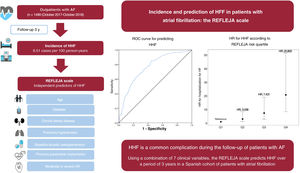
Hospitalization for heart failure (HHF) is common in patients with atrial fibrillation (AF) and is associated with increased mortality. The aims of this study were to determine the incidence of HHF, identify the clinical predictors of its occurrence, and develop a new risk scale.
MethodsThe incidence of HHF was estimated using data from the prospective single-center REFLEJA registry of outpatients with AF (October 2017-October 2018). A multivariate Cox regression model was calculated to detect HHF predictors, and a nomogram was created for individual risk assessment.
ResultsOf the 1499 patients included (mean age 73.8±11.1 years, 48.1% women), 127 had HHF (incidence rate of 8.51 per 100 persons/y) and 319 died (rate of death from any cause of 21.1 per 100 persons/y) after a 3-year follow-up. The independent predictors of HHF were age, diabetes, chronic kidney disease, pulmonary hypertension, previous pacemaker implantation, baseline use of diuretics, and moderate-severe aortic regurgitation. The c-statistic for predicting the event was 0.762 (95%CI after boostrapping resampling, 0.753-0.791). The cumulative incidences of the main outcome for the risk scale quartiles were 1.613 (Q1), 3.815 (Q2), 8.378 (Q3), and 20.436 (Q4) cases per 100 persons/y (P <.001).
ConclusionsHHF was common in this AF cohort. The combination of certain clinical characteristics can identify patients with a very high risk of HHF.
Keywords
In the context of increasing life expectancy and a general reduction in cardiovascular mortality, both the incidence and prevalence of atrial fibrillation (AF) and heart failure (HF) continue to grow at alarming rates.1,2 AF and HF often coexist because they are pathophysiologically interconnected and share multiple cardiovascular risk factors.2 Patients with AF have a 3-fold increased risk of developing HF,3 and the coexistence of these conditions is associated with a worse prognosis, characterized by an increased risk of hospitalization and death.4,5
Although some of the risk factors for HF in patients with AF are known, there is a need to elucidate the frequency and extent to which each of these factors contributes to the onset of HF.6 In addition, not all patients with AF develop symptomatic HF.4 Individualized risk stratification tools would allow tailored screening strategies, potentially delaying the onset of HF through early prevention and treatment.7,8 Although a number of scales for predicting the risk of HF exist, some of these were developed years ago,7 while others were not specifically developed for AF6,9 or Spain.8,10 Furthermore, some of the models did not include patients from real-world registries or nonpublic health care systems, or involve input from specialists other than cardiologists.
Although recent trends in the prevalence and incidence of HF in Spain have been reported,11,12 the specific incidence of HF in patients with AF is unknown.
The main aims of this study were to estimate the incidence of a first hospitalization for heart failure (HHF) in patients with AF, examine associated risk factors, and develop a new risk scale.
METHODSStudy populationThe study population comprised patients from the prospective observational single-center REFLEJA registry of consecutive patients with AF evaluated by the cardiology department at Hospital Universitario de Jaén between October 2017 and October 2018. The design and characteristics of this registry are described elsewhere.13 We included all patients older than 18 years who provided signed informed consent. The only exclusion criterion was a diagnosis of atrial flutter. The study was approved by the Provincial Research Ethics Committee of Jaén (figure 1 of the supplementary data).
Study characteristicsBaseline clinical characteristics, blood work results, and electrocardiogram and echocardiographic findings were recorded at the first visit. Mean time from the most recent echocardiogram to patient inclusion was 3.4 months. Obesity was defined as a body mass index ≥30kg/m2 and anemia as a hemoglobin level <13g/dL in men and <12g/dL in women. Patients were considered to have chronic kidney disease (CKD) when they had a glomerular filtration rate <60mL/min according to the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula. The above variables were dichotomized for practicality and simplicity. We did, however, conduct a sensitivity analysis in which the quantitative variables extracted from the data were treated as continuous (table 1 of the supplementary data).
Heart rate and QRS morphology were evaluated in the baseline electrocardiogram. Left ventricular hypertrophy was defined as an interventricular septum or posterior left ventricular wall thickness ≥12mm. Patients were considered to have a high echocardiographic probability of pulmonary hypertension (PHT) when the peak tricuspid regurgitation velocity was > 3.4m/s or >2.9m/s in the presence of dilation of the right heart chambers or other indirect echocardiographic signs of PHT. In all other cases, patients were considered to have a low probability of PHT.14 Moderate or severe aortic and mitral regurgitation were defined using the echocardiographic criteria recommended by the European Society of Cardiology.15 Because tricuspid regurgitation velocity was not reported for all patients, we conducted a sensitivity analysis excluding PHT (table 2 of the supplementary data).
Events and follow-upThree-year incidence rates for HHF were calculated up to October 2021. All events were recorded by the hospital's cardiologists using information extracted from electronic medical records or obtained during visits or telephone calls. HHF was defined as hospitalization involving at least 1 overnight stay for a patient with signs or symptoms of HF due to a structural and/or functional cardiac abnormality (eg, ejection fraction [EF] <50%, abnormal chamber volumes, left ventricular hypertrophy), elevated natriuretic peptide levels, or evidence of pulmonary or systemic congestion of cardiac origin.16 Secondary outcomes were 3-year incidence rates for all-cause and cardiovascular mortality, hospitalization for AF (defined as the need for admission to control symptomatic AF), and the incidence of stroke or transient ischemic attack.
Statistical analysisBaseline characteristics were stratified according to the occurrence or nonoccurrence of HHF during follow-up.
Incidence rates were calculated as the number of events per 100 person-years. Individual Cox proportional hazards models were constructed for each independent variable to estimate the risk factors for HHF in patients with AF. Only patients with complete records were included (ie, missing data were not imputed). A multivariate model was built by selecting variables from the individual models deemed to be the most plausible from a clinical, statistical, and biological perspective. The models were chosen using the Akaike information criterion. A Fine and Gray competing risk analysis (table 3 of the supplementary data) was conducted in addition to the Cox regression analysis.
The resulting model satisfied the assumption of proportional hazards and was internally validated through bootstrap resampling (R Core Team, Austria) with 10 000 iterations and calculation of the optimism-adjusted c-statistic. The Gronnesby and Borgan test was used to calibrate the scale.
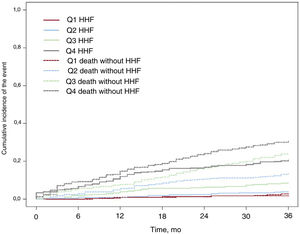
To estimate the risk of a patient with AF being hospitalized for HF for the first time, we developed a nomogram incorporating a risk scale for the occurrence of HHF at 12, 24, and 36 months. The patients were divided into risk categories based on quartiles and an individual risk calculator was created. Cumulative incidence curves for HHF were plotted for the entire cohort, with death in the absence of HHF used as the competing event. The curves were stratified by risk quartiles and independent predictors of HHF.
Statistical significance was set at a P value < .05 for all analyses. Statistical analyses were conducted in IBM SPSS (version 21; IBM Corp., USA) and R (version 4.2.1.; R Core Team, Austria).
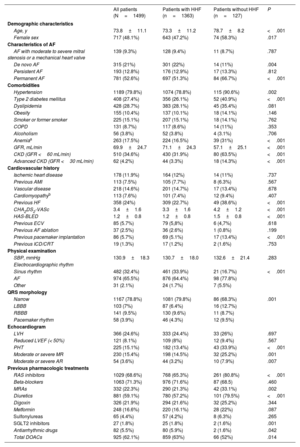
RESULTSPatient characteristicsThe baseline characteristics of the study population are summarized in table 1. We studied 1499 patients (48.1% women) with a mean age of 73.8±11.1 years; 52.6% had permanent AF. The respective prevalence of hypertension, diabetes, and dyslipidemia was 79.8%, 27.4%, and 28.7%. Patients hospitalized for HF for the first time during follow-up were older, more often women, and had a higher prevalence of diabetes, anemia, CKD, and a past history of HF.
Baseline characteristics of study population
| All patients (N=1499) | Patients with HHF (n=1363) | Patients without HHF (n=127) | P | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, y | 73.8±11.1 | 73.3±11.2 | 78.7±8.2 | <.001 |
| Female sex | 717 (48.1%) | 643 (47.2%) | 74 (58.3%) | .017 |
| Characteristics of AF | ||||
| AF with moderate to severe mitral stenosis or a mechanical heart valve | 139 (9.3%) | 128 (9.4%) | 11 (8.7%) | .787 |
| De novo AF | 315 (21%) | 301 (22%) | 14 (11%) | .004 |
| Persistent AF | 193 (12.8%) | 176 (12.9%) | 17 (13.3%) | .812 |
| Permanent AF | 781 (52.6%) | 697 (51.3%) | 84 (66.7%) | <.001 |
| Comorbidities | ||||
| Hypertension | 1189 (79.8%) | 1074 (78.8%) | 115 (90.6%) | .002 |
| Type 2 diabetes mellitus | 408 (27.4%) | 356 (26.1%) | 52 (40.9%) | <.001 |
| Dyslipidemia | 428 (28.7%) | 383 (28.1%) | 45 (35.4%) | .081 |
| Obesity | 155 (10.4%) | 137 (10.1%) | 18 (14.1%) | .146 |
| Smoker or former smoker | 225 (15.1%) | 207 (15.1%) | 18 (14.1%) | .762 |
| COPD | 131 (8.7%) | 117 (8.6%) | 14 (11%) | .353 |
| Alcoholism | 56 (3.8%) | 52 (3.8%) | 4 (3.1%) | .706 |
| Anemiaa | 263 (17.5%) | 224 (16.5%) | 39 (31%) | <.001 |
| GFR, mL/min | 69.9±24.7 | 71.1±24.3 | 57.1±25.1 | <.001 |
| CKD (GFR <60 mL/min) | 510 (34.6%) | 430 (31.9%) | 80 (63.5%) | <.001 |
| Advanced CKD (GFR <30 mL/min) | 62 (4.2%) | 44 (3.3%) | 18 (14.3%) | <.001 |
| Cardiovascular history | ||||
| Ischemic heart disease | 178 (11.9%) | 164 (12%) | 14 (11%) | .737 |
| Previous AMI | 113 (7.5%) | 105 (7.7%) | 8 (6.3%) | .567 |
| Vascular disease | 218 (14.6%) | 201 (14.7%) | 17 (13.4%) | .678 |
| Cardiomyopathyb | 113 (7.6%) | 101 (7.4%) | 12 (9.4%) | .407 |
| Previous HF | 358 (24%) | 309 (22.7%) | 49 (38.6%) | <.001 |
| CHA2DS2-VASc | 3.4±1.6 | 3.3±1.6 | 4.2±1.2 | <.001 |
| HAS-BLED | 1.2±0.8 | 1.2±0.8 | 1.5±0.8 | <.001 |
| Previous ECV | 85 (5.7%) | 79 (5,8%) | 6 (4,7%) | .618 |
| Previous AF ablation | 37 (2.5%) | 36 (2.6%) | 1 (0.8%) | .199 |
| Previous pacemaker implantation | 86 (5.7%) | 69 (5.1%) | 17 (13.4%) | <.001 |
| Previous ICD/CRT | 19 (1.3%) | 17 (1.2%) | 2 (1.6%) | .753 |
| Physical examination | ||||
| SBP, mmHg | 130.9±18.3 | 130.7±18.0 | 132.6±21.4 | .283 |
| Electrocardiographic rhythm | ||||
| Sinus rhythm | 482 (32.4%) | 461 (33.9%) | 21 (16.7%) | <.001 |
| AF | 974 (65.5%) | 876 (64.4%) | 98 (77.8%) | |
| Other | 31 (2.1%) | 24 (1.7%) | 7 (5.5%) | |
| QRS morphology | ||||
| Narrow | 1167 (78.8%) | 1081 (79.8%) | 86 (68.3%) | .001 |
| LBBB | 103 (7%) | 87 (6.4%) | 16 (12.7%) | |
| RBBB | 141 (9.5%) | 130 (9.6%) | 11 (8.7%) | |
| Pacemaker rhythm | 58 (3.9%) | 46 (4.3%) | 12 (9.5%) | |
| Echocardiogram | ||||
| LVH | 366 (24.6%) | 333 (24.4%) | 33 (26%) | .697 |
| Reduced LVEF (< 50%) | 121 (8.1%) | 109 (8%) | 12 (9.4%) | .567 |
| PHT | 225 (15.1%) | 182 (13.4%) | 43 (33.9%) | <.001 |
| Moderate or severe MR | 230 (15.4%) | 198 (14.5%) | 32 (25.2%) | .001 |
| Moderate or severe AR | 54 (3.6%) | 44 (3.2%) | 10 (7.9%) | .007 |
| Previous pharmacologic treatments | ||||
| RAS inhibitors | 1029 (68.6%) | 768 (65.3%) | 261 (80.8%) | <.001 |
| Beta-blockers | 1063 (71.3%) | 976 (71.6%) | 87 (68.5) | .460 |
| MRAs | 332 (22.3%) | 290 (21.3%) | 42 (33.1%) | .002 |
| Diuretics | 881 (59.1%) | 780 (57.2%) | 101 (79.5%) | <.001 |
| Digoxin | 326 (21.9%) | 294 (21.6%) | 32 (25.2%) | .344 |
| Metformin | 248 (16.6%) | 220 (16.1%) | 28 (22%) | .087 |
| Sulfonylureas | 65 (4.4%) | 57 (4.2%) | 8 (6.3%) | .265 |
| SGLT2 inhibitors | 27 (1.8%) | 25 (1.8%) | 2 (1.6%) | .001 |
| Antiarrhythmic drugs | 82 (5.5%) | 80 (5.9%) | 2 (1.6%) | .042 |
| Total DOACs | 925 (62.1%) | 859 (63%) | 66 (52%) | .014 |
ACE, angiotensin converting enzyme; AF, atrial fibrillation; AMI, acute myocardial infarction; AR, aortic regurgitation; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled], vascular disease, age 65 to 74 years, and sex category [female]; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; DOACs, direct-acting oral anticoagulants; ECV, electrical cardioversion; GFR, glomerular filtration rate; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly; HHF, hospitalization for heart failure; ICD, implantable cardioverter defibrillator; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonists; PHT, pulmonary hypertension; RAS, renin-angiotensin system; RBBB, right bundle branch block; SGLT-2, sodium-glucose cotransporter 2.
Values are expressed as No. (%) or mean±standard deviation.
At the end of the 3-year follow-up period, 23 patients (1.5%) had been lost to follow-up, 127 (8.5% of the total population) had been hospitalized for HF for the first time, and 319 had died. The incidence rate for the main event (HHF) was 8.51 events per 100 person-years. The respective rates for all-cause and cardiovascular mortality were 21.10 and 5.09 deaths per 100 person-years (table 2). The incidence curves for HHF during follow-up, with death in the absence of HHF as the competing event, are shown in figure 1. The 3-year incidence rate for hospitalization for AF and hospitalization for stroke or transient ischemic attack was 2.88 per 100 patient-years in both cases (table 2).
Incidence of events in the study population at 3 years
| Incidence of events per 100 person-years at 3 years | Cumulative incidence with death as the competing event | ||||||
|---|---|---|---|---|---|---|---|
| First year | Second year | Third year | 3-year period | First year | Second year | Third year | |
| Hospitalization for HF | 4.35 | 2.55 | 2.13 | 8.51 | 4.34 | 6.68 | 8.48 |
| Overall mortality | 8.24 | 7.45 | 7.10 | 21.10 | — | — | — |
| Cardiovascular mortality | 1.94 | 1.68 | 1.89 | 5.09 | — | — | — |
| Noncardiovascular mortality | 4.76 | 3.80 | 4.10 | 11.72 | — | — | — |
| Mortality of unknown cause | 1.54 | 1.97 | 1.10 | 4.29 | — | — | — |
| Hospitalization for AF | 1.00 | 1.82 | 0.24 | 2.88 | 1.00 | 2.75 | 2.85 |
| Stroke/TIA | 1.07 | 1.09 | 0.95 | 2.88 | 1.07 | 1.94 | 2.74 |
AF, atrial fibrillation; HF, heart failure; TIA, transient ischemic attack.
Incidence curves showing the probability of HHF and death in the absence of HHF during follow-up. A: entire cohort. B: stratified by age. C: stratified by presence/absence of diabetes. D: stratified by presence/absence of CKD. E: previous pacemaker implantation. F: PHT. G: moderate or severe aortic regurgitation. H: baseline diuretic use. AR, aortic regurgitation; CKD, chronic kidney disease; HF, heart failure; HHF, hospitalization for heart failure; PHT, pulmonary hypertension.
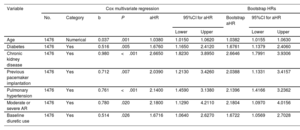
The variables significantly associated with HHF in the final multivariate analysis were age, diabetes, CKD, PHT, previous pacemaker implantation, baseline diuretic use, and moderate or severe aortic regurgitation. The cumulative incidence of HHF according to these independent predictors is shown in figure 1. The proposed multivariate model was statistically significant (P<.001) and had a c-statistic of 0.772 for the prediction of HHF at 3 years; the optimism-corrected c-statistic was 0.762. The CI calculated after bootstrap resampling with 10 000 iterations was 0.753 to 0.791. The results of the individual and multivariate Cox regression models are shown in table 4 of the supplementary data. A previous history of HF was independently associated with HHF when PHT was eliminated from the model (table 2 of the supplementary data). No significant variations were observed in the Fine and Gray multivariate analysis (table 3 of the supplementary data).
REFLEJA risk scale for HHFThe nomogram was built using the 7 independent predictors of HHF (figure 2). Based on the associated hazard ratios, the maximum score that could be assigned to any patient was 240 points. The scores assigned to each of the predictors are shown in table 5 of the supplementary data. The REFLEJA risk scale provides a simple means of predicting HHF at 12, 24, and 36 months of follow-up. The scale showed adequate calibration, with a P value of .2616 on the Gronnesby and Borgan test (figure 2 of the supplementary data).
The cumulative incidence curves, with death in the absence of HHF as the competing event, are shown in figure 3. The risk of HHF is shown according to the quartiles of the scale. The 3-year cumulative incidence of HHF was 1.613 cases per 100 person-years for quartile 1 (Q1), 3.815 cases per 100 person-years for Q2, 8.378 cases per 100 person-years for Q3, and 20.436 cases per 100 person-years for Q4 (P<.001). Higher quartiles were associated with an increased risk of all-cause mortality (even in the absence of HHF). Patients hospitalized for HF for the first time were 2.54 times more likely to die of any cause than those not requiring hospitalization (95%CI, 1.90-3.40; P<001). HHF was also associated with an increased risk of cardiovascular death (HR, 8.57; 95%CI, 5.41-13.58; P<.001) (table 3).
Cox multivariate regression for hospitalization for heart failure at 3 years
| Variable | Cox multivariate regression | Bootstrap HRs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Category | b | P | aHR | 95%CI for aHR | Bootstrap aHR | 95%CI for aHR | |||
| Lower | Upper | Lower | Upper | |||||||
| Age | 1476 | Numerical | 0.037 | .001 | 1.0380 | 1.0150 | 1.0620 | 1.0382 | 1.0155 | 1.0630 |
| Diabetes | 1476 | Yes | 0.516 | .005 | 1.6760 | 1.1650 | 2.4120 | 1.6761 | 1.1379 | 2.4060 |
| Chronic kidney disease | 1476 | Yes | 0.980 | <.001 | 2.6650 | 1.8230 | 3.8950 | 2.6646 | 1.7991 | 3.9306 |
| Previous pacemaker implantation | 1476 | Yes | 0.712 | .007 | 2.0390 | 1.2130 | 3.4260 | 2.0388 | 1.1331 | 3.4157 |
| Pulmonary hypertension | 1476 | Yes | 0.761 | <.001 | 2.1400 | 1.4590 | 3.1380 | 2.1396 | 1.4166 | 3.2362 |
| Moderate or severe AR | 1476 | Yes | 0.780 | .020 | 2.1800 | 1.1290 | 4.2110 | 2.1804 | 1.0970 | 4.0156 |
| Baseline diuretic use | 1476 | Yes | 0.514 | .026 | 1.6716 | 1.0640 | 2.6270 | 1.6722 | 1.0569 | 2.7028 |
95%CI, 95% confidence interval; aHR, adjusted hazard ratio; AR, aortic regurgitation.
The initial multivariate model included the following variables: sex, age, hypertension, diabetes mellitus, past history of acute myocardial infarction, alcoholism, past history of cancer, chronic kidney disease, past history of heart failure, electrocardiographic rhythm, type of atrial fibrillation (paroxysmal, persistent, or permanent), CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled], vascular disease, age 65 to 74 years, and sex category [female]), HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly), anemia, moderate or severe mitral regurgitation, moderate or severe AR, left ventricular hypertrophy, pulmonary hypertension, left ventricular ejection fraction, QRS morphology, previous pacemaker implantation, previous implantable cardioverter defibrillator or cardiac resynchronization therapy, and treatment with metformin, sulfonylureas, sodium-glucose cotransporter 2 inhibitors, renin-angiotensin system inhibitors, beta-blockers, aldosterone antagonists, diuretics, digoxin, direct anticoagulants, and antiarrhythmic drugs.
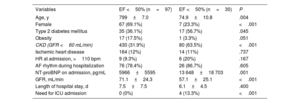
In total, 127 patients (58.3% women) with a mean age of 78.7±8.3 years were hospitalized for HF; 80.3% were in AF rhythm on admission. Mean EF was 56% (75.6% of the patients had a preserved ejection fraction). The mean N-terminal fragment of pro-B-type natriuretic peptide level was 7822±10 811 (median 5400) pg/mL. Patients with a preserved EF were older, mostly women, and less likely to have CKD (table 4). The 3-year mortality rate in patients hospitalized for HF was 44.1%. The cause of death was noncardiovascular in 66.9% of patients, cardiovascular in 25.2%, and unknown in almost 8%.
Characteristics of patients hospitalized for HF
| Variables | EF <50% (n=97) | EF <50% (n=30) | P |
|---|---|---|---|
| Age, y | 799±7.0 | 74.9±10.8 | .004 |
| Female | 67 (69.1%) | 7 (23.3%) | <.001 |
| Type 2 diabetes mellitus | 35 (36.1%) | 17 (56.7%) | .045 |
| Obesity | 17 (17.5%) | 1 (3.3%) | .051 |
| CKD (GFR <60 mL/min) | 430 (31.9%) | 80 (63.5%) | <.001 |
| Ischemic heart disease | 164 (12%) | 14 (11%) | .737 |
| HR at admission, >110 bpm | 9 (9.3%) | 6 (20%) | .167 |
| AF rhythm during hospitalization | 76 (78.4%) | 26 (86.7%) | .605 |
| NT-proBNP on admission, pg/mL | 5966±5595 | 13 648±18 703 | .001 |
| GFR, mL/min | 71.1±24.3 | 57.1±25.1 | <.001 |
| Length of hospital stay, d | 7.5±7.5 | 6.1±4.5 | .400 |
| Need for ICU admission | 0 (0%) | 4 (13.3%) | <.001 |
AF, atrial fibrillation; CKD, chronic kidney disease; ECG, electrocardiogram; EF, ejection fraction; GFR, glomerular filtration rate; HR, heart rate; ICU, intensive care unit; N-terminal pro-B-type natriuretic peptide.
Values are expressed as No. (%) or mean±standard deviation.
During a 3-year follow-up, HHF was common in this prospective cohort of patients with AF. It was more common than hospitalization for AF and for stroke or transient ischemic attack and was also associated with an increased risk of all-cause and cardiovascular mortality.
Age, diabetes, CKD, HTP, previous pacemaker implantation, baseline diuretic use, and moderate or severe aortic regurgitation were each independently associated with an increased risk of HHF. A previous history of HF was not predictive of hospitalization for this cause (figure 4).
Central illustration. Incidence and prediction of hospitalization for HF in patients with AF: the REFLEJA scale. AF, atrial fibrillation; AR, aortic regurgitation; HF, heart failure; HHF, hospitalization for heart failure; HR, hazard ratio; Q, quartile; ROC, receiver operating characteristics.
The REFLEJA registry is the first registry used to design a scale for predicting HF in patients with AF in Spain. The scale was developed using data from all consecutive patients treated by the cardiology department at Hospital Universitario de Jaén. No exclusion criteria were applied. If shown to have adequate external validity, the REFLEJA risk scale could be a useful tool for predicting HF in patients with AF. The discriminative ability of the scale, with a c-statistic of 0.7 to 0.8, is good, but the model cannot be considered fully robust.
Incidence of HHFThe incidence of HHF in this cohort of patients with AF was high, at around 4% a year. This rate is similar to rates reported in classic observational studies4,17,18 and higher than those observed in more recent registries showing an incidence of HF of approximately 1 to 2 cases per 100 person-years.8,10 The higher incidence detected in the REFLEJA registry could be related to the older age of the cohort and the higher prevalence of comorbidities and cardiovascular risk factors. The incidence of HHF was 4.35% at 1 year and 8.51% at 3 years, indicating that HHF is more common in the early stages of AF diagnosis. This finding supports previous reports by Suzuki et al.19
Overall mortality at 3 years was 21.1%. Most of the deaths were noncardiovascular, probably in relation to the older patient age and the high rate of noncardiovascular comorbidities. Higher rates of noncardiovascular deaths have also been observed in hospitalized patients with HF and a preserved EF, who tend to be older and have more comorbid conditions.20
Predictors of HHF and calculation of risk scoresMost of the aforementioned studies have reported an association between HHF and both age6,7,9,10,21–23 and diabetes.6–8,10,19,22,24–26 Additional predictors identified in other studies, such as CKD8,10,19,24 and valvular heart disease,8,10,27 were also predictive of HHF in our cohort. In our case, however, only moderate or severe aortic regurgitation was significant, potentially positioning it as a more specific predictor.
PHT is frequently associated with progression to right HF and increased mortality.14 It has also been linked to a higher prevalence of AF, with onset indicative of disease progression and clinical worsening.28 To our knowledge, however, no studies have found an independent association between a high probability of PHT and HHF. The presence of PHT probably identifies a subset of patients who, notwithstanding the etiology of PHT, have a higher risk of right ventricular failure and, therefore, clinical onset of HF. Notably, a past history of HF emerged as an independent predictor of HHF when PHT was excluded from the model, which was unsurprising given the significant association between the 2 variables (table 6 of the supplementary data).
Supporting previous findings,19,27 baseline diuretic use was also predictive of HHF, probably because it is an indirect marker of HF severity and even a past history of HF.
The final independent predictor of HHF to emerge in the model was prior pacemaker implantation. Although we found no reports of this association in our review of the literature, one study described an association between intraventricular conduction disorders (QRS width) and HHF.8 A history of pacemaker implantation and intraventricular conduction disorders are interconnected, as both intraventricular and interventricular dyssynchrony often lead to a decrease in left ventricular EF (LVEF) and consequently HF symptoms. Dyssynchrony is the most common phenomenon observed in patients requiring single-chamber right ventricular pacing, which is the most common type of pacing used in patients with AF.29
The absence of an association between HHF and reduced LVEF and a previous history of HF is noteworthy, although it could be due to the predominance of patients hospitalized for HF who had preserved LVEF, a condition typically observed in older patients with a higher prevalence of noncardiac comorbidities.20
Despite various scales for predicting mortality in patients with HF, such as the MAGGIC Risk Score30 and the BIOSTAT-CHF31,31 the REFLEJA scale helps predict the onset of HF in patients with AF, regardless of whether or not they have a previous history of HF. These scales, unlike ours, were tested using data from clinical trials,8 smaller retrospective registries,22 or studies with a shorter follow-up time (on average, 2 years).8,19 In addition, some of the studies were conducted in Asian patients, who, on average, were younger than those in the REFLEJA registry. In a recent study of a larger cohort of Spanish patients aged ≥ 80 years, Melendo-Viu et al.32 reported a higher incidence of HF than that observed in our cohort, although it should be noted that their study included patients diagnosed in both hospital and outpatient settings. Significant predictors of HF were age, diabetes, CKD, and significant valvular heart disease.
Phenotypes of patients with AF hospitalized for HFMost (3/4) of the patients from the REFLEJA AF registry admitted for HF had preserved EF. As expected, these patients were older and more likely to be women, supporting previous reports by Pandey et al.10 and Potpara et al.26 The exact reasons for the higher frequency of this phenotype in patients with AF remain to be determined, but a possible explanation is that the 2 conditions may share factors such as older age, hypertension, diabetes, and obesity.
Clinical implications and limitationsOnset of HF is common in patients with AF. Tools capable of identifying at-risk patients will facilitate the implementation of interventions that help delay onset. Possible strategies include clinical follow-up and proactive diagnosis, lifestyle changes, and initiation of pharmacologic treatments with proven cardiovascular benefit, such as sodium-glucose cotransporter 2 inhibitors for patients with diabetes, finerenone for patients with diabetes and CKD, and glucagon-like peptide-1 agonists for patients with diabetes and obesity.33,34
The REFLEJA scale has a number of limitations, primarily that it was designed using data from a single center, hampering adequate external validity and, pending this validation, limiting its use in other settings. As the study was observational, it is subject to potential biases and unidentified confounders. In addition, the scale cannot be used to predict the risk of HF according to AF duration. To overcome the problem of missing data, we had to characterize certain continuous variables (body mass index and pulmonary systolic arterial pressure) as dichotomous (obesity, pulmonary hypertension, etc.), potentially limiting out ability to identify better cutoffs. We were also missing information on other variables that might have improved the discriminative ability of the scale, such as frailty, dementia, social support, and echocardiographic findings (eg, right ventricular systolic dysfunction and left atrial volume). In addition, the width of the CIs for HHF risk prediction in the scale may have impeded the development of a more accurate risk prediction model.
By using a follow-up period of just 3 years, we may have underestimated the long-term risk of HF in patients with AF. In addition, details of clinical events were not collected by an independent external committee. Finally, the inclusion of patients treated by cardiologists only (and not, for example, by primary care physicians or internists) may have biased our results.
CONCLUSIONSThe 3-year incidence of HHF in this cohort of patients with AF was high. The discriminative ability of the REFLEJA scale (with 7 clinical variables) to predict HHF over a 3-year period is similar to that described for other scales,7–9,18 but it is tailored to an exclusively Spanish population under the care of cardiologists.
FUNDINGThe project was partly funded by an unconditional grant from Bayer and Daiichi-Sankyo.
ETHICAL CONSIDERATIONSThe study was approved by the Provincial Ethics Committee of Jaén. Informed consent was obtained from all patients included. Potential gender issues were taken into account during the reporting of this article.
USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSAll the authors contributed equally to the preparation of this manuscript. The manuscript was drafted by J. Torres-Llergo and revised by the other authors.
CONFLICTS OF INTERESTThe authors declare that they do not have any conflicts of interest in relation to this article.
- -
HHF is a common complication in patients with AF, and the coexistence of both conditions is associated with increased mortality.
- -
Patients with AF have certain clinical characteristics associated with an increased risk of HF.
- -
Several scales for predicting HF in patients with AF have been produced outside Spain.
- -
The incidence of HHF in a recent cohort of patients with AF is higher than that described in other series.
- -
Previous pacemaker implantation and a high probability of PHT were identified as 2 new independent predictors of HHF in patients with AF.
- -
To our knowledge, this is the first scale for predicting HHF in a Spanish population with AF.
We thank María del Carmen Rosa Garrido from the Methodology and Statistics Unit of Fundación para la Investigación Biosanitaria de Andalucía Oriental «Alejandro Otero» for her invaluable methodological and statistical guidance for this study.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2024.02.001