Mortality remains high in cardiogenic shock (CS), especially in refractory CS involving the use of mechanical circulatory support (MCS) devices. The aim of this study was to analyze the results of a care program for patients in CS after the creation of a multidisciplinary team in our center and a regional network of hospitals in our area.
MethodsObservational and retrospective study of patients attended in this program from September 2014 to January 2019. We included patients in refractory CS who required MCS and those who, because of their age and absence of comorbidities, were candidates for advanced therapies. The primary endpoint was survival to discharge.
ResultsA total of 130 patients were included (69 local and 61 transferred patients). The mean age was 52±15 years (72% men). The most frequent causes of CS were acute decompensated heart failure (29%), acute myocardial infarction (26%), and postcardiotomy CS (25%). MCS was used in 105 patients (81%), mostly extracorporeal membrane oxygenation (58%). Survival to discharge was 57% (74 of 130 patients). The most frequent destinations were myocardial recovery and heart transplant. Independent predictors of in-hospital mortality were SAPS II score, lactate level, acute myocardial infarction etiology, and vasoactive-inotropic score.
ConclusionsThe creation of multidisciplinary teams for patients with mainly refractory CS and a regional network is feasible and allows survival to discharge in more than a half of attended patients with CS.
Keywords
Cardiogenic shock (CS) continues to show elevated mortality.1 The only available treatment with clear clinical efficacy is early revascularization of patients with CS after acute myocardial infarction (AMI).2 However, in recent years there has been an exponential increase in the use of mechanical circulatory support (MCS) devices for highly severe patients who are refractory to medical therapy, who have a mortality rate of almost 100%.3,4 These devices have 2 main objectives: a) to support the functioning and recovery of the affected ventricles; and b) to ensure adequate perfusion and oxygenation of vital organs to avoid multiple organ failure and death.
The use of these and other complex therapies in refractory patients with CS supports the creation of multidisciplinary teams specialized in its management. These teams, primarily comprising advanced heart failure (HF) cardiologists, interventional cardiologists, cardiac surgeons, and critical care specialists, are integrated into hospitals with the specialized focus and resources to manage patients with CS.5,6 In addition to their internal coordination, they are designed to act as referral centers for a hierarchical network of hospitals with different care levels, analogous to those used for AMI and stroke, which can provide better coverage of the referral population of an area.7 Similar experiences in other countries and contexts have achieved satisfactory results.4,8,9
The objective of the present study was to analyze the initial outcomes of a care program for patients with CS, mainly refractory CS, after the creation of a multidisciplinary team in our hospital and coordination with a network of hospitals in our referral region.
METHODSContextIn recent years, one of the objectives of our center has been to improve the management of patients with CS, achieved through the creation of a CS unit at the end of 2014. This unit, comprising a multidisciplinary team, is available 24hours a day 7 days a week and is based on 4 main specialties: advanced HF, cardiac surgery, interventional cardiology, and critical care. This unit has 4 basic aims: rapid identification and characterization of CS, early revascularization of patients with CS after AMI, use of MCS in refractory patients, and implementation of a process exit strategy.
Clinical process and decision-makingSince the implementation of this network, the patients considered for treatment via this pathway have been those with CS criteria with refractory indicators and need for short-term MCS or those who, due to age and vital situation, are candidates for advanced HF treatments, such as a long-term ventricular assist device (LT-VAD) or heart transplant (HTx).
CS is defined as systolic blood pressure <90mmHg for more than 30minutes or the use of catecholamines to maintain a pressure of at least 90mmHg, clinical signs of pulmonary congestion, and signs of poor organ perfusion with at least 1 of the following: altered mental status, cold and clammy skin, oliguria with diuresis <30mL/h, or arterial lactate> 2.0 mmol/L.10 Patients are considered to have refractory CS when they have CS indicators despite initial treatment with vasopressors, inotropic agents, and, in some patients, an intra-aortic balloon pump (IABP). Patients with refractory CS are candidates for MCS once the presence has been ruled out of absolute contraindications (patient or family refusal, more than 30minutes of aborted sudden cardiac death, septic shock, or short life expectancy due to age or comorbidities). Other candidates for short-term MCS as a bridge to HTx are inotrope-dependent (INTERMACS 3) patients with CS who are not good candidates for a LT-VAD (eg, severe right ventricular failure, hypertrophic/restrictive cardiomyopathy, or irreparable ventricular septal defect after AMI).
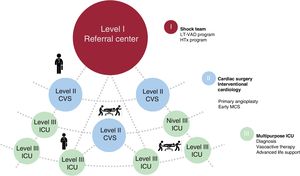
Patients can access the process via 2 pathways: patients directly treated in our center (local patients) or patients referred from other hospitals (transferred patients). Within our region, hospitals are divided into 3 care levels based on their resources (figure 1):
Level III: generally equipped with a multipurpose intensive care unit. They are able to diagnose patients with CS, apply advanced life support measures and initiate initial drug therapy, and, in some cases, implant an IABP.
Level II: in addition to the previous resources, these hospitals have a 24-hour, 7-day a week, primary angioplasty program and their interventional cardiologists and cardiac surgeons are often able to implant an MCS.
Level I: a tertiary center with the possibility of primary angioplasty and MCS implantation 24hours a day 7 days a week. These centers have a multidisciplinary team for CS treatment. In addition, they have LT-VAD and HTx programs.
When a patient is a possible candidate for management via this route, the treating physician contacts a member of the CS team (usually an on-call advanced HF cardiologist, in person or by telephone, thereby activating the “shock code”). After collecting basic information, this member reports the data to the rest of the team to decide patient management (generally involving a cardiac surgeon, intensivist/critical care cardiologist, and advanced HF cardiologist). For some of the referred patients, a member of the CS team of the level I hospital goes to the hospital treating the patient for an in situ assessment. Patients who meet the above-mentioned criteria are transferred to the referral hospital. There are also intermediate transfers between level II and level III hospitals. Patients who are in level II hospitals with refractory CS should ideally be stabilized with MCS for a safer subsequent transfer. In some cases, a team from the level I hospital comprising a cardiac surgeon, perfusionist, and intensivist may travel to the center treating the patient for implantation of an MCS device and the subsequent transfer of the patient to the referral hospital. Every day at 12:30, the physicians of the CS unit conduct a clinical session aimed at deciding patient management (figure 1).
Once the use of an MCS device has been decided for patients with refractory CS, it is implanted as an early “bridge to decision”. The MCS devices used are the following: short-term ventricular assist devices (ST-VADs) (univentricular or biventricular Impella CP [Abiomed, Germany] or Levitronix CentriMag [Abbott, United States]) or venoarterial extracorporeal membrane oxygenation (VA-ECMO) (CardioHelp [Maquet, Germany] or Levitronix CentriMag with membrane oxygenation). VA-ECMO is generally preferred when patients have any of the following conditions: a) doubtful neurological status or prolonged cardiopulmonary arrest; b) severe hemodynamic instability with biventricular involvement and respiratory compromise; and c) severe coagulopathy secondary to CS or also the use of dual antiplatelet therapy or glycoprotein IIb/IIIa inhibitors (for surgical devices).
The Impella CP is the ST-VAD of choice for patients with CS after AMI and left ventricular involvement in the context of primary angioplasty, as long as the patients have none of the above-mentioned conditions. These devices are also used as a “bridge to decision” in some patients with acute decompensated HF who progress to CS with predominant LV involvement and for LV unloading in patients with peripheral VA-ECMO. In the remaining patients, the Levitronix CentriMag is used in any of its modalities (univentricular or biventricular, with or without membrane oxygenation, and through surgical cannulation or a minimally invasive procedure).11
Study design and endpointsThis retrospective observational study analyzed all consecutive patients treated in the CS unit from September 2014 to January 2019. Since the implementation of this program, patient data have been prospectively collected and stored in a local registry. Hemodynamic and biochemical variables are recorded at CS diagnosis in local patients and upon arrival at our center in transferred patients. Demographic and treatment-related variables are also collected. To improve patient characterization, the Vasoactive-Inotropic Score (VIS) was calculated 24 and 48hours after admission. Also calculated were the Sepsis-related Organ Failure Assessment (SOFA), Simplified Acute Physiology Score (SAPS) II, and Acute Physiology And Chronic Health Evaluation II (APACHE II) scores in the first 24hours of admission. In patients with various contributing factors, the condition with the strongest influence on the final event was considered the cause of death.
The main study objective was to evaluate survival to discharge. The other objectives were as follows: a) to determine the destination of patients with CS; b) to identify independent and early predictors of in-hospital mortality; and c) to ascertain the mid- to long-term prognosis of survivors.
Statistical analysisContinuous variables are expressed as mean±standard deviation or as median [interquartile range] if nonnormally distributed. Categorical variables are expressed as frequency and percentage. In hypothesis tests, the Wilcoxon test was used for continuous variables and the chi-square test with Yates correction (in the case of values <5 in any of the cells) for categorical variables. The Fisher exact test was also used.
Univariate and multivariate analyses were performed with logistic regression to compare patients who did and did not survive to hospital discharge. Variables associated with mortality with P <.1 in the univariate analysis were included in the multivariate analysis. Variables were included in the prognostic model based on the stepwise-backward method and the variables considered pertinent were those recorded in the first 24hours of patient admission to the level I hospital. Kaplan-Meier curves were used for survival analysis. P <.05 was considered statistically significant and analyses were performed with STATA IC/15 software. This study was approved by the Research Ethics Committee of the Autonomous University of Madrid and Hospital Universitario Puerta de Hierro Majadahonda.
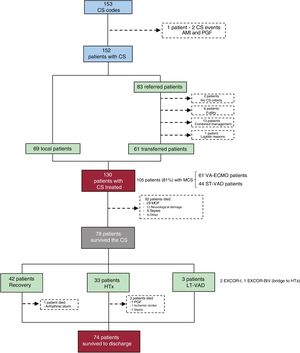
RESULTSIn total, the “shock code” was activated in 152 patients (153 events; 1 patient had 2 CS processes but only the first was considered). Of these, 69 were local patients and 83 were telephone consultations from other hospitals. Finally, of these 83 patients, 61 were transferred to our center. The main reasons for not transferring patients were a possible adequate and agreed treatment in the hospital treating the patient (n=13) and futility (n=6). A total of 130 patients (69 local and 61 transferred) were treated and included in the present analysis (figure 2).
Flow chart of patients included in the program. AMI, acute myocardial infarction; CS, cardiogenic shock; HTx, heart transplantation; LT-VAD, longterm ventricular assist device; MCS, mechanical circulatory support; MOF, multiple organ failure; PGF, primary graft failure; ST-VAD, short-term ventricular assist device; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
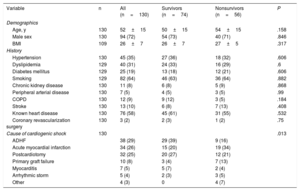
The patients’ demographic characteristics are reported in table 1. The mean patient age was 52±15 years (72% were men). The predominant causes of the CS were acute decompensated HF (29%), AMI (26%), and postcardiotomy CS (25%). The causes differed between local and transferred patients: more than half of local patients had postoperative CS (postcardiotomy and after primary graft failure), whereas transferred patients tended to have AMI and acute decompensated HF.
Baseline and demographic characteristics of the population
| Variable | n | All (n=130) | Survivors (n=74) | Nonsurvivors (n=56) | P |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 130 | 52±15 | 50±15 | 54±15 | .158 |
| Male sex | 130 | 94 (72) | 54 (73) | 40 (71) | .846 |
| BMI | 109 | 26±7 | 26±7 | 27±5 | .317 |
| History | |||||
| Hypertension | 130 | 45 (35) | 27 (36) | 18 (32) | .606 |
| Dyslipidemia | 129 | 40 (31) | 24 (33) | 16 (29) | .6 |
| Diabetes mellitus | 129 | 25 (19) | 13 (18) | 12 (21) | .606 |
| Smoking | 129 | 82 (64) | 46 (63) | 36 (64) | .882 |
| Chronic kidney disease | 130 | 11 (8) | 6 (8) | 5 (9) | .868 |
| Peripheral arterial disease | 130 | 7 (5) | 4 (5) | 3 (5) | .99 |
| COPD | 130 | 12 (9) | 9 (12) | 3 (5) | .184 |
| Stroke | 130 | 13 (10) | 6 (8) | 7 (13) | .408 |
| Known heart disease | 130 | 76 (58) | 45 (61) | 31 (55) | .532 |
| Coronary revascularization surgery | 130 | 3 (2) | 2 (3) | 1 (2) | .75 |
| Cause of cardiogenic shock | 130 | .013 | |||
| ADHF | 38 (29) | 29 (39) | 9 (16) | ||
| Acute myocardial infarction | 34 (26) | 15 (20) | 19 (34) | ||
| Postcardiotomy | 32 (25) | 20 (27) | 12 (21) | ||
| Primary graft failure | 10 (8) | 3 (4) | 7 (13) | ||
| Myocarditis | 7 (5) | 5 (7) | 2 (4) | ||
| Arrhythmic storm | 5 (4) | 2 (3) | 3 (5) | ||
| Other | 4 (3) | 0 | 4 (7) | ||
ADHF, acute decompensated heart failure; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Categorical variables are expressed as No. (%) and continuous variables as mean±standard deviation.
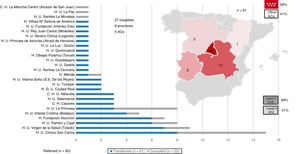
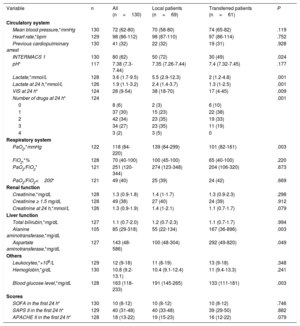
Transferred patients were mainly referred from level II hospitals (69%) and from within the autonomous community itself (59%). The median time from CS diagnosis to transfer was 2 [1-4] days. Eighteen patients were transferred with an IABP, 14 with VA-ECMO (10 of the 11 patients with peripheral cannulation also had an IABP), and 6 with ST-VAD (all with Levitronix CentriMag). No notable complications were recorded during the transfer (figure 3).
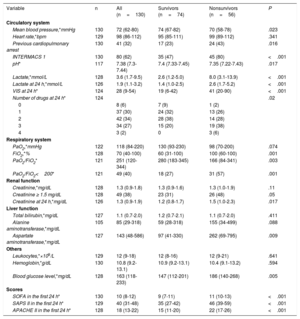
The patients’ clinical situation at admission and in the first 24hours in our intensive care unit is described in table 2 and table 3; 41 patients (32%) had previously experienced a cardiopulmonary arrest and 80 (62%) had an INTERMACS profile of 1. The lactate, creatinine, aspartate aminotransferase, and alanine aminotransferase levels and SOFA, SAPS II, and APACHE II scores reflect the multiple organ failure experienced by the patients in the first hours of admission to our center. At 24hours, 63% of the patients needed 2 or more vasoactive drugs to maintain hemodynamic stability. Transferred patients had lower lactate levels at admission (5.5 [2.9-12.3] vs 2.0 [1.2-4.8] mmol/L; P=.001) but higher transaminase levels (aspartate aminotransferase, 100 [48-304] vs 292 [49-820] mg/dL; P=.049; alanine aminotransferase, 55 [22-134] vs 167 [36-896] mg/dL; P=.003) (table 3).
Organ failure variables at first contact and during the first 24hours based on survival to discharge
| Variable | n | All (n=130) | Survivors (n=74) | Nonsurvivors (n=56) | P |
|---|---|---|---|---|---|
| Circulatory system | |||||
| Mean blood pressure,*mmHg | 130 | 72 (62-80) | 74 (67-82) | 70 (58-78) | .023 |
| Heart rate,*bpm | 129 | 98 (86-112) | 95 (85-111) | 99 (89-112) | .341 |
| Previous cardiopulmonary arrest | 130 | 41 (32) | 17 (23) | 24 (43) | .016 |
| INTERMACS 1 | 130 | 80 (62) | 35 (47) | 45 (80) | <.001 |
| pH* | 117 | 7.38 (7.3-7.44) | 7.4 (7.33-7.45) | 7.35 (7.22-7.43) | .017 |
| Lactate,*mmol/L | 128 | 3.6 (1.7-9.5) | 2.6 (1.2-5.0) | 8.0 (3.1-13.9) | <.001 |
| Lactate at 24 h,*mmol/L | 126 | 1.9 (1.1-3.2) | 1.4 (1.0-2.5) | 2.6 (1.7-5.2) | <.001 |
| VIS at 24 h* | 124 | 28 (9-54) | 19 (6-42) | 41 (20-90) | <.001 |
| Number of drugs at 24 h* | 124 | .02 | |||
| 0 | 8 (6) | 7 (9) | 1 (2) | ||
| 1 | 37 (30) | 24 (32) | 13 (26) | ||
| 2 | 42 (34) | 28 (38) | 14 (28) | ||
| 3 | 34 (27) | 15 (20) | 19 (38) | ||
| 4 | 3 (2) | 0 | 3 (6) | ||
| Respiratory system | |||||
| PaO2,*mmHg | 122 | 118 (84-220) | 130 (93-230) | 98 (70-200) | .074 |
| FiO2,*% | 128 | 70 (40-100) | 60 (31-100) | 100 (60-100) | .001 |
| PaO2/FiO2* | 121 | 251 (120-344) | 280 (183-345) | 166 (84-341) | .003 |
| PaO2/FiO2<200* | 121 | 49 (40) | 18 (27) | 31 (57) | .001 |
| Renal function | |||||
| Creatinine,*mg/dL | 128 | 1.3 (0.9-1.8) | 1.3 (0.9-1.6) | 1.3 (1.0-1.9) | .11 |
| Creatinine ≥ 1.5 mg/dL | 128 | 49 (38) | 23 (31) | 26 (48) | .05 |
| Creatinine at 24 h,*mg/dL | 126 | 1.3 (0.9-1.9) | 1.2 (0.8-1.7) | 1.5 (1.0-2.3) | .017 |
| Liver function | |||||
| Total bilirubin,*mg/dL | 127 | 1.1 (0.7-2.0) | 1.2 (0.7-2.1) | 1.1 (0.7-2.0) | .411 |
| Alanine aminotransferase,*mg/dL | 105 | 85 (29-318) | 59 (28-318) | 155 (34-499) | .088 |
| Aspartate aminotransferase,*mg/dL | 127 | 143 (48-586) | 97 (41-330) | 262 (69-795) | .009 |
| Others | |||||
| Leukocytes,*×109/L | 129 | 12 (9-18) | 12 (8-16) | 12 (9-21) | .641 |
| Hemoglobin,*g/dL | 130 | 10.8 (9.2-13.1) | 10.9 (9.2-13.1) | 10.4 (9.1-13.2) | .594 |
| Blood glucose level,*mg/dL | 128 | 163 (118-233) | 147 (112-201) | 186 (140-268) | .005 |
| Scores | |||||
| SOFA in the first 24 h* | 130 | 10 (8-12) | 9 (7-11) | 11 (10-13) | <.001 |
| SAPS II in the first 24 h* | 129 | 40 (31-48) | 35 (27-42) | 46 (39-59) | <.001 |
| APACHE II in the first 24 h* | 128 | 18 (13-22) | 15 (11-20) | 22 (17-26) | <.001 |
APACHE II, Acute Physiology And Chronic Health Evaluation II; FiO2, fraction of inspired oxygen; INTERMACS, The Interagency Registry for Mechanically Assisted Circulatory Support; PaO2, partial pressure of oxygen; SAPS II, Simplified Acute Physiology Score; SOFA, Sepsis-related Organ Failure Assessment; VIS, Vasoactive-Inotropic Score.
Categorical variables are expressed as No. (%) and continuous variables as mean (range).
Organ failure variables at first contact and during the first 24hours according to patient origin
| Variable | n | All (n=130) | Local patients (n=69) | Transferred patients (n=61) | P |
|---|---|---|---|---|---|
| Circulatory system | |||||
| Mean blood pressure,*mmHg | 130 | 72 (62-80) | 70 (58-80) | 74 (65-82) | .119 |
| Heart rate,*bpm | 129 | 98 (86-112) | 98 (87-110) | 97 (86-114) | .752 |
| Previous cardiopulmonary arrest | 130 | 41 (32) | 22 (32) | 19 (31) | .928 |
| INTERMACS 1 | 130 | 80 (62) | 50 (72) | 30 (49) | .024 |
| pH* | 117 | 7.38 (7.3-7.44) | 7.35 (7.26-7.44) | 7.4 (7.32-7.45) | .177 |
| Lactate,*mmol/L | 128 | 3.6 (1.7-9.5) | 5.5 (2.9-12.3) | 2 (1.2-4.8) | .001 |
| Lactate at 24 h,*mmol/L | 126 | 1.9 (1.1-3.2) | 2.4 (1.4-3.7) | 1.3 (1-2.5) | .001 |
| VIS at 24 h* | 124 | 28 (9-54) | 38 (18-70) | 17 (4-45) | .009 |
| Number of drugs at 24 h* | 124 | .001 | |||
| 0 | 8 (6) | 2 (3) | 6 (10) | ||
| 1 | 37 (30) | 15 (23) | 22 (38) | ||
| 2 | 42 (34) | 23 (35) | 19 (33) | ||
| 3 | 34 (27) | 23 (35) | 11 (19) | ||
| 4 | 3 (2) | 3 (5) | 0 | ||
| Respiratory system | |||||
| PaO2,*mmHg | 122 | 118 (84-220) | 139 (84-299) | 101 (82-161) | .003 |
| FiO2,*% | 128 | 70 (40-100) | 100 (45-100) | 65 (40-100) | .220 |
| PaO2/FiO2* | 121 | 251 (120-344) | 274 (123-348) | 204 (106-320) | .673 |
| PaO2/FiO2<200* | 121 | 49 (40) | 25 (39) | 24 (42) | .669 |
| Renal function | |||||
| Creatinine,*mg/dL | 128 | 1.3 (0.9-1.8) | 1.4 (1-1.7) | 1.3 (0.9-2.3) | .298 |
| Creatinine ≥ 1.5 mg/dL | 128 | 49 (38) | 27 (40) | 24 (39) | .912 |
| Creatinine at 24 h,*mmol/L | 126 | 1.3 (0.9-1.9) | 1.4 (1-2.1) | 1.1 (0.7-1.7) | .079 |
| Liver function | |||||
| Total bilirubin,*mg/dL | 127 | 1.1 (0.7-2.0) | 1.2 (0.7-2.3) | 1.1 (0.7-1.7) | .994 |
| Alanine aminotransferase,*mg/dL | 105 | 85 (29-318) | 55 (22-134) | 167 (36-896) | .003 |
| Aspartate aminotransferase,*mg/dL | 127 | 143 (48-586) | 100 (48-304) | 292 (49-820) | .049 |
| Others | |||||
| Leukocytes,*×109/L | 129 | 12 (9-18) | 11 (8-19) | 13 (9-18) | .348 |
| Hemoglobin,*g/dL | 130 | 10.8 (9.2-13.1) | 10.4 (9.1-12.4) | 11 (9.4-13.3) | .241 |
| Blood glucose level,*mg/dL | 128 | 163 (118-233) | 191 (145-265) | 133 (111-181) | .003 |
| Scores | |||||
| SOFA in the first 24 h* | 130 | 10 (8-12) | 10 (8-12) | 10 (8-12) | .746 |
| SAPS II in the first 24 h* | 129 | 40 (31-48) | 40 (33-48) | 39 (29-50) | .882 |
| APACHE II in the first 24 h* | 128 | 18 (13-22) | 19 (15-23) | 16 (12-22) | .079 |
APACHE II, Acute Physiology And Chronic Health Evaluation II; FiO2, fraction of inspired oxygen; INTERMACS, The Interagency Registry for Mechanically Assisted Circulatory Support; PaO2, partial pressure of oxygen; SAPS II, Simplified Acute Physiology Score; SOFA, Sepsis-related Organ Failure Assessment; VIS, Vasoactive-Inotropic Score.
Categorical variables are expressed as No. (%) and continuous variables as mean (range).
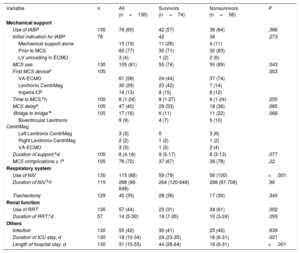
An MCS device was used in 105 of the 130 patients (81%). Escalation of support from the IABP was required in 57% of these patients. Only 10 patients (8% of the series) did not receive an IABP or MCS device. VA-ECMO was the first device used in more than half of the patients (61 of 105; 58%). The first device was the Levitronix CentriMag in 30 patients (8 left ventricular, 2 right ventricular, and 20 biventricular) and the Impella CP in 14. In 17 patients, a second MCS device was used to stabilize the patient, mainly due to respiratory and hemodynamic problems in patients with a peripheral VA-ECMO as a bridge to a Levitronix CentriMag ST-VAD (table 4).
Management and course of patients with cardiogenic shock
| Variable | n | All (n=130) | Survivors (n=74) | Nonsurvivors (n=56) | P |
|---|---|---|---|---|---|
| Mechanical support | |||||
| Use of IABP | 130 | 78 (60) | 42 (57) | 36 (64) | .386 |
| Initial indication for IABP | 78 | 42 | 36 | .273 | |
| Mechanical support alone | 15 (19) | 11 (26) | 4 (11) | ||
| Prior to MCS | 60 (77) | 30 (71) | 30 (83) | ||
| LV unloading in ECMO | 3 (4) | 1 (2) | 2 (6) | ||
| MCS use | 130 | 105 (81) | 55 (74) | 50 (89) | .043 |
| First MCS devicea | 105 | .003 | |||
| VA-ECMO | 61 (58) | 24 (44) | 37 (74) | ||
| Levitronix CentriMag | 30 (29) | 23 (42) | 7 (14) | ||
| Impella CP | 14 (13) | 8 (15) | 6 (12) | ||
| Time to MCS,ah | 105 | 6 (1-24) | 8 (1-27) | 6 (1-24) | .255 |
| MCS delaya | 105 | 47 (45) | 29 (53) | 18 (36) | .085 |
| “Bridge to bridge”a | 105 | 17 (16) | 6 (11) | 11 (22) | .068 |
| Biventricular Levitronix CentriMag | 9 (9) | 4 (7) | 5 (10) | ||
| Left Levitronix CentriMag | 3 (3) | 0 | 3 (6) | ||
| Right Levitronix CentriMag | 2 (2) | 1 (2) | 1 (2) | ||
| VA-ECMO | 3 (3) | 1 (2) | 2 (4) | ||
| Duration of support,ad | 105 | 8 (4-16) | 9 (5-17) | 8 (3-13) | .077 |
| MCS complications ≥ 1a | 105 | 76 (72) | 37 (67) | 39 (78) | .22 |
| Respiratory system | |||||
| Use of NIV | 130 | 115 (88) | 59 (79) | 56 (100) | <.001 |
| Duration of NIV,bh | 115 | 288 (96-648) | 264 (120-648) | 288 (87-708) | .96 |
| Tracheotomy | 129 | 45 (35) | 28 (38) | 17 (30) | .345 |
| Renal function | |||||
| Use of RRT | 130 | 57 (44) | 23 (31) | 34 (61) | .002 |
| Duration of RRT,cd | 57 | 14 (5-30) | 19 (7-35) | 10 (3-24) | .093 |
| Others | |||||
| Infection | 130 | 55 (42) | 30 (41) | 25 (45) | .639 |
| Duration of ICU stay, d | 130 | 18 (10-34) | 24 (23-35) | 16 (6-31) | .021 |
| Length of hospital stay, d | 130 | 31 (15-55) | 44 (28-64) | 16 (6-31) | <.001 |
ECMO, extracorporeal membrane oxygenation; IABP, intra-atrial balloon pump; ICU, intensive care unit; LV, left ventricular; MCS, mechanical circulatory support; NIV, noninvasive ventilation; RRT, renal replacement therapy; VA-ECMO, venoarterial ECMO.
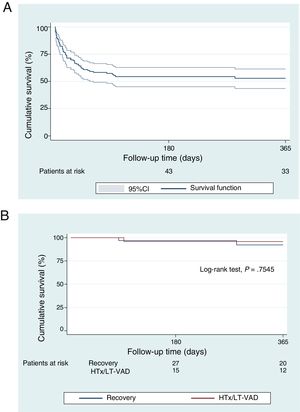
In total, 78 patients (60%) survived the acute phase: 42 achieved myocardial recovery, 33 underwent an urgent transplant, and 3 received an EXCOR ST-VAD as a “bridge to transplant”. The overall survival to discharge rate was 57% (74 of 130 patients; 51% for local patients and 64% for transferred patients). Among the patients who survived the acute CS phase, the survival to discharge rate was 95% (74 of 78 patients). Only 1 patient died of arrhythmic storm after recovery and 3 after HTx (primary graft failure, ischemic stroke, and sepsis). The survival to discharge rate after urgent HTx in this series was 91% (30 of 33 patients). The 3 patients with an EXCOR LT-VAD underwent HTx 85, 90, and 140 days after the implantation. In Kaplan-Meier analysis, the 1-year survival rate of the entire cohort was 53%. In the survivors, after a median follow-up of 221 [44-699] days after discharge, there were only 5 deaths, with a 1-year actuarial survival of 94%, without differences according to patient destination (figure 4).
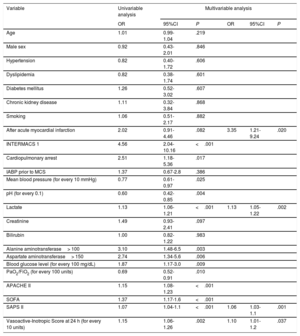
The results of univariable and multivariable analyses of in-hospital mortality are reported in table 5. Independent predictors of in-hospital mortality were CS after AMI (odds ratio [OR]=3.35; 95% confidence interval [95%CI], 1.21-9.24; P=.02), the initial lactate level (OR=1.13; 95%CI, 1.05-1.22; P=.002), the SAPS II score in the first 24hours (OR=1.06; 95%CI, 1.03-1.1; P=.001), and the VIS at 24hours after admission (OR=1.1; 95%CI, 1.01-1.2; P=.037). The C statistic of the multivariable model that included the clinical parameters from the first hours of admission was 0.82 (95%CI, 0.75-0.90).
Univariable and multivariable analysis of in-hospital mortality
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |
| Age | 1.01 | 0.99-1.04 | .219 | |||
| Male sex | 0.92 | 0.43-2.01 | .846 | |||
| Hypertension | 0.82 | 0.40-1.72 | .606 | |||
| Dyslipidemia | 0.82 | 0.38-1.74 | .601 | |||
| Diabetes mellitus | 1.26 | 0.52-3.02 | .607 | |||
| Chronic kidney disease | 1.11 | 0.32-3.84 | .868 | |||
| Smoking | 1.06 | 0.51-2.17 | .882 | |||
| After acute myocardial infarction | 2.02 | 0.91-4.46 | .082 | 3.35 | 1.21-9.24 | .020 |
| INTERMACS 1 | 4.56 | 2.04-10.16 | <.001 | |||
| Cardiopulmonary arrest | 2.51 | 1.18-5.36 | .017 | |||
| IABP prior to MCS | 1.37 | 0.67-2.8 | .386 | |||
| Mean blood pressure (for every 10 mmHg) | 0.77 | 0.61-0.97 | .025 | |||
| pH (for every 0.1) | 0.60 | 0.42-0.85 | .004 | |||
| Lactate | 1.13 | 1.06-1.21 | <.001 | 1.13 | 1.05-1.22 | .002 |
| Creatinine | 1.49 | 0.93-2.41 | .097 | |||
| Bilirubin | 1.00 | 0.82-1.22 | .983 | |||
| Alanine aminotransferase> 100 | 3.10 | 1.48-6.5 | .003 | |||
| Aspartate aminotransferase> 150 | 2.74 | 1.34-5.6 | .006 | |||
| Blood glucose level (for every 100 mg/dL) | 1.87 | 1.17-3.0 | .009 | |||
| PaO2/FiO2 (for every 100 units) | 0.69 | 0.52-0.91 | .010 | |||
| APACHE II | 1.15 | 1.08-1.23 | <.001 | |||
| SOFA | 1.37 | 1.17-1.6 | <.001 | |||
| SAPS II | 1.07 | 1.04-1.1 | <.001 | 1.06 | 1.03-1.1 | .001 |
| Vasoactive-Inotropic Score at 24 h (for every 10 units) | 1.15 | 1.06-1.26 | .002 | 1.10 | 1.01-1.2 | .037 |
95%CI, 95% confidence interval; APACHE II, Acute Physiology And Chronic Health Evaluation II; FiO2, fraction of inspired oxygen; IABP, intra-atrial balloon pump; INTERMACS, The Interagency Registry for Mechanically Assisted Circulatory Support; MCS, mechanical circulatory support; OR, odds ratio; PaO2, partial pressure of oxygen; SAPS II, Simplified Acute Physiology Score; SOFA, Sepsis-related Organ Failure Assessment.
The main causes of death during the acute CS phase were multiple organ failure in 29 of the 52 patients who died (56%), neurological damage in 12 (23%; 8 anoxic encephalopathies, 2 ischemic strokes, and 2 hemorrhagic strokes), and sepsis in 5 (10%). Of the 105 patients with MCS, 76 (72%) had at least 1 related complication. The main complications were bleeding requiring transfusion or reintervention in 47 patients (45%) and stroke in 13 (12%; 9 ischemic and 4 hemorrhagic), as well as peripheral arterial disease related to an MCS device in 9 patients (9%). Just 2 of the 13 patients with stroke and 3 of the 9 with peripheral arterial disease survived to hospital discharge.
DISCUSSIONThis work shows the outcomes of a care program for patients with CS, mainly refractory CS, after the creation of a multidisciplinary team for CS and the organization of a network comprising regional hospitals. The main findings are that a) 57% of patients survived to discharge; b) patient survival was mainly due to myocardial recovery or urgent HTx; c) CS cause, lactate level, SAPS II score, and VIS were independent predictors of in-hospital mortality; and d) patients who survived to discharge had excellent mid-term survival (94% at 1 year).
In recent years, there has been a surge of interest in improving the management of patients with CS. In addition to an increased use of MCS devices,3 a large part of the effort has been focused on organizational aspects, with the creation of experienced multidisciplinary teams and hospital networks for patient transfer to referral centers.5–7 These networks permit a) better and more homogeneous care of all patients in a specific region; b) centralized activity, increased team experience, and, accordingly, improved outcomes for this condition; and c) a care headquarters that enables the generation of the scientific evidence lacking in this field. As far as we know, this series is the first reported from Spain with this organizational model and also has unique characteristics vs other published series.
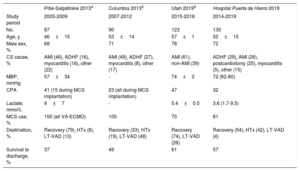
First, the results were obtained from a strategy based on a priori defined selection criteria, in contrast to other series that have been built around the use of MCS devices or the patients’ severity profile. Although this may increase the heterogeneity of the studied population, we believe that it has the virtue of showing the “real life” activity and the possibilities of a program with these characteristics. The activity and outcome data could be useful for the planning of other teams in other regions. During the study period, an average of 2 to 3 patients were treated per month and the outcomes have been stable, possibly due to the educational and training activities of the team in the years prior to program initiation. Despite the difficulty of comparing the distinct CS series published, the survival to discharge rate reported in our work is in line with those of other groups with similar programs (table 6).4,8,9 Although it might appear that no advances have been made in the prognosis of these patients in recent decades, given that their mortality is still around 50%, we believe that the characteristics of the patients treated in our center and others reflect an increasingly less healthy population that would previously not have had any other treatment options.
Comparison of the characteristics and outcomes of our care program for patients with CS vs others in the literature
| Pitié-Salpêtriére 20134 | Columbia 20138 | Utah 20199 | Hospital Puerta de Hierro 2019 | |
|---|---|---|---|---|
| Study period | 2005-2009 | 2007-2012 | 2015-2018 | 2014-2019 |
| No. | 87 | 90 | 123 | 130 |
| Age, y | 46±15 | 53±14 | 57±1 | 52±15 |
| Male sex, % | 68 | 71 | 78 | 72 |
| CS cause, % | AMI (46), ADHF (16), myocarditis (16), other (22) | AMI (49), ADHF (27), myocarditis (8), other (17) | AMI (61), non-AMI (39) | ADHF (29), AMI (26), postcardiotomy (25), myocarditis (5), other (15) |
| MBP, mmHg | 57±34 | - | 74±2 | 72 (62-80) |
| CPA | 41 (15 during MCS implantation) | 23 (all during MCS implantation) | 47 | 32 |
| Lactate, mmol/L | 9±7 | - | 5.4±0.5 | 3.6 (1.7-9.5) |
| MCS use, % | 100 (all VA-ECMO) | 100 | 70 | 81 |
| Destination, % | Recovery (79), HTx (8), LT-VAD (13) | Recovery (33), HTx (19), LT-VAD (48) | Recovery (74), LT-VAD (26) | Recovery (54), HTx (42), LT-VAD (4) |
| Survival to discharge, % | 37 | 49 | 61 | 57 |
ADHF, acute decompensated heart failure; AMI, acute myocardial infarction; CPA, cardiopulmonary arrest; CS, cardiogenic shock; HTx, heart transplantation; LT-VAD, long-term ventricular assist device; MBP, mean blood pressure; MCS, mechanical circulatory support; VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Second, and this point is related to the first, this program included all causes of CS, with no exclusion criteria. Although the patients’ pathophysiology and clinical context are distinct, particularly in the postoperative patients, CS patients’ treatment and destination are often the same. In our series, 74% of the patients included had CS that was not secondary to AMI. These activity data, with less than one third of all patients having CS after AMI, are in line with those of other recent publications, highlighting the change in the profile of CS patients now treated in intensive care units.12
Finally, in this series, urgent HTx was the destination of 42% of the patients who survived the acute CS phase because they showed no signs of myocardial recovery. This elevated use of HTx in patients with CS is possible only in countries with a high rate of donations and with relatively short waiting periods, such as ours.13 Although the HTx outcomes in this type of patient can be inferior to those obtained with elective HTx, which has been an object of study and source of controversy,14,15 centers with experience and with adequate selection of recipients after the resolution of multiple organ failure can achieve> 90% survival to discharge rates, as in our series (30 of 33 patients, 91%).
SAPS II score, CS after AMI, lactate level at admission, and IVS were powerful independent predictors of in-hospital mortality in our series. These findings are in line with the available evidence. The SAPS II score, which includes patients’ age, blood pressure, heart rate, Glasgow Coma Scale, and other biochemical and respiratory variables, has already been shown to have predictive value for CS patients’ mortality.4,16 In addition, CS after AMI and lactate level have been correlated with poor prognosis in these patients.4,17 Finally, Na et al.18 recently demonstrated that an elevated VIS in the first 48hours after CS diagnosis is associated with higher in-hospital mortality, as seen in this study.
LimitationsThe present work has the typical limitations inherent to retrospective studies. In addition, the study does not represent all patients with CS due to the selection criteria applied. Moreover, we must note a probable selection bias, due to the a priori transfer and treatment of more stable patients with a better prognosis. Thus, the applicability of this series should be considered within the clinical context described, taking into account aspects such as access to HTx and the profile of the patients included. This characteristic complicates comparisons among series. Another limitation is the absence of a historic control, which would help to clarify the usefulness of this organizational model in the MCS era.
CONCLUSIONSThe creation of multidisciplinary teams for the management of patients with CS, predominantly refractory CS, and coordination with a network of hospitals in a specific region are feasible and achieve survival to discharge in more than half of patients treated. In Spain, most of the patients survive due to myocardial recovery or urgent HTx. These outcomes, together with other published experiences, support the application of this internal and regional organizational model to all geographic regions. Multicenter and larger studies are required to better determine the benefits of this strategy and improve outcomes.
FUNDINGThe present work has been partially funded by a grant awarded by the Spanish Society of Cardiology for “Proyectos de investigación SEC-ROVI para promoción de la investigación en insuficiencia cardiaca de la Sección de Insuficiencia Cardiaca 2017” (“SEC-ROVI research projects for the promotion of research into heart failure of the Heart Failure Section 2017”).
CONFLICTS OF INTERESTNone.
- –
Cardiogenic shock continues to show high mortality and has an increasingly complex management due to the growing use of mechanical circulatory support devices in refractory patients. Initial experiences and analogy with other “time-sensitive” diseases such as infarction and stroke support the creation of specialized teams in referral centers and coordination with a network of regional hospitals to optimize outcomes.
- –
We present the first experience in Spain with the implementation of this organizational model in our region. With this network, survival to discharge was achieved in more than half of patients. In Spain, in contrast to other regions, urgent heart transplant was one of the main destinations for survivors. Patients who survived the acute phase of cardiogenic shock had an excellent mid-term prognosis.