There have been no analyses of the influence of cardiovascular risk as a predictor of events in patients with exercise echocardiography (EE) without ischemia. Our objective was to determine the predictors of cardiac events, paying special attention to cardiovascular risk.
MethodsThis study included 1640 patients with EE without ischemia. Of these, there were 1206 with no previously known coronary artery disease (CAD), whose risk of a fatal cardiovascular disease event was estimated according to the European SCORE (Systematic COronary Risk Evaluation) risk assessment system, and 434 with known CAD. The primary endpoint was cardiac event-free survival (EFS) (cardiac death, nonfatal acute coronary syndrome, and coronary revascularization).
ResultsAfter a median follow-up of 35 [23-54] months, no differences were found in cardiac EFS between patients with a SCORE ≥ 10 or diabetes and patients with previous CAD (89.8% vs 87.1%). In the first year, cardiac EFS was high in all groups (99.4% if SCORE < 5; 100% if 5-9; 98% if ≥ 10 or diabetes and 97% in patients with CAD). In the third year, cardiac EFS was similar in the group with SCORE ≥ 10 or diabetes (94.5%) and patients with CAD (91.1%, P = NS). In these patients, the annualized event rate was 2.8% and 2.55%, respectively, and was significantly higher than in groups with SCORE < 5 (0.6%) and SCORE 5-9 (0.12%). The most frequent events were non—ST-segment elevation acute coronary syndrome and late revascularization. Predictors of cardiac events were previous CAD, SCORE ≥ 10 or diabetes mellitus, creatinine clearance, left ventricular ejection fraction, and chest pain during EE.
ConclusionsInitial outcome after an EE without ischemia is favorable but is subsequently modulated by cardiovascular risk.
Keywords
Exercise echocardiography (EE) is the most physiological imaging technique for the detection of ischemia and offers advantages such as its low cost and absence of radiation. Although its limited diagnostic value has been questioned compared with anatomical techniques,1,2 its high prognostic value has been demonstrated in various clinical settings.3–9 Several advances have facilitated its implementation (second harmonic, continuous acquisition, simultaneous comparison of baseline with exercise, or the use of contrast). Indications and training for its use have been established by expert consensus.10 The aim of this study was to identify the predictors of events in patients with EE without inducible ischemia, paying special attention to cardiovascular risk (CVR) and its possible role in modifying the risk of cardiac events.
METHODSAn observational retrospective cohort study was conducted with 2307 consecutive patients with EE using a Bruce or Naughton treadmill with 12-lead electrocardiogram monitoring. Image acquisition was performed in 4-, 3-, and 2-chamber views and short-axis orientations or in 5-chamber view (depending on the quality of the parasternal window or the use of contrast) at baseline and immediately after exercise or peak exercise with continuous acquisition, side-by-side comparison, and digital storage. Contractile abnormalities were assessed in a 16-segment left ventricular model. Contrast was used in 329 patients (20.1%). Inclusion criteria were age > 18 years and EE without induced ischemia (ie, without new segmental contractile abnormalities with exercise compared with the patient at rest, regardless of baseline abnormality in patients with heart disease). EE testing without inducible ischemia was based on segmental contractility and not on clinical or electrocardiographic abnormalities. Positive, doubtful, or inconclusive echocardiographic studies (noninterpretable due to poor window or defective acquisition) were excluded. There were no fatal complications. Demographic data, clinical variables, and data derived from EE were collected.
Known coronary artery disease (CAD) was defined as a history of myocardial infarction, previous percutaneous or surgical revascularization, or severe coronary lesion on previous angiography. In patients without CAD and without diabetes mellitus (DM), CVR was estimated according to the European SCORE (Systematic Coronary Risk Evaluation) scale calibrated for the Spanish population for a risk of cardiovascular death at 10 years by age, sex, systolic blood pressure, total cholesterol, and smoking.11 Patients without CAD were classified into 3 groups: SCORE < 5% (low-medium risk), 5% to 9% (high risk) and ≥ 10% or DM (very high risk). Follow-up was conducted by electronic and paper history, telephone call, and death register.
The primary outcome variable was the composite of fatal event (sudden death of unexplained origin and cardiac death due to heart failure or acute coronary syndrome [ACS]), nonfatal ACS (due to ACS with or without ST-segment elevation), and late revascularization. Acute coronary syndrome was defined as symptoms or ECG abnormalities compatible with ischemia with increased cardiac markers (troponin T). Late revascularization refers to that performed during follow-up, rather than to early revascularization with cardiac catheterization, despite normal EE. “Short-term prognosis” was arbitrarily defined as 1 year after EE and “long-term prognosis” as 3 years after EE.
Statistical AnalysisIn the descriptive analysis, categorical variables are expressed as frequencies and percentages and continuous variables are expressed as mean ± standard deviation. The descriptive analysis was performed using the total sample and each CVR group. The association between the variable CVR and the primary endpoint variable and the types of events that comprised it was assessed. The annualized event rate and the log-rank test were used to compare events between groups. Multiple comparisons were adjusted using the Bonferroni correction. In the univariable analysis, univariable Cox regression models were used to measure the association between the study variables and the primary outcome variable. All variables that were significant at a level of .20 were considered to be potential independent variables for the multivariable survival model (Cox regression). The final predictors were variables statistically significant at a level of .05. At follow-up, the model was calibrated using the Hosmer-Lemeshow test and its predictive capacity was assessed by calculating the area under the ROC (Receiver Operating Characteristic) curve. Kaplan-Meier curves were used to measure the survival of patients with negative EE by risk group. This procedure was conducted using the log-rank test. All effects were considered statistically significant at a P value of < .05. All analyses were conducted using the SAS software package, version 9.4 (SAS Institute Inc). Figures were constructed using R v3.0.1.
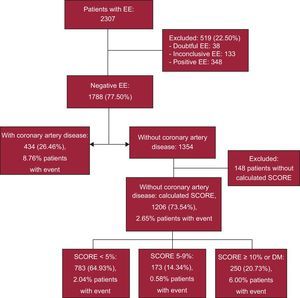
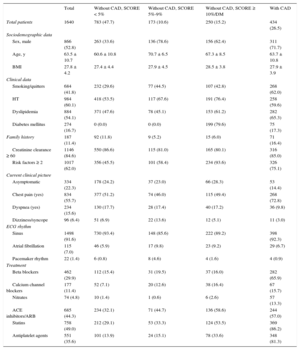
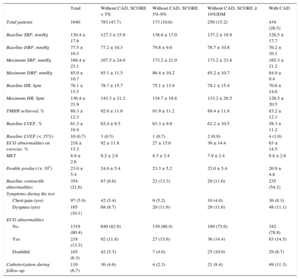
RESULTSA total of 2307 EE studies were conducted between January 2007 and December 2012, leading to the exclusion of 348 patients with a positive EE and 171 with doubtful or inconclusive EE. Of the remaining, we included 1788 patients without ischemia, only 1640 (434 with CAD and 1206 without CAD for whom data were available for European SCORE calculation). Mean age was 63.5 ± 10.7 years and 52.8% were men. Of the 1206 patients without CAD, there were 783 with SCORE < 5% (65%), 173 with SCORE 5% to 9% (14.3%), and 250 with SCORE ≥ 10%, or 250 with DM (20.7%) (Figure 1). Of the 434 CAD patients, 242 had a previous history of myocardial infarction and 356 of percutaneous or surgical revascularization. Demographic and clinical characteristics and pharmacological treatment are shown in Table 1. The EE data are shown in Table 2. Of the patients, 21.6% had contractile abnormalities at baseline. Obviously, the segmental contractility index did not increase during EE without ischemia. Mean left ventricular ejection fraction (LVEF) was 61.3% ± 10.4%. Of the 1640 study patients, 84 (5.12%) had an LVEF < 50% and less than 1% had an LVEF < 35%.
Demographic and Clinical Characteristics of the Total Group and According to Cardiovascular Risk
| Total | Without CAD, SCORE < 5% | Without CAD, SCORE 5%-9% | Without CAD, SCORE ≥ 10%/DM | With CAD | |
|---|---|---|---|---|---|
| Total patients | 1640 | 783 (47.7) | 173 (10.6) | 250 (15.2) | 434 (26.5) |
| Sociodemographic data | |||||
| Sex, male | 866 (52.8) | 263 (33.6) | 136 (78.6) | 156 (62.4) | 311 (71.7) |
| Age, y | 63.5 ± 10.7 | 60.6 ± 10.8 | 70.7 ± 6.5 | 67.3 ± 8.5 | 63.7 ± 10.8 |
| BMI | 27.8 ± 4.2 | 27.4 ± 4.4 | 27.9 ± 4.5 | 28.5 ± 3.8 | 27.9 ± 3.9 |
| Clinical data | |||||
| Smoking/quitters | 684 (41.8) | 232 (29.6) | 77 (44.5) | 107 (42.8) | 268 (62.0) |
| HT | 984 (60.1) | 418 (53.5) | 117 (67.6) | 191 (76.4) | 258 (59.6) |
| Dyslipidemia | 884 (54.1) | 371 (47.6) | 78 (45.1) | 153 (61.2) | 282 (65.3) |
| Diabetes mellitus | 274 (16.7) | 0 (0.0) | 0 (0.0) | 199 (79.6) | 75 (17.3) |
| Family history | 187 (11.4) | 92 (11.8) | 9 (5.2) | 15 (6.0) | 71 (16.4) |
| Creatinine clearance ≥ 60 | 1146 (84.6) | 550 (86.6) | 115 (81.0) | 165 (80.1) | 316 (85.0) |
| Risk factors ≥ 2 | 1017 (62.0) | 356 (45.5) | 101 (58.4) | 234 (93.6) | 326 (75.1) |
| Current clinical picture | |||||
| Asymptomatic | 334 (22.3) | 178 (24.2) | 37 (23.0) | 66 (28.3) | 53 (14.4) |
| Chest pain (yes) | 834 (55.7) | 377 (51.2) | 74 (46.0) | 115 (49.4) | 268 (72.8) |
| Dyspnea (yes) | 234 (15.6) | 130 (17.7) | 28 (17.4) | 40 (17.2) | 36 (9.8) |
| Dizziness/syncope | 96 (6.4) | 51 (6.9) | 22 (13.6) | 12 (5.1) | 11 (3.0) |
| ECG rhythm | |||||
| Sinus | 1498 (91.6) | 730 (93.4) | 148 (85.6) | 222 (89.2) | 398 (92.3) |
| Atrial fibrillation | 115 (7.0) | 46 (5.9) | 17 (9.8) | 23 (9.2) | 29 (6.7) |
| Pacemaker rhythm | 22 (1.4) | 6 (0.8) | 8 (4.6) | 4 (1.6) | 4 (0.9) |
| Treatment | |||||
| Beta blockers | 462 (29.9) | 112 (15.4) | 31 (19.5) | 37 (16.0) | 282 (65.9) |
| Calcium channel blockers | 177 (11.4) | 52 (7.1) | 20 (12.6) | 38 (16.4) | 67 (15.7) |
| Nitrates | 74 (4.8) | 10 (1.4) | 1 (0.6) | 6 (2.6) | 57 (13.3) |
| ACE inhibitors/ARB | 685 (44.3) | 234 (32.1) | 71 (44.7) | 136 (58.6) | 244 (57.0) |
| Statins | 758 (49.0) | 212 (29.1) | 53 (33.3) | 124 (53.5) | 369 (86.2) |
| Antiplatelet agents | 551 (35.6) | 101 (13.9) | 24 (15.1) | 78 (33.6) | 348 (81.3) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; ECG, electrocardiogram; HT, hypertension; SCORE, Systematic COronary Risk Evaluation.
Values are expressed as no. (%) or mean ± standard deviation.
Data Derived From Exercise Echocardiography and Catheterization in the Total Group During Follow-up and According to Cardiovascular Risk
| Total | Without CAD, SCORE < 5% | Without CAD, SCORE 5%-9% | Without CAD, SCORE ≥ 10%/DM | With CAD | |
|---|---|---|---|---|---|
| Total patients | 1640 | 783 (47.7) | 173 (10.6) | 250 (15.2) | 434 (26.5) |
| Baseline SBP, mmHg | 130.4 ± 17.6 | 127.3 ± 15.9 | 138.8 ± 17.0 | 137.2 ± 18.9 | 128.5 ± 17.7 |
| Baseline DBP, mmHg | 77.5 ± 10.3 | 77.2 ± 10.3 | 79.8 ± 9.6 | 78.7 ± 10.8 | 76.2 ± 10.1 |
| Maximum SBP, mmHg | 168.4 ± 23.1 | 167.5 ± 24.0 | 173.2 ± 21.0 | 173.2 ± 23.8 | 165.3 ± 21.2 |
| Maximum DBP, mmHg | 85.0 ± 10.7 | 85.1 ± 11.5 | 86.4 ± 10.2 | 85.2 ± 10.7 | 84.0 ± 9.4 |
| Baseline HR, bpm | 76.1 ± 15.5 | 78.7 ± 15.7 | 75.1 ± 13.9 | 78.1 ± 15.4 | 70.6 ± 14.6 |
| Maximum HR, bpm | 136.4 ± 21.9 | 143.3 ± 21.2 | 134.7 ± 19.8 | 133.2 ± 20.5 | 126.5 ± 20.5 |
| TMHR achieved, % | 89.3 ± 12.3 | 92.0 ± 11.6 | 91.9 ± 11.2 | 89.4 ± 11.8 | 83.2 ± 12.1 |
| Baseline LVEF, % | 61.3 ± 10.4 | 62.4 ± 9.5 | 63.3 ± 9.9 | 62.2 ± 10.5 | 58.3 ± 11.2 |
| Baseline LVEF (< 35%) | 10 (0.7) | 3 (0.5) | 1 (0.7) | 2 (0.9) | 4 (1.0) |
| ECG abnormalities on exercise, % | 218 ± 13.3 | 92 ± 11.8 | 27 ± 15.6 | 36 ± 14.4 | 63 ± 14.5 |
| MET | 8.8 ± 2.6 | 9.2 ± 2.6 | 8.3 ± 2.4 | 7.9 ± 2.4 | 8.8 ± 2.6 |
| Double product (× 103) | 23.0 ± 5.4 | 24.0 ± 5.4 | 23.3 ± 5.2 | 23.0 ± 5.4 | 20.9 ± 4.8 |
| Baseline contractile abnormalities | 354 (21.6) | 67 (8.6) | 23 (13.3) | 29 (11.6) | 235 (54.2) |
| Symptoms during the test | |||||
| Chest pain (yes) | 97 (5.9) | 42 (5.4) | 9 (5.2) | 10 (4.0) | 36 (8.3) |
| Dyspnea (yes) | 165 (10.1) | 68 (8.7) | 20 (11.6) | 29 (11.6) | 48 (11.1) |
| ECG abnormalities | |||||
| No | 1319 (80.4) | 649 (82.9) | 139 (80.4) | 189 (75.6) | 342 (78.8) |
| Yes | 218 (13.3) | 92 (11.8) | 27 (15.6) | 36 (14.4) | 63 (14.5) |
| Doubtful | 103 (6.3) | 42 (5.3) | 7 (4.0) | 25 (10.0) | 29 (6.7) |
| Catheterization during follow-up | 110 (6.7) | 36 (4.6) | 4 (2.3) | 21 (8.4) | 49 (11.3) |
CAD, coronary heart disease; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiogram; HR, heart rate; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; SCORE, Systematic COronary Risk Evaluation; TMHR, theoretical maximum heart rate.
Values are expressed as no. (%) or mean ± standard deviation.
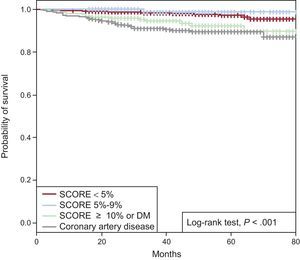
In patients with EE without ischemia after a median follow-up of 35 [23-54] months, event-free survival (EFS) estimated by Kaplan-Meier curves was 93.1% of the total group at the end of follow-up. Based on CVR, EFS was 95.4%, 98.7%, and 89.8% of patients with SCORE < 5%, 5% to 9%, or ≥ 10% or DM, respectively, with no significant differences at the end of follow-up between the patients with known CAD and those with SCORE ≥ 10 or DM (87.1% vs 89.8%; P = .193). In the 2 groups with higher CVR, cardiac EFS was significantly lower (P < .001) than in the 2 groups with SCORE < 10% (Figure 2).
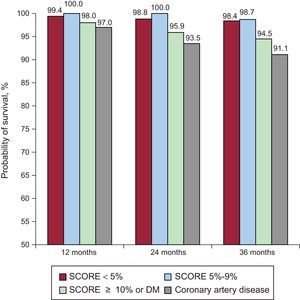
Event-free survival was assessed at different times during follow-up to determine if there had been an increase in risk after EE based on CVR. In patients without CAD, EFS was 99.2%, 98.4%, and 97.6% at 1, 2, and 3 years, respectively. Based on short-term CVR (1 year of follow-up), cardiac EFS was high in all groups (99.4% if SCORE < 5%, 100% if SCORE 5% to 9%, 98% if SCORE ≥ 10% or DM, and 97% with known CAD). At 2 years, cardiac EFS was 98.8%, 100%, 95.9%, and 93.5%, respectively. Based on long-term CVR (3 years of follow-up), cardiac EFS was similar in the 2 low-CVR groups (98.4% if SCORE < 5% and 98.7% if SCORE 5% to 9%; P = .40). However, in the group with SCORE ≥ 10% or DM, cardiac EFS at 3 ≥ years had significantly decreased compared with the group with SCORE < 5% (94.5%, P < .001) and was similar to that of known CAD patients (91.1%; P = .24) (Figure 3).
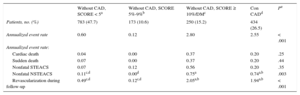
Event TypesOf the 1640 patients with EE without ischemia, 70 (4.3%) experienced at least 1 cardiac event. Mean time from EE to the first event in the total group was 18 (range, 6-30) months. The cardiac mortality rate was 0.12% (2 patients) in the first year and 0.79% (13 patients with fatal cardiac events) during overall follow-up. Among the nonfatal events, there were 27 (1.6%) cases of ACS (9 with ST-segment elevation and 18 without ST-segment elevation) and 54 (3.3%) cases of late revascularization during follow-up. Cardiac catheterization was performed during follow-up in 6.7% of patients and was significantly more frequent in the CAD group (11.3%) than in the 2 groups with SCORE < 10% (Table 2). During follow-up, 16.67% of patients underwent revascularization within 6 months of EE. Annualized event rates were 0.6%, 0.12%, 2.8%, and 2.55% in the 4 risk groups, respectively. Regarding the type of CVR-based events, there were no differences between groups in fatal events or nonfatal ST–segment-elevation ACS. Acute coronary syndrome was significantly more frequent in patients with SCORE ≥ 10% or DM than in patients with SCORE < 5% (annualized rate, 0.75% vs 0.11%; P < .008) and was similar to that in CAD patients (annualized rate, 0.74%). Late revascularization was significantly more frequent in patients with SCORE ≥ 10% or DM and in the CAD group than in the 2 groups at lower risk, but was similar in the first 2 groups (annualized rates of 2.05% and 1.94%, respectively) (Table 3).
Annualized Event Rate According to Cardiovascular Risk
| Without CAD, SCORE < 5a | Without CAD, SCORE 5%-9%b | Without CAD, SCORE ≥ 10%/DMc | Con CADd | Pe | |
|---|---|---|---|---|---|
| Patients, no. (%) | 783 (47.7) | 173 (10.6) | 250 (15.2) | 434 (26.5) | |
| Annualized event rate | 0.60 | 0.12 | 2.80 | 2.55 | < .001 |
| Annualized event rate: | |||||
| Cardiac death | 0.04 | 0.00 | 0.37 | 0.20 | .25 |
| Sudden death | 0.07 | 0.00 | 0.37 | 0.20 | .44 |
| Nonfatal STEACS | 0.07 | 0.12 | 0.56 | 0.20 | .35 |
| Nonfatal NSTEACS | 0.11c,d | 0.00d | 0.75a | 0.74a,b | .003 |
| Revascularization during follow-up | 0.49c,d | 0.12c,d | 2.05a,b | 1.94a,b | < .001 |
CAD, coronary heart disease; DM, diabetes mellitus; NSTEACS: non—ST-segment elevation acute coronary syndrome; SCORE, Systematic COronary Risk Evaluation; STEACS: ST-segment elevation acute coronary syndrome.
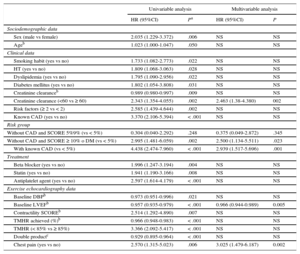
In the univariable analysis, predictors of events were age, male sex, classic CVR factors (smoking, dyslipidemia, hypertension, and DM). The risk of cardiac events was significantly increased by the presence of 2 or more CVR factors, or known CAD, or a SCORE ≥ 10% or DM in patients without CAD. The risk of cardiac events was also significantly increased by creatinine clearance < 60mL/min. A predictor of cardiac events was treatment with antiplatelet agents, beta-blockers, or statins. These treatments are common in patients with known CAD (81.3% with antiplatelet agents, 65.89% beta-blockers, and 86.21% with statins). Among the variables derived from EE, the risk of cardiac events was significantly higher in patients with chest pain during EE, a lower baseline LVEF, the lowest percentage of achieved maximum heart rate, and the lowest double product (Table 4). Electrocardiographic abnormalities during EE were not predictive of cardiac events. In the multivariable analysis, predictors of cardiac events were known CAD, a very high cardiovascular risk (SCORE ≥ 10 or DM), creatinine clearance < 60mL/min, low baseline LVEF, and chest pain during EE. Patients with CAD and those without CAD with SCORE ≥ 10% or DM were 2.9 (P = .001) and 2.5 (P = .023) times more at risk of cardiac events than patients with SCORE < 5%, respectively. Patients with creatinine clearance < 60mL/min had a 2.5-fold increased risk of cardiac events than those with clearance ≥ 60mL/min. Regarding the variables related to EE, a higher baseline LVEF decreased the risk of cardiac events (Table 4). The final model showed adequate goodness-of-fit (Hosmer-Lemeshow, P = .100) and adequate discrimination (area under the curve, 0.746; 95% confidence interval, 0.685-0.807).
Predictors of Events With Exercise Echocardiography Without Ischemia: Univariable and Multivariable Analysis
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95%CI) | Pa | HR (95%CI) | P | |
| Sociodemographic data | ||||
| Sex (male vs female) | 2.035 (1.229-3.372) | .006 | NS | NS |
| Ageb | 1.023 (1.000-1.047) | .050 | NS | NS |
| Clinical data | ||||
| Smoking habit (yes vs no) | 1.733 (1.082-2.773) | .022 | NS | NS |
| HT (yes vs no) | 1.809 (1.068-3.063) | .028 | NS | NS |
| Dyslipidemia (yes vs no) | 1.795 (1.090-2.956) | .022 | NS | NS |
| Diabetes mellitus (yes vs no) | 1.802 (1.054-3.808) | .031 | NS | NS |
| Creatinine clearanceb | 0.989 (0.980-0.997) | .009 | NS | NS |
| Creatinine clearance (<60 vs ≥ 60) | 2.343 (1.354-4.055) | .002 | 2.463 (1.38-4.380) | 002 |
| Risk factors (≥ 2 vs < 2) | 2.585 (1.439-4.644) | .002 | NS | NS |
| Known CAD (yes vs no) | 3.370 (2.106-5.394) | < .001 | NS | NS |
| Risk group | ||||
| Without CAD and SCORE 5%9% (vs < 5%) | 0.304 (0.040-2.292) | .248 | 0.375 (0.049-2.872) | .345 |
| Without CAD and SCORE ≥ 10% o DM (vs < 5%) | 2.995 (1.481-6.059) | .002 | 2.500 (1.134-5.511) | .023 |
| With known CAD (vs < 5%) | 4.438 (2.474-7.960) | < .001 | 2.939 (1.517-5.696) | .001 |
| Treatment | ||||
| Beta blocker (yes vs no) | 1.996 (1.247-3.194) | .004 | NS | NS |
| Statin (yes vs no) | 1.941 (1.190-3.166) | .008 | NS | NS |
| Antiplatelet agent (yes vs no) | 2.597 (1.614-4.179) | < .001 | NS | NS |
| Exercise echocardiography data | ||||
| Baseline DBPb | 0.973 (0.951-0.996) | .021 | NS | NS |
| Baseline LVEFb | 0.957 (0.935-0.979) | < .001 | 0.966 (0.944-0.989) | 0.005 |
| Contractility SCOREb | 2.514 (1.292-4.890) | .007 | NS | NS |
| TMHR achieved (%)b | 0.966 (0.948-0.983) | < .001 | NS | NS |
| TMHR (< 85% vs ≥ 85%) | 3.366 (2.092-5.417) | < .001 | NS | NS |
| Double productc | 0.929 (0.895-0.964) | < .001 | NS | NS |
| Chest pain (yes vs no) | 2.570 (1.315-5.023) | .006 | 3.025 (1.479-6.187) | 0.002 |
95%CI, 95% confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CAD, coronary artery disease; CCB, calcium channel blockers; DBP, diastolic blood pressure; DM, diabetes mellitus; ECG, electrocardiogram; HR, hazard ratio; HT, hypertension; LVEF, left ventricular ejection fraction; NS, nonsignificant in the multivariate model; SBP, systolic blood pressure; SCORE, Systematic COronary Risk Evaluation; TMHR, theoretical maximum heart rate.
P was calculated using univariable and multivariable Cox regression models. The variables chest pain, treatment with CCB, nitrates, and ACE inhibitors/ARB, baseline SBP, MET, ECG abnormalities, and dyspnea were also analyzed in the univariable analysis. None of these variables reached statistical significance (P > .05) For the multivariable model, the area under the curve = 0.746 (95%CI, 0.685 to 0.807; PHosmer-Lemeshow = .10) (both were estimated at the end of follow-up).
The results show that patients without CAD with EE without ischemia have excellent prognosis. McCully et al.12 estimated that overall survival at 1 year and 3 years was 99.5% and 98.6%, respectively, in 1325 patients with normal EE without CAD and ventricular dysfunction. Prognostic data were very similar (99.2% and 97.6% at 1 year and 3 years, respectively) in the 1206 patients without CAD. Therefore, the favorable prognostic value of EE without ischemia reported in our setting is similar to that of hospitals with extensive experience. Our results are comparable to those reported in a meta-analysis conducted by Metz et al.,13 which included 3021 patients with negative EE and whose cardiac death or myocardial infarction rate was 1.56% at a mean follow-up of 33 months. In the present study, the composite rate of cardiac or sudden death and nonfatal ACS was higher (2.3% after a median follow-up of 35 months). This disparity may be related to the follow-up time and the differential classification of acute myocardial infarction after troponins were included in the study.
The finding of predictors of cardiac events in patients with EE without ischemia is of special interest because such patients are frequently discharged from cardiology clinics. In other studies, most of the predictors of events in patients with negative EE were obtained from the test itself. Thus, a normal but submaximal EE (HR < 85%)14,15 or decreased functional capacity (< 7 MET in men or < 5 MET in women) entails a higher rate of events.8,16 In the univariable analysis, an achieved maximum heart rate < 85% was also a predictor of cardiac events. In line with previous studies,17 electrocardiographic abnormalities during the test were not predictive of events. In the multivariable analysis, chest pain during EE was the only predictor of cardiac events derived from the test. Other predictors in the multivariate analysis were clinical factors (high CVR and kidney function) and baseline LVEF, indicating the importance of patient characteristics in prognosis in EE without induced ischemia.
The influence of the risk profile of CAD on the risk of cardiac events in patients with negative EE was barely addressed. Some studies have shown that patients with negative EE have a cardiac event rate < 1% per year regardless of pretest probability.12,18 The pretest probability of coronary heart disease was not used in this study, because it is estimated using clinical scores that include the type of pain (typical angina, atypical angina, or nonanginal pain). The retrospective nature of the study made it impossible to reliably assess the characteristics of pain for its appropriate classification. Furthermore, only 55% of the patients had been seen for chest pain. Therefore, we chose CVR, which was estimated using CVR factors to determine its influence on cardiac events. To our knowledge, this is the first study to establish the risk of events and their timing in patients with negative EE based on CVR. Despite the excellent prognosis initially indicated by a negative EE in all groups, the data obtained suggest that the number of long-term events is modulated by CVR. Thus, the decrease in long-term EFS was slightly modified if SCORE was < 10%, and was maintained if > 98% at 3 years, but was most striking in the group with SCORE ≥ 10 or DM or patients with CAD (< 95%). These results have clear implications for patient treatment. First, regardless of CVR, the favorable prognostic effect of negative EE is maintained for 1 year then decreases over time in patients with CAD (91.1% at 3 years) or SCORE ≥ 10% or DM (94.5% at 3 years). Second, although we cannot recommend the use of additional diagnostic methods or anti-ischemic treatment, primary prevention measures should definitely be applied in patients with SCORE ≥ 10% or DM and secondary prevention should be applied in patients with coronary disease despite the absence of induced ischemia.
Strengths and LimitationsThis retrospective observational study was conducted in a population with limited geographical mobility, which facilitated follow-up. Our hospital was their only reference center. The study population is representative of the type of patients referred to a nontertiary general hospital for the detection of ischemia. Only 0.95% of the patients were followed up at < 6 months and 3.3% at < 1 year. EE is limited by technique, in which the interpretation of contractility depends on the operator. Regarding the data on coronary events during follow-up, and in contrast to older studies, the use of troponins clearly differentiated anginal or noncoronary episodes from an acute myocardial infarction. The low primary event rate reflects the reliability of the technique, but is itself a limitation. It should be noted that diagnostic catheterization and revascularization performed despite negative EE were not considered to be events and did not invalidate the results. The treatment described corresponds to that received by patients when they attended the EE study, without knowing if it was changed after negative EE or its effect on prognosis.
CONCLUSIONSInitial prognosis after an EE without ischemia is favorable but is subsequently modulated by cardiovascular risk.
FUNDINGThis study was possible due to the consent of the Ethics Committee of the Hospital of Galdakao and a grant from the Department of Health of the Basque Regional Government: No. 2012111009.
CONFLICTS OF INTERESTNone declared.
- –
Several studies have shown and concurred that EE without inducible ischemia has a favorable prognosis.
- –
Clinical variables and those derived from the test have also been found that indicate which patients may be at the most risk of events.
- –
However, no studies have investigated the association between prognosis and CVR profile in these patients.
- –
In the present study, predictors of events were previous CAD, SCORE ≥ 10% or DM, kidney failure, left ventricular dysfunction, and chest pain during EE. Some of these predictors are the same as those found in other studies.
- –
The novelty of this study is the role of cardiovascular risk in the prognosis of patients with EE without ischemia, such that those with SCORE < 10% have a favorable prognosis during follow-up, whereas patients with SCORE ≥ 10% or diabetes have a risk of events that increases with time since EE and resembles that of patients with known ischemic heart disease.