.
Aortic stenosis (AS) is the most common valve disease, and a considerable increase in its incidence is foreseen for the coming decades because of the progressive aging of the population. Aortic valve replacement (AVR) surgery has been associated with a substantial improvement in the survival, functional capacity, and quality of life of patients with symptomatic AS and, at the present time, is the treatment of choice for this condition. However, it has been reported that up to 30% of patients with symptomatic AS do not undergo surgical AVR due to a very high or prohibitive surgical risk.1 The first percutaneous transcatheter implantation of a prosthetic valve in the aortic position (TAVI) was performed in 2002 in a patient in cardiogenic shock who was not considered a suitable candidate for AVR surgery2 and, over the following years, TAVI was further developed as a less invasive alternative to surgical AVR in patients with severe symptomatic AS and very high or prohibitive surgical risk. The expansion of this new technology for the treatment of AS has been very rapid and, to date, more than 40000 percutaneous aortic valve implantations have been performed. The two prostheses on which the cumulative clinical experience with TAVI has been based up to now are the balloon expandable Edwards SAPIEN valve (Edwards Lifesciences, Irvine, California, United States) and the self-expanding CoreValve prosthesis (Medtronic, Minneapolis, Minnesota, United States) (Figure 1). In recent years, a series of multicenter registries including about 4000 patients have been created in Europe and Canada.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 The most relevant findings of these studies are summarized in Table 1. The success rate of the TAVI procedure was systematically higher than 90%, with mortality rates after 30 days and after 1 year of follow-up of 9% (6.6% to 12.7%) and 20% (15% to 24%), respectively. However, these studies have a series of important limitations, such as the relative heterogeneity of the populations included due to the lack of uniform criteria for patient inclusion, the lack of standardized definitions of the clinical events and complications arising after the procedure, the lack of monitoring and adjudication of the clinical events by an independent committee (with the exception of the SAPIEN Aortic Bioprosthesis European Outcome [SOURCE] registry), and the lack of randomization with respect to AVR surgery or medical treatment.
Figure 1. A: Edwards SAPIEN XT transcatheter valve. B: CoreValve transcatheter valve (by courtesy of Dr. Marc Ruel, Ottawa Heart Institute, Ottawa, Ontario, Canada).
Table 1. Summary of the Most Recent Registries of Transcatheter Aortic Valve Implantation.
| Registry | n | Logistic EuroSCORE, % | Prosthesis, % | Access, % | Success, % | 30-Day Follow-up | One-year Follow-up | |||
| Mortality, % | Stroke, % | Vascular complications, % | Pacemaker, % | Mortality, % | ||||||
| European registry 3 | 646 | 23.1±13.8 | CoreValve(100) | TF (100) | 97 | 8 | 1.9 | NA | 9.3 | NA |
| Canadian registry 4 | 339 | 27.7±16.3 | Cribier-EdwardsEdwards SAPIEN (100) | TF (50)TA (50) | 93.3 | 10.4 | 2.3 | 13 | 4.9 | 24 |
| Spanish registry 5 | 108 | 16±13.9 | CoreValve (100) | TF (95.4)SC (4.6) | 98.1 | 7.4 | 0 | 5.6 | 35.2 | 17.7 |
| SOURCE registry 6,7 | 1038 | 27.6±15.5 | Edwards SAPIEN (100) | TF (45)TA (55) | 93.8 | 8.5 | 2.5 | 12.8 | 7 | 23.9 |
| German registry 8 | 697 | 20.5±13.2 | Edwards SAPIEN (26)CoreValve (84) | TF (92)SC (3)TA (4)TAo (1) | 98.4 | 12.4 | 2.8 | NA | 39.3 | NA |
| Italian registry 9 | 656 | 23±14 | CoreValve (100) | TF (90)SC (10) | 98 | 6.6 | 1.2 | NA | 18.5 | 14.9 |
| Belgian registry 10 | 328 | 28±16 | Edwards SAPIEN (57)CoreValve (43) | TF (71)TA (27)SC (2) | 97 | 11 | 5 | NA | 13 | 17 |
| French registry 11 | 244 | 25.6±11.4 | Edwards SAPIEN (68)CoreValve (32) | TF (66)TA (29)SC (5) | 98.3 | 12.7 | 3.6 | 6.9 | 4.826.9 | NA |
| United Kingdom registry 12 | 870 | 18.5 (11.7-27.9) | Edwards SAPIEN (47)CoreValve (52) | TA (65)TF (31)SC (4) | 97.2 | 7.1 | 4.1 | 6.3 | 7.424.4 | 21.4 |
NA, not available; SC, subclavian; SOURCE, SAPIEN Aortic Bioprosthesis European Outcome; TA, transapical; TAo, transaortic; TF, transfemoral.
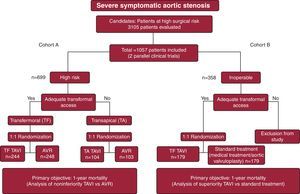
The PARTNER study is a multicenter, randomized clinical trial involving 2 treatment cohorts undertaken to compare TAVI with conventional AVR surgery (cohort A)13 and with standard medical treatment or aortic valvuloplasty (cohort B)14 in patients with severe symptomatic AS. The details of the study design are summarized in Figure 2. Cohort A included patients at high surgical risk according to an estimated risk of mortality within 30 days of surgery of 15% or greater (a surgical risk greater than 10% according to the Society of Thoracic Surgeons [STS] predicted mortality risk score was used as a guideline for patient inclusion). All the patients included in cohort B were considered by at least 2 cardiac surgeons to be inoperable on the basis of an estimated 30-day risk of mortality or serious irreversible morbidity of 50% or more. Most of the patients included in the study were octogenarians, and among the most important comorbidities were a history of stroke (∼27%), chronic obstructive pulmonary disease (∼40%), and coronary artery disease (∼70%), which, together with other comorbidities, resulted in very high mean estimated surgical risk scores (STS ∼11% and logistic EuroSCORE ∼29%). The Edwards SAPIEN transcatheter prosthesis was employed. The major findings of the PARTNER trial are summarized in Figure 3.
Figure 2. Main features of the design of the PARTNER trial. AVR, aortic valve replacement; TAVI, percutaneous transcatheter aortic valve implantation.
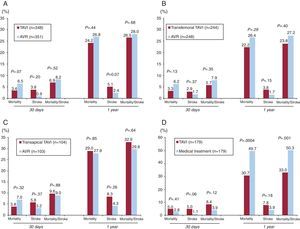
Figure 3. Main outcomes in mortality and stroke in the PARTNER trial after 30 days and 1 year of follow-up. A: cohort A, patients at high surgical risk who underwent percutaneous transcatheter aortic valve implantation versus aortic valve replacement surgery. B: cohort A, candidates for percutaneous transcatheter aortic valve implantation via the transfemoral approach versus aortic valve replacement surgery. C: cohort A, patients in whom percutaneous transthoracic aortic valve implantation via transfemoral access was not possible, who were treated using the transapical approach, versus aortic valve replacement surgery. D: cohort B, inoperable patients who underwent percutaneous transcatheter aortic valve implantation versus medical treatment. AVR, aortic valve replacement; TAVI, percutaneous transcatheter aortic valve implantation.
Patients at High Surgical Risk (Cohort A)After a year of follow-up, no differences were observed between TAVI and surgical AVR in terms of overall mortality (TAVI, 24.2%; AVR, 26.8%; P=.44) or cardiovascular mortality (TAVI, 14.3%; AVR, 13%; P=.63). Percutaneous transcatheter aortic valve implantation was associated with a higher incidence of stroke (8.3% vs 4.3%; P=.04), although the combined endpoint of death and major stroke was similar after 1 year of follow-up (TAVI, 26.5%; AVR, 28%; P=.68%). The TAVI group developed more major vascular complications (11% vs 3.2%; P<.001), but fewer severe hemorrhagic complications (9.3% vs 19.5%; P<.001) and less new-onset atrial fibrillation (8.6% vs 16%; P<.001). There were no differences between the groups with respect to the need for a permanent pacemaker following the procedure (TAVI, 3.8%; AVR, 3.6%). The analysis of the subgroups revealed that a history of previous coronary artery surgery favored AVR over TAVI, whereas TAVI was associated with better results (with respect to AVR) in women. Finally, a year after the procedure, a functional improvement was observed in both groups, although it took place earlier in the TAVI group, with more patients in New York Heart Association (NYHA) functional classes I and II covering a greater distance in the 6-min walk test at 1 month after the procedure.
Inoperable Patients (Cohort B)Percutaneous aortic valve implantation was associated with a reduction in all-cause mortality of 20% after 1 year of follow-up (30.7% vs 50.7%; P<.001), which means that only 5 patients required treatment with TAVI in order that 1 more patient survive 1 year of follow-up, compared to medical/ aortic valvuloplasty standard treatment. The investigators also observed a significant decrease in cardiovascular mortality (20.5% vs 44.6%; P<.001) and hospital readmissions (22.3% vs 44.1%;P<.001). It should be pointed out that up to 84% of the patients in the medical treatment group underwent aortic valvuloplasty, a fact that confirms the relative ineffectiveness of this treatment over the medium term in elderly patients with severe AS. Percutaneous transcatheter aortic valve implantation was also associated with a significant improvement in the functional capacity (NYHA functional class I or II: 75% vs 42%; P<.001) and quality of life after 1 year of follow-up.15 The cost-effectiveness analysis demonstrated that TAVI involved a cost of approximately $50 000 per additional life-year.
As a result of the PARTNER trial, the following recommendations were established:
1. Treatment with TAVI should be offered to patients with severe symptomatic AS and prohibitive surgical risk following evaluation by a multidisciplinary team consisting of cardiologists and cardiac surgeons. This multidisciplinary team should evaluate, probably with the aid of other specialists (geriatricians, internists, pulmonologists, etc.), the life expectancy and potential for improving the functional class and quality of life of each patient (aside from the AS). Only those patients with a life expectancy of at least 1 year (criterion for inclusion in the PARTNER trial) and the capacity for improving his or her functional class and quality of life should undergo TAVI treatment.
2. Percutaneous transcatheter aortic valve implantation should be considered an alternative to AVR for patients found to have a high surgical risk. Until there is a risk scale specific to candidates for TAVI, the guideline should be a risk >8% according to the STS score (approximately equivalent to a logistic risk score higher than 20) in order to consider a patient as being at high surgical risk. Evaluation of life expectancy and the potential for improved functional class and quality of life should be carried out in accordance with the same recommendations as those followed for patients considered inoperable (see the preceding paragraph).
The PARTNER trial is, beyond all doubt, one of the most important studies ever carried out in the treatment of AS, and represents the confirmation of TAVI as an alternative to AVR. However, there still remain a number of doubts and points to be clarified in its wake.
Patients Excluded From the PARTNER TrialIt is important to bear in mind that the PARTNER trial involved numerous exclusion criteria, several of them important. Among them, we underscore the presence of bicuspid aortic valve, severe mitral regurgitation, severely depressed left ventricular function, and severe chronic renal failure. Patients with previous valve surgery were also excluded, and thus there were no cases in which TAVI was performed to treat dysfunction of a surgically implanted prosthesis (“valve-in-valve” procedure). Despite the fact that a number of observational studies have reported promising results in the different subgroups of patients not included in the PARTNER trial,3, 4, 5, 6, 7, 8, 9, 10, 16 there is no evidence that TAVI is superior to medical therapy or aortic valvuloplasty, or equivalent to AVR, for the treatment of these patients. It is also important to point out that many patients were not included in the PARTNER trial because the surgical risk was not considered to be high enough (or prohibitive in the case of cohort B).
The good results obtained in the PARTNER trial would encourage the extension of the indication for TAVI to groups of patients at lower risk, a step that has begun to be taken with promising results in some European centers. However, it is important to consider that in patients at intermediate or low risk, AVR surgery is associated with excellent outcomes in most centers. In the coming years, 2 randomized studies (SURTAVI and PARTNER II) are going to be undertaken in which TAVI and surgical AVR results will be compared in patients with symptomatic AS and intermediate surgical risk (STS score between 4% and 8%). The findings of these studies should demonstrate whether or not TAVI is equivalent to AVR for the treatment of these patients.
Finally, the results of the PARTNER trial are limited to TAVI involving the use of the Edwards SAPIEN transcatheter valve. At the present time, a prospective, randomized study is underway to compare the CoreValve transcatheter aortic valve with AVR in patients at high surgical risk (clinicaltrials.gov Identifier#NCT01240902).
High Incidence of StrokeStroke is one of the most feared complications associated with the TAVI procedure. The PARTNER trial has confirmed: a) that the incidence of stroke following TAVI is one of the highest recorded in interventional cardiology, and b) that this high rate of stroke surpasses that observed following AVR surgery (cohort A) and aortic valvuloplasty (cohort B). Transcranial Doppler studies have demonstrated that cerebral embolism can occur at any time during the procedure, but it appears to be more frequent during the percutaneous positioning and implantation of the prosthetic valve, a circumstance indicating that the embolization of particles that become detached from the leaflets of the calcified native aortic valve is an important mechanism in TAVI-related cerebral embolism.17 This would also explain the absence of differences between transfemoral and transapical implantation in terms of the rate of stroke, despite the fact that the latter route avoids the use of large-bore catheters in the aortic arch and ascending aorta and retrograde crossing of the native aortic valve. Preliminary results regarding the use of devices to protect against cerebral embolism during TAVI are available and in the coming years their effectiveness in reducing the incidence during these procedures of cerebral ischemic events (those detected on the basis of clinical evidence and those examined by brain magnetic resonance imaging) will be evaluated. However, data from the PARTNER trial13, 14 have revealed that approximately 50% of the strokes occur more than 24h after the procedure, which indicates that mechanisms not directly related to the percutaneous intervention could also play an important role in a high percentage of the strokes occurring after TAVI. Amat-Santos et al.18 recently pointed out that the development of atrial arrhythmias (atrial fibrillation) following TAVI may be the most important factor related to strokes occurring more than 24h after the procedure. Although empiric treatment with a combination of aspirin and clopidogrel is recommended after TAVI, future studies should determine the optimal antithrombotic therapy following these procedures. Finally, one of the substudies of the PARTNER trial (cohort A) indicated that strokes occurring in the acute phase following TAVI were related to a smaller valve area prior to the procedure, whereas late events were related to a greater cardiovascular risk factor burden and, again, the investigators point out that the mechanisms of the acute and late events are probably different.19 This substudy also confirmed that the percutaneous implantation of aortic prostheses is not associated with a higher incidence of neurological events than the surgical placement of these devices.
High Rate of Vascular ComplicationsThe utilization of large catheters in very elderly patients has been associated with a high incidence of vascular complications, as is also reflected in the PARTNER trial data with rates of severe vascular complications in cohorts B and A of 16% and 11%, respectively. It is important to point out that the PARTNER trial employed 22-Fr and 24-Fr catheters, rather than the 18-Fr catheters that are now being used. The absence of an alternative to the transfemoral route in cohort B of the PARTNER trial may also have led to the inclusion of patients with a small arterial diameter or extremely severe calcification of the iliofemoral arteries, two factors that are related to a greater number of vascular complications. In the future, the meticulous evaluation of the femoral access, the use of routes other than the transfemoral approach (transapical, transaortic, subclavian, axillary) in cases in which the iliac and femoral arteries are borderline, the utilization of smaller catheters, and greater experience in TAVI procedures should be associated with a significant decrease in the rate of vascular complications.
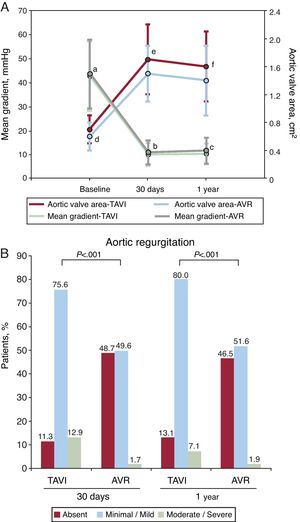
Residual Aortic RegurgitationThe different studies in the field of TAVI in the pre-PARTNER era had already demonstrated that the Edwards and CoreValve transcatheter valves were usually associated with excellent hemodynamic results, although residual paravalvular regurgitation is common, being minimal or mild in most cases and moderate in ∼10% of the patients.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 It is important to point out that the PARTNER trial includes, for the first time in a study dealing with TAVI, analysis of the ultrasound data in an independent echocardiographic core lab. The results confirm the excellent hemodynamics of the Edwards SAPIEN transcatheter valve, even better than the hemodynamics of the surgically implanted prostheses (cohort A, Figure 4). However, residual aortic regurgitation, mainly paravalvular, was detected in 89% of the cases (moderate in 13%), a percentage that is much higher than that observed following surgical valve replacement. Undoubtedly, the existence of at least moderate aortic regurgitation in approximately 10% of the patients represents a significant limitation of TAVI, as it is associated with a poorer prognosis after the procedure and can generate doubts with respect to the expansion of this technology for the treatment of a younger patient population with lower surgical risk. However, a number of factors predictive of the onset of at least moderate aortic regurgitation following TAVI have been identified, including more severe calcification of the native valve, greater valve annulus diameter, disproportion between annulus and prosthetic valve sizes, prosthesis implantation too low or too high, and excessive angulation between ascending aorta and left ventricular outflow tract. Thus, over the next few years, risk scales that will enable us to determine which patients are at a higher risk for the onset of moderate or severe aortic regurgitation following TAVI will very probably be developed. Research is also being carried out in the improvement of the design of prosthetic valves for percutaneous implantation in order to reduce the incidence of this complication. Finally, it is important to highlight that the echocardiographic assessment of the severity of paravalvular regurgitation following TAVI is not a simple task. In the PARTNER trial, the only criterion employed was the color jet area in short-axis views, a strategy that appears to be insufficient for a correct evaluation of the severity of this condition. The parameters for the proper evaluation of the degree of residual paravalvular regurgitation after TAVI should also be validated in the future.
Figure 4. Hemodynamic findings in the PARTNER trial (cohort A). A: mean transaortic gradient and valve area following percutaneous transcatheter aortic valve implantation versus surgical aortic valve replacement. B: residual aortic regurgitation after percutaneous transcatheter aortic valve implantation versus surgical aortic valve replacement. AVR, aortic valve replacement; TAVI, transcatheter aortic valve implantation. aP=.51. bP=.04. cP=.008 (TAVI vs AVR). dP=.032. eP=.001. fP=.002 (TAVI vs AVR).
High One-Year Mortality RateDespite the fact that the majority of the TAVI procedures were carried out at the beginning of the learning curve in most of the participating centers and that the devices employed were less advanced than those available at the present time, the PARTNER trial was associated with an excellent 30-day survival rate, with a mortality of less than 6% (3.4% in intention to treat and 5.2% in patients who underwent TAVI) in the transfemoral TAVI group of cohort A, much lower than the estimated mortality according to the STS score (∼11%). However, the mortality rate was relatively high after 1 year of follow-up (30.7% in cohort B and 24.2% in cohort A). Despite the fact that patients with a life expectancy of less than 1 year were excluded by the protocol, this finding indicates that patients with cardiac and/or noncardiac conditions that were too advanced to benefit from any invasive treatment, including TAVI, ended up being enrolled. In fact, patients that survive TAVI with no major complications but die a few months after the procedure belong to the so-called “cohort C” (as compared to PARTNER cohorts A and B), which corresponds to those patients with probably too high a risk to undergo TAVI who should only receive medical treatment. The acceptance of these patients for TAVI excessively penalizes the cost-effectiveness ratio associated with this technique. It should be pointed out that some studies have shown that late mortality (more than 30 days) after TAVI is basically related to noncardiac causes, mainly respiratory and renal disorders.4, 7, 20 Unfortunately, the surgical risk scales, especially the logistic EuroSCORE, do not appear to be appropriate for short- and medium-term risk determination in patients who undergo TAVI and, in the coming years, a risk scale specific to TAVI should be designed. Several studies have evaluated the predictive factors of medium-term mortality associated with TAVI,4, 7, 9 which can be divided into 3 groups: a) cardiac comorbidity (severely depressed left ventricular function, pulmonary hypertension, severe mitral regurgitation); b) complications during the procedure (stroke, severe vascular complications, sepsis, moderate-to-severe residual aortic regurgitation), and c) noncardiac comorbidity (chronic obstructive pulmonary disease, chronic renal failure, liver cirrhosis). The validation of these factors and others in studies with larger numbers of patients should make it possible in the coming years to design a risk scale specific for TAVI, a circumstance that undoubtedly would make it possible to improve the selection process of this difficult patient population. This is an essential step if the aim is to improve the medium-term results of TAVI.
Absence of Data on the Long-term Durability of Percutaneously Implanted ProsthesesThe echocardiographic findings in the PARTNER trial after 1 year of follow-up confirm the good results of previous observational studies on the hemodynamic stability associated with percutaneously implanted valves. However, to date we have little data on their long-term durability and the absence of structural failure in these devices. The longest follow-up period up to now is that reported by the Vancouver group,20 in a series of 70 patients with a mean follow-up of nearly 4 years, during which there were no cases of structural dysfunction or failure of the percutaneously implanted valve. Nevertheless, structural failure is detected in less than 5% of the surgically placed prostheses over the long term (more than 5 to 10 years of follow-up) and, thus, we will still have to wait a few years to determine whether the same results in terms of durability are achieved with the percutaneously implanted prostheses. This information is of the utmost importance for the possible expansion of this technique to the treatment of younger patients with a life expectancy of more than 10 years after the procedure.
ConclusionsPercutaneous transcatheter aortic valve implantation is a less invasive alternative to AVR for the treatment of severe symptomatic AS. The PARTNER trial has confirmed that, at the present time, TAVI is the treatment of choice for patients with prohibitive surgical risk, and a valid alternative to AVR for patients considered to be at high surgical risk. However, over the coming years, we should improve the process of patient selection and reduce some of the complications associated with the procedure, such as stroke, vascular complications, and moderate-to-severe residual aortic regurgitation. There are promising preliminary data concerning the results of TAVI in patients at moderate surgical risk, its use in the treatment of dysfunction of surgically implanted prostheses (“valve-in-valve”), and the medium- and long-term follow-up of percutaneously implanted prostheses. The confirmation of these results in future studies should enable the expansion of treatment with TAVI to a much wider spectrum of patients with severe symptomatic AS.
FundingDr. Urena is the recipient of a grant for research in foreign centers from the Sociedad Española de Cardiología (Spanish Society of Cardiology). Dr. Nombela-Franco is the recipient of a research grant from Fundación Alfonso Martín Escudero, Madrid, Spain.
Conflict of interestsDr. Josep Rodés-Cabau is a consultant for Edwards Lifesciences and St. Jude Medical.
Acknowledgements
The authors wish to thank Melanie Côté, MSc, Quebec Heart & Lung Institute, for her work in the preparation of the figures.
Corresponding author: Quebec Heart & Lung Institute, 2725 chemin Ste-Foy, G1V 4G5 Quebec city, Quebec, Canada. josep.rodes@criucpq.ulaval.ca