18 | <3 | <6 | <3 | ≥ 120 | ≥ 12 |
| hs-cTn I (Access; Beckman Coulter)15 | <4 | <5 | <4 | ≥ 50 | ≥ 15 |
| hs-cTn I (Clarity; Singulex)19 | <1 | <2 | <1 | ≥ 30 | ≥ 6 |
| hs-cTn I (Vitros; Clinical Diagnostic)29 | <1 | <2 | <1 | ≥ 40 | ≥ 4 |
| hs-cTn I (Pathfast; LSI Medicine)34 | <3 | <4 | <3 | ≥ 90 | ≥ 20 |
| hs-cTn I (Triage True; Quidel)36 | <4 | <5 | <3 | ≥ 60 | ≥ 8 |
| 0 h/2 h algorithm | Very low | Low | No 2 h Δ | High | Very high |
| hs-cTn T (Elecsys; Roche)35 | <5 | <14 | <4 | ≥ 52 | ≥ 10 |
| hs-cTn I (Architect; Abbott)17 | <4 | <6 | <2 | ≥ 64 | ≥ 15 |
| hs-cTn I (Centaur; Siemens)18 | <3 | <8 | <7 | ≥ 120 | ≥ 20 |
| hs-cTn I (Access; Beckman Coulter)30 | <4 | <5 | <5 | ≥ 50 | ≥ 20 |
| hs-cTn I (Clarity; Sing ulex)19 | <1 | TBD | TBD | ≥ 30 | TBD |
| hs-cTn I (Vitros; Clinical Diagnostic)29 | <1 | TBD | TBD | ≥ 40 | TBD |
| hs-cTn I (Pathfast; LSI Medicine)34 | <3 | TBD | TBD | ≥ 90 | TBD |
| hs-cTn I (Triage True; Quidel)36 | <4 | TBD | TBD | ≥ 60 | TBD |
hs-cTn, high-sensitivity cardiac troponin; TBD, to be determined.
The advantage of these algorithms lies in the possibility of excluding an MI within the first few hours after symptom onset with very high safety.
Rule-out zoneIf chest pain onset is> 3hours from presentation to the ED and hs-cTn values at presentation are very low (undetectable levels), a diagnosis of MI can already be excluded with a single blood sample. Similarly, an MI can also be excluded if the hs-cTn levels at presentation are low and there is no relevant dynamic within the first hour (figure 1). The negative predictive value of this pathway was> 99% in several validation studies,4,10,11 without taking into consideration clinical judgement or electrocardiogram changes.
In the rule-out zone, after exclusion of other life-threatening diagnoses (such as pneumothorax, unstable angina aortic dissection), the patient may be a candidate for early discharge and outpatient management. Even after MI rule-out, further investigations may be necessary (cardiac coronary computed tomography, or noninvasive stress testing for low-to-intermediate risk patients and invasive coronary angiography for very high clinical likelihood of unstable angina). All these assessments can be scheduled for the ambulatory setting, depending on clinical judgement and the patient's risk profile.2
Rule-in zoneIn contrast, patients with very high concentrations of hs-cTn at presentation or a relevant dynamic within the first hour, can be classified in the rule-in zone.
The positive predictive value for this zone was between 70%-75% in the studies. However, most patients triaged toward the rule-in group without having an MI had other life-threatening conditions such as tako-tsubo syndrome and myocarditis and were therefore candidates for early invasive coronary angiography and admission to a coronary care unit.2
Observe zonePatients not meeting the rule-in or rule-out criteria are assigned to the observe zone. This is a widely heterogeneous group of patients which has been shown to have high mortality (comparable to that of rule-in patients). An individual assessment based on the patient's particular risk profile (including risk scores, for example) is of paramount importance. Additionally, a third measurement of cardiac troponin at 3hours and echocardiography as the next steps are usually required.37,38
Recently, specific cutoffs for patients assigned to the observe zone using the hs-cTn T assay (a combination of a 3-hour hs-cTnT concentration [< 15 ng/L] and a 0h/3h absolute change [< 4 ng/L]) have been derived and validated with acceptable safety and efficacy for further decision-making.38 Specific cutoffs for other hs-cTn I assays in the observe zone are currently missing.
Invasive coronary angiography should be considered in patients with a high degree of clinical suspicion of NSTEACS (eg, relevant increase in cardiac troponin from presentation to 3 hours), while in patients with a low-to-intermediate likelihood for this condition according to clinical judgement, noninvasive imaging with coronary computed tomography angiography or, when not readily available, stress testing should be considered after the patient has been discharged from the ED to the ward. No further diagnostic testing is indicated when alternative conditions have been identified, such as rapid ventricular rate response to atrial fibrillation, profound anemia, or a hypertensive emergency.2
CLINICAL USE OF THE ESC 0h/1h hs-cTn ALGORITHMAlthough these strategies simplify the triage of patients in the ED considerably, proper training of clinicians and nurses is of paramount importance for their successful implementation in practice. Several factors that should be highlighted when using one of these rapid diagnostic algorithms for NSTEMI:
Assay-specific algorithmsThe derived and validated decision points are assay-specific (figure 1 and table 1). They can only be used for the suggested assays for which the algorithms have been validated. Hence, the first step when using these strategies is to elucidate which assay is being used in the institution.1 Colleagues from the laboratory department can usually help us to find this information. If none of these assays is available, an alternative strategy must be considered.
Be aware of the turnaround timeThe time to decision is the result of the time of the blood draw together and the turnaround time. The use of the ESC 0h/1h algorithms is irrespective of the local turnaround-time (time from blood draw to blood results). Zero hour and 1 hour refer to the time point at which blood is taken. Eventually, the second blood draw needs to be obtained even before the results of the first are available (the results are usually obtained within 60minutes of blood sampling), but this does not affect the interpretation of the algorithms. In many institutions, the turnaround time often is longer than 1 hour. The application of the ESC 0h/1h algorithm is also possible in these institutions since 1 hour only refers to the time point of the second blood sample. To maximize efficacy, the nursing team needs to be trained to obtain the 1 hour blood draw in all patients independently of the reporting of the 0 hour result.
Documentation of the time of the 0 hour blood draw allows exact determination of the time window (± 10 minutes) of the 1 hour blood draw. If, for whatever reason, the 1 h (± 10 minutes) blood draw was not feasible, then blood should be drawn at 2hours and the ESC 0h/2h algorithm applied (if validated for the assay in use).
Triage algorithmsThese algorithms are triage algorithms and are not discharge/admit algorithms. Even if an MI has been excluded, the diagnosis still needs to be worked out. In addition, patients with a clear pattern of crescendo or unstable angina should undergo further investigation.
Additionally, the algorithms should be used only in conjunction with full clinical assessment, including detailed assessment of chest pain characteristics and electrocardiogram, and should be applied only following exclusion of ST-segment elevation MI or other life-threatening conditions (ie, aortic dissection).
Similarly, the rapid algorithms using hs-cTn should be used only in patients presenting to the ED with suspected acute MI and should not be applied in an unselected ED population (ie, patients with stroke or sepsis).2,3,39
FUNDINGThe current work received no funding.
AUTHORS’ CONTRIBUTIONSAll authors drafted and reviewed the manuscript.
CONFLICTS OF INTERESTM. Rubini Gimenez reports research grants from the Swiss Heart Foundation and Swiss National Foundation (P400PM_180828) and speaker honoraria from Roche, Ortho Clinical Diagnostics, Quidel, and Siemens. J. Boeddinghaus has received research grants from the University of Basel and the Division of Internal Medicine, the Swiss Academy of Medical Sciences, the Gottfried and Julia Bangerter-Rhyner-Foundation, and speaker honoraria from Siemens, Roche Diagnostics, and Ortho Clinical Diagnostics, outside the submitted work. T. Nestelberger has received research support from the Swiss National Science Foundation (P400PM_191037/1), the Swiss Heart Foundation (FF20079), the Prof. Dr Max Cloëtta Foundation, the Margarete und Walter Lichtenstein-Stiftung (3MS1038), the University of Basel and Basel University Hospital as well as speaker honoraria/consulting honoraria from Siemens, Beckman Coulter, Bayer, Ortho Clinical Diagnostics and Orion Pharma, all outside the submitted work. l. Koechlin received a research grant from the Swiss Heart Foundation, the University of Basel, the Swiss Academy of Medical Sciences and the Gottfried and Julia Bangerter-Rhyner Foundation, as well as the “Freiwillige Akademische Gesellschaft Basel” and speaker honoraria from Roche Diagnostics, Siemens, and Abbott outside the submitted work. P. López-Ayala has received research grants from the Swiss Heart Foundation (FF20079 and FF21103) and speaker honoraria from Quidel, paid to the institution, outside the submitted work. C. Mueller has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the European Union, the University of Basel, Basel University Hospital, Abbott, Beckman Coulter, Biomerieux, Idorsia, Ortho Cinical Diagnostics, Quidel, Roche, Siemens, Singulex, and Sphingotec, as well as speaker honoraria/consulting honoraria from Acon, Amgen, Astra Zeneca, Boehringer Ingelheim, Bayer, BMS, Idorsia, Novartis, Osler, Roche, and Sanofi, outside of the submitted work.
.