Short-term mechanical circulatory support is frequently used as a bridge to heart transplant in Spain. The epidemiology and prognostic impact of infectious complications in these patients are unknown.
MethodsSystematic description of the epidemiology of infectious complications and analysis of their prognostic impact in a multicenter, retrospective registry of patients treated with short-term mechanical devices as a bridge to urgent heart transplant from 2010 to 2015 in 16 Spanish hospitals.
ResultsWe studied 249 patients, of which 87 (34.9%) had a total of 102 infections. The most frequent site was the respiratory tract (n=47; 46.1%). Microbiological confirmation was obtained in 78 (76.5%) episodes, with a total of 100 causative agents, showing a predominance of gram-negative bacteria (n=58, 58%). Compared with patients without infection, those with infectious complications showed higher mortality during the support period (25.3% vs 12.3%, P=.009) and a lower probability of receiving a transplant (73.6% vs 85.2%, P=.025). In-hospital posttransplant mortality was similar in the 2 groups (with infection: 28.3%; without infection: 23.4%; P=.471).
ConclusionsPatients supported with temporary devices as a bridge to heart transplant are exposed to a high risk of infectious complications, which are associated with higher mortality during the organ waiting period.
Keywords
In recent years, there has been a progressive increase in the number of heart transplant (HTx) candidates requiring mechanical circulatory support (MCS). The use of short-term MCS devices predominates in Spain, partly due to the characteristics of our national organ distribution system, which awards the highest waiting list priority (ONT status 0) to such patients.1
The main drawback of MCS devices is the high incidence of adverse clinical events, namely, thromboembolism, bleeding, and infection. There are extensive data in the literature on the complications associated with long-term MCS devices,2 derived from clinical trials and multicenter registries. However, very few studies have systematically analyzed the incidence and clinical impact of complications associated with short-term MCS devices.
Infectious complications can ultimately affect slightly more than half of all patients treated with extracorporeal membrane oxygenation (ECMO)3 and short-term ventricular assist devices (VADs).4 Nonetheless, the information published on this topic is largely derived from small single-center studies, which are affected by local practices and specific epidemiological environments. No systematic analysis has explored the potential impact of infectious complications in the specific case of patients receiving short-term MCS as a direct bridge to HTx.
Given this knowledge gap, we decided to systematically analyze the causative agents, risk factors, and prognostic impact of the infectious complications associated with short-term MCS devices in urgent HTx candidates in Spain. To do so, we examined clinical data collected from a multicenter registry.
METHODSStudy descriptionThe ASIS-TC study (Empleo de los dispositivos de asistencia circulatoria mecánica de corta duración como puente a trasplante cardiaco urgente en España [Use of short-term mechanical circulatory support devices as a bridge to urgent heart transplantation in Spain]) is a retrospective registry including consecutive patients on the urgent waiting list of the Spanish National Transplant Organization (ONT status 0) for a first, single-organ HTx, who were treated with a short-term MCS device between January 1, 2010, and December 31, 2015.1 The short-term MCS devices included were venoarterial ECMO devices and surgically implanted or percutaneous short-term VADs. All 16 transplant centers in Spain participated in the registry. The protocol was approved by the Clinical Research Ethics Committee of the Autonomous Community of Galicia.
The present article describes a subanalysis of the data collected in the ASIS-TC study that are related to the incidence and prognostic impact of infectious complications in patients treated with short-term MCS devices as a bridge to HTx. It thus involves a retrospective analysis of a pre-existing database that was not specifically designed for this use.
Patients were excluded from the registry if they were supported with more than 1 MCS device or had experienced any infection treated with antibiotics during the hospital admission period prior to the MCS device implantation.
Definition of infectious complicationsThe main outcome variable of the study was infection present during support with a short-term MCS device, defined as any episode of infection identified on culture and diagnosed after device insertion and before device removal or HTx surgery, independently of the treatment used. Episodes of suspected infection diagnosed by clinical, analytical, and typical imaging findings but without microbiological confirmation of a causative pathogen were also considered infectious events but only when the patients received empirical intravenous antibiotic therapy. The infections recorded were a posteriori classified as MCS-specific infections, MCS-related infections, and non-MCS infections, in line with the nomenclature of the International Society for Heart and Lung Transplantation.5
The assignment of infection episodes, foci, and causative agents was the responsibility of the local research team and was based on information recorded in the patients’ medical records, given the retrospective nature of the registry.
Statistical analysisQuantitative variables are reported as mean ± standard deviation and qualitative variables as proportions. For analysis of differences between groups, a t test was used for quantitative variables and a chi-square test for qualitative variables. Due to a skewed distribution, MCS duration was reported as median [interquartile range].
The infection incidence rate (episodes/1000 patient-days) during the MCS period was estimated in both the total cohort and the patient subgroups treated with each type of device. The cumulative probability of infection by MCS duration was estimated using the Kaplan-Meier method.
A multivariable backward stepwise logistic regression model with an exit criterion of P < .05 was used to identify clinical factors independently associated with infection risk during MCS. In the first step of this model, we entered variables showing a significant (P < .05) association with the event under study.
Logistic regression was also used to adjust for the effect of MCS-related infection on mortality during the same support period. In this case, adjustment was performed by including as covariables various clinical factors that were considered potential confounding factors based on clinical reasoning (age, sex, type of underlying heart disease, INTERMACS profile, and type of MCS device).
Statistical analysis was performed with SPSS 20 and Epidat 4.1. Significance was set at P < .05 for all comparisons.
RESULTSIncidence of infectionThe ASIS-TC registry included 291 patients from 16 Spanish hospitals. We excluded from the present analysis 21 patients who had been treated with more than 1 device and another 21 who experienced an infectious complication during the hospital stay prior to the implantation. Accordingly, the final sample comprised 249 individuals. Of these, 151 (60.6%) were treated with a venoarterial ECMO (146 with peripheral cannulation and 5 with central cannulation), 11 (4.4%) with a percutaneous VAD (all Impella Recover, implanted via femoral access), and 87 (34.9%) with a surgically implanted VAD (67 Levitronix Centrimag, 17 Abiomed BVS 5000, 1 Abiomed AB5000, 1 Sorin Revolution, and 1 Maquet Rotaflow).
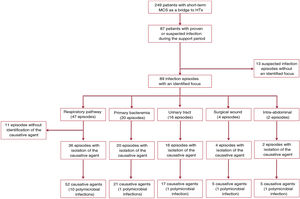
The mean duration of MCS was 12.1 ± 11.3 days, with a median of 9 [4-17] days. In this period, 87 patients (34.9%) had a total of 102 infection episodes: 3 episodes were recorded in 2 patients, 2 in 11 patients, and 1 in 74 patients. figure 1 shows a flow chart detailing the distribution of nosocomial infection episodes diagnosed in the study patients.
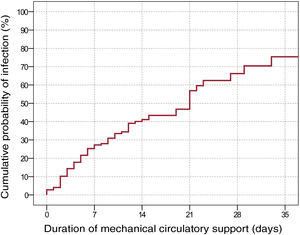
We estimated a total incidence rate of nosocomial infection of 33.8 episodes/1000 patient-days of support (95% confidence interval [95%CI], 27.6-41.4 episodes/1000 patient-days). The cumulative probability of infection by MCS duration, estimated using the Kaplan-Meier method, is shown in figure 2.
Infection foci and causative agentsMicrobiological confirmation was obtained by culture in 78 (76.5%) of the 102 nosocomial infection episodes; in 14 of them (17.9%), the etiology was polymicrobial. Among the 24 cases of suspected infection without microbiological confirmation, 11 were attributed to a probable respiratory focus based on clinical data (figure 1).
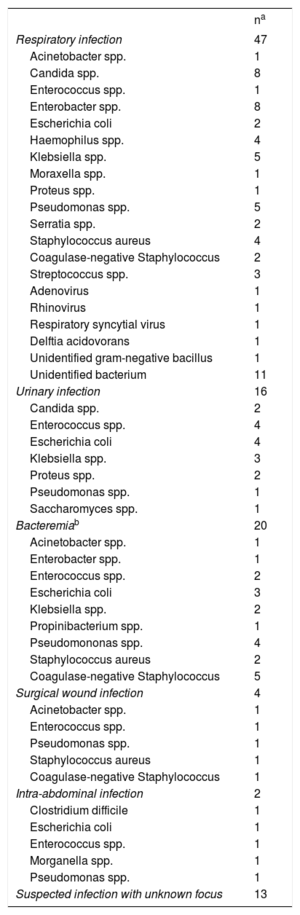
In total, 100 causative agents were identified: 58 cases (58%) of Gram-negative bacteria, 28 (28%) of Gram-positive bacteria, 11 (11%) of fungi, and 3 (3%) of viruses. Information on the causative agents isolated is provided in table 1.
Infection foci and pathogens isolated in cultures. The number of infection episodes recorded for each focus is represented by n
| na | |
|---|---|
| Respiratory infection | 47 |
| Acinetobacter spp. | 1 |
| Candida spp. | 8 |
| Enterococcus spp. | 1 |
| Enterobacter spp. | 8 |
| Escherichia coli | 2 |
| Haemophilus spp. | 4 |
| Klebsiella spp. | 5 |
| Moraxella spp. | 1 |
| Proteus spp. | 1 |
| Pseudomonas spp. | 5 |
| Serratia spp. | 2 |
| Staphylococcus aureus | 4 |
| Coagulase-negative Staphylococcus | 2 |
| Streptococcus spp. | 3 |
| Adenovirus | 1 |
| Rhinovirus | 1 |
| Respiratory syncytial virus | 1 |
| Delftia acidovorans | 1 |
| Unidentified gram-negative bacillus | 1 |
| Unidentified bacterium | 11 |
| Urinary infection | 16 |
| Candida spp. | 2 |
| Enterococcus spp. | 4 |
| Escherichia coli | 4 |
| Klebsiella spp. | 3 |
| Proteus spp. | 2 |
| Pseudomonas spp. | 1 |
| Saccharomyces spp. | 1 |
| Bacteremiab | 20 |
| Acinetobacter spp. | 1 |
| Enterobacter spp. | 1 |
| Enterococcus spp. | 2 |
| Escherichia coli | 3 |
| Klebsiella spp. | 2 |
| Propinibacterium spp. | 1 |
| Pseudomononas spp. | 4 |
| Staphylococcus aureus | 2 |
| Coagulase-negative Staphylococcus | 5 |
| Surgical wound infection | 4 |
| Acinetobacter spp. | 1 |
| Enterococcus spp. | 1 |
| Pseudomonas spp. | 1 |
| Staphylococcus aureus | 1 |
| Coagulase-negative Staphylococcus | 1 |
| Intra-abdominal infection | 2 |
| Clostridium difficile | 1 |
| Escherichia coli | 1 |
| Enterococcus spp. | 1 |
| Morganella spp. | 1 |
| Pseudomonas spp. | 1 |
| Suspected infection with unknown focus | 13 |
The most frequent sites of infection were the respiratory tract (n = 47; 46.1%) and urinary tract (n = 16; 15.6%). In total, 20 episodes of bacteremia were documented: 11 related to central line infection, 3 to MCS device infection, and 6 to primary bacteremias or bacteremias of unknown origin. In addition, another 5 patients showed bloodstream dissemination from a primary infection focus. Four patients had superficial infections from the surgical wound: 3 patients had venoarterial ECMO with peripheral cannulation and 1 had VAD implanted via sternotomy.
Of the 89 infection episodes whose focus could be identified, 3 (3.4%) met the criteria to be classified as MCS-specific infections, 21 (23.6%) as MCS-related infections, and 65 (73%) as non-MCS infections, in agreement with the nomenclature recommended by the International Society for Heart and Lung Transplantation.5
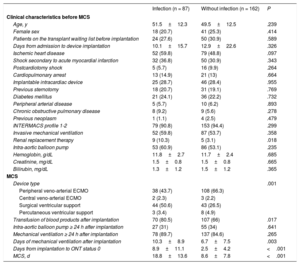
Clinical characteristics of patients with and without nosocomial infectionTable 2 shows the clinical characteristics of patients by whether they experienced an infection during the MCS period. The group of patients who had an infection exhibited a higher frequency of surgical VADs and a lower frequency of ECMO, a greater need for renal replacement therapy before device implantation, a greater need for periprocedural blood product transfusion, and a longer duration of mechanical ventilation after device implantation.
Comparison of the clinical characteristics of the study patients based on the presence or absence of mechanical circulatory support-related infections
| Infection (n = 87) | Without infection (n = 162) | P | |
|---|---|---|---|
| Clinical characteristics before MCS | |||
| Age, y | 51.5±12.3 | 49.5±12.5 | .239 |
| Female sex | 18 (20.7) | 41 (25.3) | .414 |
| Patients on the transplant waiting list before implantation | 24 (27.6) | 50 (30.9) | .589 |
| Days from admission to device implantation | 10.1±15.7 | 12.9±22.6 | .326 |
| Ischemic heart disease | 52 (59.8) | 79 (48.8) | .097 |
| Shock secondary to acute myocardial infarction | 32 (36.8) | 50 (30.9) | .343 |
| Postcardiotomy shock | 5 (5.7) | 16 (9.9) | .264 |
| Cardiopulmonary arrest | 13 (14.9) | 21 (13) | .664 |
| Implantable intracardiac device | 25 (28.7) | 46 (28.4) | .955 |
| Previous sternotomy | 18 (20.7) | 31 (19.1) | .769 |
| Diabetes mellitus | 21 (24.1) | 36 (22.2) | .732 |
| Peripheral arterial disease | 5 (5.7) | 10 (6.2) | .893 |
| Chronic obstructive pulmonary disease | 8 (9.2) | 9 (5.6) | .278 |
| Previous neoplasm | 1 (1.1) | 4 (2.5) | .479 |
| INTERMACS profile 1-2 | 79 (90.8) | 153 (94.4) | .299 |
| Invasive mechanical ventilation | 52 (59.8) | 87 (53.7) | .358 |
| Renal replacement therapy | 9 (10.3) | 5 (3.1) | .018 |
| Intra-aortic balloon pump | 53 (60.9) | 86 (53.1) | .235 |
| Hemoglobin, g/dL | 11.8±2.7 | 11.7±2.4 | .685 |
| Creatinine, mg/dL | 1.5±0.8 | 1.5±0.8 | .665 |
| Bilirubin, mg/dL | 1.3±1.2 | 1.5±1.2 | .365 |
| MCS | |||
| Device type | .001 | ||
| Peripheral veno-arterial ECMO | 38 (43.7) | 108 (66.3) | |
| Central veno-arterial ECMO | 2 (2.3) | 3 (2.2) | |
| Surgical ventricular support | 44 (50.6) | 43 (26.5) | |
| Percutaneous ventricular support | 3 (3.4) | 8 (4.9) | |
| Transfusion of blood products after implantation | 70 (80.5) | 107 (66) | .017 |
| Intra-aortic balloon pump ≥ 24 h after implantation | 27 (31) | 55 (34) | .641 |
| Mechanical ventilation ≥ 24 h after implantation | 78 (89.7) | 137 (84.6) | .265 |
| Days of mechanical ventilation after implantation | 10.3±8.9 | 6.7±7.5 | .003 |
| Days from implantation to ONT status 0 | 8.9±11.1 | 2.5±4.2 | <.001 |
| MCS, d | 18.8±13.6 | 8.6±7.8 | <.001 |
ECMO, extracorporeal membrane oxygenation; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; MCS, mechanical circulatory support.
Values represent No. (%) or mean ± standard deviation.
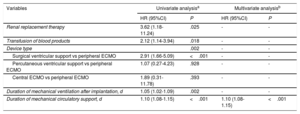
On multivariate logistic regression analysis (table 3), the only variable showing a significant and independent association with infection risk during MCS was the duration of support (for each day of support: odds ratio = 1.11; 95%CI, 1.08-1.15; P < .001).
Risk factors for short-term mechanical circulatory support-related infection: logistic regression analysis
| Variables | Univariate analysisa | Multivariate analysisb | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Renal replacement therapy | 3.62 (1.18-11.24) | .025 | - | - |
| Transfusion of blood products | 2.12 (1.14-3.94) | .018 | - | - |
| Device type | .002 | - | - | |
| Surgical ventricular support vs peripheral ECMO | 2.91 (1.66-5.09) | <.001 | - | - |
| Percutaneous ventricular support vs peripheral ECMO | 1.07 (0.27-4.23) | .928 | - | - |
| Central ECMO vs peripheral ECMO | 1.89 (0.31-11.78) | .393 | - | - |
| Duration of mechanical ventilation after implantation, d | 1.05 (1.02-1.09) | .002 | - | - |
| Duration of mechanical circulatory support, d | 1.10 (1.08-1.15) | <.001 | 1.10 (1.08-1.15) | <.001 |
95%CI, 95% confidence interval; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio.
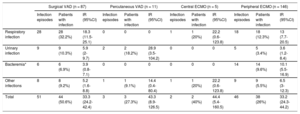
The cumulative infection incidence rates during the MCS period in patients with surgically implanted VAD, percutaneous VAD, central ECMO, and peripheral ECMO were 50.6%, 27.3%, 40%, and 26%, respectively (P = .002). The higher cumulative infection incidence observed in patients treated with a surgically implanted VAD was largely due to a higher number of respiratory infections (table 4).
Cumulative incidences and incidence rates of the main infection foci by type of mechanical circulatory support device
| Surgical VAD (n = 87) | Percutaneous VAD (n = 11) | Central ECMO (n = 5) | Peripheral ECMO (n = 146) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection episodes | Patients with infection | IR (95%CI) | Infection episodes | Patients with infection | IR (95%CI) | Infection episodes | Patients with infection | IR (95%CI) | Infection episodes | Patients with infection | IR (95%CI) | |
| Respiratory infection | 28 | 28 (32.2%) | 18.3 (11.5-25.1) | 0 | 0 | 0 | 1 | 1 (20%) | 22.2 (0.6-123.8) | 18 | 18 (12.3%) | 13 (7.7-20.5) |
| Urinary infection | 9 | 9 (10.3%) | 5.9 (2-9.7) | 2 | 2 (18.2%) | 28.9 (3.5-104.2) | 0 | 0 | 0 | 5 | 5 (3.4%) | 3.6 (1.2-8.4) |
| Bacteremia* | 6 | 6 (6.9%) | 3.9 (0.8-7.1) | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 14 (9.6%) | 10.1 (5.5-16.9) |
| Other infections | 8 | 8 (9.2%) | 5.2 (1.6-8.8) | 1 | 1 (9.1%) | 14.4 (0.4-80.4) | 1 | 1 (20%) | 22.2 (0.6-123.8) | 9 | 9 (5.5%) | 6.5 (3-12.3) |
| Total | 51 | 44 (50.6%) | 33.3 (24.2-42.4) | 3 | 3 (27.3%) | 43.3 (8.9-126.5) | 2 | 2 (40%) | 44.4 (5.4-160.5) | 46 | 38 (26%) | 33.2 (24.3-44.2) |
95%CI, 95% confidence interval; ECMO, extracorporeal membrane oxygenation; IR, incidence rate (episodes/1000 patient-days); VAD, ventricular assist device.
The mean duration of MCS was significantly longer in patients with a surgically implanted VAD (17.6 ± 14.5 days) than in patients with peripheral ECMO (9.5 ± 7.8 days), central ECMO (9 ± 7.5 days), and percutaneous VAD (6.3 ± 5.8 days) (P < .001). The infection incidence rate during MCS was similar in the 4 groups of patients, as shown in table 4 (surgically implanted VAD, 33.3 episodes/1000 patient-days; percutaneous VAD, 43.3/1000 patient-days; central ECMO, 44.4/1000 patient-days; peripheral ECMO, 33.2/1000 patient-days; P > .05 for all between-group comparisons).
Clinical impact of infectionDuring the MCS period, 202 patients (81.1%) received a transplant while 42 (16.9%) died without HTx. In addition, 2 patients (0.8%) received a long-term MCS, whereas 3 (1.2%) were weaned from the MCS due to recovery and were managed with medication alone.
Patients who had infectious complications exhibited higher mortality during the MCS period (25.3% vs 12.3%; P = .009) and a lower probability of undergoing HTx (73.6% vs 85.2%; P = .025). The infection was judged, according to the researcher's criteria, to be the main cause of death in 6 cases. The mortality rates during the MCS period were 27.7%, 25%, 30%, and 26.3% in patients who had respiratory infections, urinary infections, bacteremias, and other infectious symptoms, respectively.
After multivariable adjustment by age, sex, INTERMACS clinical profile, type of MCS device, and type of underlying heart disease, infectious complications were independently associated with mortality during the MCS period (odds ratio = 2.47; 95%CI, 1.21-5.05; P = .013).
Patients with infections during the MCS period also had a higher cumulative incidence of renal failure requiring renal replacement therapy (24.1% vs 14.2%; P = .05) and a higher cumulative incidence of thromboembolic complications (17.6% vs 6.1%; P = .025). The cumulative incidence of bleeding complications was numerically but nonsignificantly higher in patients with infection (46% vs 34.6%; P = .078).
Heart transplantIn total, 202 patients (81.1%) received a HTx after a mean wait of 7.2 ± 7.2 days. Of these patients, 64 (31.7%) had experienced infectious complications during the MCS period.
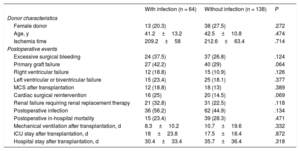
No significant differences were seen in terms of in-hospital postoperative mortality between patients with and without a history of infectious complications during the MCS period (with infection, 23.4%; without infection, 28.3%; P = .471) (table 5). Neither were significant differences detected between the 2 groups of patients regarding the incidence of other adverse clinical events during the postoperative hospitalization period after HTx.
Characteristics of donors and adverse clinical outcomes during the in-hospital postoperative period in the 202 study patients transplanted according to the presence or absence of mechanical circulatory support-related infection
| With infection (n = 64) | Without infection (n = 138) | P | |
|---|---|---|---|
| Donor characteristics | |||
| Female donor | 13 (20.3) | 38 (27.5) | .272 |
| Age, y | 41.2±13.2 | 42.5±10.8 | .474 |
| Ischemia time | 209.2±58 | 212.6±63.4 | .714 |
| Postoperative events | |||
| Excessive surgical bleeding | 24 (37.5) | 37 (26.8) | .124 |
| Primary graft failure | 27 (42.2) | 40 (29) | .064 |
| Right ventricular failure | 12 (18.8) | 15 (10.9) | .126 |
| Left ventricular or biventricular failure | 15 (23.4) | 25 (18.1) | .377 |
| MCS after transplantation | 12 (18.8) | 18 (13) | .389 |
| Cardiac surgical reintervention | 16 (25) | 20 (14.5) | .069 |
| Renal failure requiring renal replacement therapy | 21 (32.8) | 31 (22.5) | .118 |
| Postoperative infection | 36 (56.2) | 62 (44.9) | .134 |
| Postoperative in-hospital mortality | 15 (23.4) | 39 (28.3) | .471 |
| Mechanical ventilation after transplantation, d | 8.3±10.2 | 10.7±19.6 | .332 |
| ICU stay after transplantation, d | 18±23.8 | 17.5±18.4 | .872 |
| Hospital stay after transplantation, d | 30.4±33.4 | 35.7±36.4 | .318 |
ICU, intensive care unit; MCS, mechanical circulatory support.
Values represent No. (%) or mean ± standard deviation.
This article presents a systematic description of the infectious complications associated with short-term MCS devices based on a Spanish multicenter registry of urgent HTx candidates. In our cohort, 34.9% of the patients had infectious complications during a mean support duration of 12 days, giving an infection incidence rate of 33.8 cases per 1000 patient-days of support.
Few data in the literature concern the incidence of nosocomial infection in patients requiring short-term MCS; in addition, most of the available data are derived from studies examining a single type of device. The reported cumulative incidence of nosocomial infection ranges from 9% to 65% in patients treated with ECMO,3,6–12 from 24% to 59% in those treated with surgical VAD,4,13 and from 12.9% to 35.3% in those treated with percutaneous VAD.14,15 This variability is explained by the significant heterogeneity of the abovementioned studies in terms of their criteria for defining infection and the rigor with which infections were recorded, as well as by the different clinical characteristics and severity of the patients treated and the different durations of support. Nonetheless, and with all due reservations, we can conclude that the incidence of infections observed in our series is generally within the expected range.
Device typeIn our work, we found no major impact of the type of short-term MCS device used (central ECMO, peripheral ECMO, percutaneous VAD, or surgical VAD) on the risk of nosocomial infection. The higher cumulative incidence of infections observed in the subgroup of patients treated with surgical VAD is solely attributable to a longer duration of support in these patients, as indicated by the absence of significant differences in the infection incidence rates by type of MCS device used. In addition, the type of MCS device was also not an independent predictor of infection risk in a multivariate logistic regression model; in this analysis, only the duration of MCS maintained a significant association with infection risk.
As in our work, other studies8,15–17 have found that the duration of support is probably the main determinant of infection risk in patients treated with short-term MCS. In contrast to other adverse clinical events, such as stroke and reintervention for bleeding, which tend to present in the first few days after device implantation, the timeframe of nosocomial infection episodes is more gradual and exhibits a delayed plateau, with the episodes dispersed throughout the entire support period.4
EpidemiologyThe epidemiological characteristics of the infectious complications described in our cohort are in line with the standard nosocomial infection pathology of critically ill patients requiring a high degree of instrumentation and life support measures.18 As in other series,4,12 most infections were attributed to foci of respiratory origin, which is in agreement with the elevated frequency of invasive mechanical ventilation and the difficult weaning of many patients.
The microbiological profile of the causative agents associated with MCS devices in our cohort was as expected for nosocomially acquired symptoms in patients with prolonged hospital stays, whose etiology is determined by the hospital flora.19 Among the bacterial agents, Gram-negative bacteria predominated, regardless of the focus, with this microbiological group present in more than half of isolates, similar to that seen by other authors.12,16 Gram-positive bacteria were also relatively frequent, mainly Staphylococcus spp., particularly in isolates from patients with bacteremia or respiratory infections. About 1 in every 10 isolates corresponded to Candida spp., again, particularly in the respiratory pathway. Although identification of the cause of this pathogen can be difficult, other authors have also described a significant incidence of Candida spp. infections in patients treated with ECMO.9
Prognostic impact of infectionA pertinent finding of our work, also reported previously,12 is the elevated mortality in patients who had MCS-related nosocomial infections. This increased risk of death was independent of baseline clinical characteristics, device type, and the patient's profile according to the INTERMACS scale. In addition, the infection episodes tended to occur in patients who also had a higher incidence of other associated complications, such as thromboembolic events and renal failure. The design of our study precludes establishment of a temporal association between the different adverse clinical events; nonetheless, other authors have observed a similar aggregation of complications in these patients and have even presented hierarchical models that better illustrate the complex causal relationships among them.20
A distinctive characteristic of our study is that, per protocol, it exclusively included urgent HTx candidates. Thus, an additional consequence of the onset of infectious complications was loss of the chance to receive an organ. Active infection is an absolute contraindication for HTx,21 at least until it is controlled; for this reason, these patients are often temporarily excluded from the urgent waiting list and lose priority placement. A French multicenter study revealed that, in patients with a similar profile to those of our series, performance of HTx is itself a variable with a major impact on survival22 because the possibility of weaning from MCS due to recovery is rare in this situation.
Finally, history of nosocomial infection was not associated with a significant increase in mortality or the incidence of postoperative complications after HTx in patients who did eventually receive the organ. In our opinion, this observation is probably explained by a selection bias; it is reasonable to suppose that patients who ultimately undergo HTx generally have successfully overcome the active phase of the infectious complication.
Implications for clinical practiceThe results of our study are relevant for routine clinical practice and highlight the importance of stricter prophylactic, surveillance, and early treatment protocols for infectious complications in patients treated with short-term MCS devices. Nonetheless, we believe that transplant teams will have to consider the matter more deeply in coming years. It is likely that the growing increase in waiting times for HTx will lead to ever longer support times and, consequently, a higher risk of MCS-related complications and prolonged critical care unit stays. In this regard, early implantation of a long-term VAD as a bridge to HTx becomes an attractive option that would enable the stabilization of some candidates and their transfer to an outpatient waiting regimen.
LimitationsThis study has some limitations. First, the work is based on an analysis of secondary data collected from a multicenter registry whose main objective was not the focus of this article. The determination of infection episodes was the responsibility of the local researchers, in agreement with the predefined criteria, and the episodes were not confirmed by an independent committee. In addition, some of the heterogeneity in the results may be explained by local differences in management protocols for MCS devices, including those related to the diagnosis and treatment of infectious complications, as well as in selection criteria for recipients of urgent HTx. The lack of information on the type and duration of the prophylactic antibiotics used during the peri-implantation period is a pertinent limitation that complicates the epidemiological interpretation of the microbiological findings. Finally, the exclusion of patients who required multiple different mechanical devices may have introduced a selection bias that would lead to underestimation of the incidence of MCS-related infection, given that these patients constitute a subpopulation with elevated risk.
CONCLUSIONSPatients treated with short-term MCS devices as a bridge to HTx are exposed to a high risk of infectious complications specific to critically ill patients, such as respiratory infections, urinary infections, and bacteremias. These types of complications decrease the probability that the patient receives the transplant and are associated with elevated mortality in patients on organ waiting lists. The results of our study reveal the need for stricter prophylactic, surveillance, and early treatment protocols for infectious complications in candidates for urgent HTx.
FUNDINGThe present study was supported by a Health Research Grant from Fundación Mutua Madrileña, XI Call for Grants, Madrid, 2014.
CONFLICTS OF INTERESTE. Barge-Caballero received an academic grant from Abbot Vascular to pursue a postgraduate course in heart failure (University of Zurich) in the 2016 to 2017 period.
- -
There is abundant literature on the epidemiological characteristics of infections associated with long-term mechanical circulatory support devices; nonetheless, little information is available on the infections associated with short-term devices.
- -
The implications of the infections related to short-term mechanical circulatory support in candidates for urgent heart transplantation are unknown.
- -
This study provides detailed information on the epidemiological characteristics and causative agents of infectious complications in patients treated with short-term mechanical circulatory support devices.
- -
This work highlights the negative prognostic impact of infectious complications associated with mechanical circulatory support in candidates for urgent heart transplant.