In Spain, 0.3% of patients with hypertension are refractory to conventional treatment. The complications resulting from deficient control of this condition can lead to poor quality of life for the patient and considerable health care costs. Barostim is an implantable device designed to lower blood pressure in these patients. The aim of this study was to analyze the cost-effectiveness of Barostim compared with drug therapy in hypertensive patients refractory to conventional treatment (at least 3 antihypertensive drugs, including 1 diuretic agent).
MethodsWe used a Markov model adapted to the epidemiology of the Spanish population to simulate the natural history of a cohort of patients with refractory hypertension over their lifetime. Data on the effectiveness of the treatments studied were obtained from the literature, and data on costs were taken from hospital administrative databases and official sources. Deterministic and probabilistic sensitivity analyses were conducted.
ResultsBarostim increased the number of quality-adjusted life years by 0.78 and reduced the number of hypertension-associated clinical events. The incremental cost-effectiveness ratio in a cohort of men reached 68 726 euros per year of quality-adjusted life. One of the main elements that makes this technology costly is the need for battery replacement. The results were robust.
ConclusionsBarostim is not a cost-effective strategy for the treatment of refractory hypertension in Spain. The cost-effectiveness ratio could be improved by future reductions in the cost of the battery.
Keywords
Within the health sector, innovation is the key to progress in scientific research, patient care, and business-related concerns. Nonetheless, the introduction of new medical technology should provide substantial added value in regular clinical practice. For this reason, the Spanish Society of Cardiology (Sociedad Española de Cardiología [SEC]) has implemented the strategic initiative, InnovaSEC, to analyze the value of new technology contemplated for use in the Spanish health care setting.1 The first new product evaluated under the auspices of InnovaSEC is the Barostim medical device.
Barostim (CVRx Inc., Minneapolis, Minnesota, United States) is an implantable system that lowers blood pressure by electrical stimulation of the carotid baroreceptors. It is indicated as a second-line treatment for hypertensive patients resistant to conventional medical therapy (established on systolic blood pressure [SBP] values ≥ 140mmHg, despite the use of at least 3 antihypertensive drugs, including a diuretic). Refractory hypertension affects 0.3% of hypertensive Spanish patients.2 Considering that the prevalence of hypertension in the Spanish population older than 30 years is around 30%,3 there would be approximately 29 000 patients with hypertension refractory to drug therapy in Spain.
Carotid baroreceptor stimulation is considered a possible therapeutic option for refractory hypertension in the guidelines of the European Society of Hypertension and the European Society of Cardiology.4 Furthermore, the indication for Barostim in patients refractory to conventional treatment was found to be cost-effective for the German population (cost-effectiveness ratio, 7797 euros/quality-adjusted life year [QALY]).5 However, the epidemiologic profile of the Spanish population and the cost of treating hypertension and its complications in Spain differ from those observed in northern European countries, and both these variables could have a considerable impact on the cost-effectiveness of this technology in our country.
The aim of this study was to analyze the cost-effectiveness of Barostim as a second-line treatment compared with an adequate pharmacologic regimen (at least 3 antihypertensive drugs, including a diuretic agent) in the population of adults with hypertension (SBP ≥ 140mmHg), from the perspective of the publically-funded Spanish Health System.
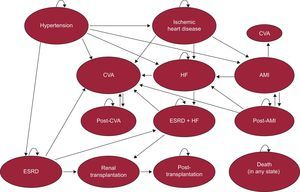
METHODSDesignA cost-effectiveness analysis was conducted using a model developed by Markov6 in which patients start in hypertensive status refractory to drug therapy and progress over time toward several possible health states (Figure 1). Time is represented as fixed cycles of 1 month's duration up to the end of the patients’ lives. The probability of transition to a new state depends on the patients’ initial characteristics and later ones, their health status, and the treatment received. Different quality of life levels and costs are associated with each health state. The model was based on a study carried out in the German population,5 and was adapted to the epidemiologic characteristics and health costs of Spain for the present study.
The following health states were included in the model: high blood pressure, ischemic heart disease, heart failure, acute myocardial infarction (AMI), cerebrovascular accident (CVA), end-stage renal disease, and death. Acute myocardial infarction and CVA were represented as states lasting for 1 cycle. In the cycle immediately following an AMI or CVA, patients could transition toward one of the above-mentioned states (including death) or progress toward post-AMI or post-CVA status. Patients in the post-AMI state could experience CVA, heart failure, recurrent AMI, or death. Post-CVA patients could only experience recurrent CVA or death. Finally, patients with end-stage renal disease could receive a renal transplant, and if they survived, transition to post-transplantation status.
The analysis compared drug therapy alone with treatment by the Barostim device plus pharmacologic therapy. It was assumed that patients would receive a combination of diuretics, beta-blockers, calcium-channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers. This combination follows the recommendations of the European Society of Hypertension and European Society of Cardiology.4 If one of the adverse events included in the model occurred, it was assumed that drug therapy would be interrupted.
As measures of clinical effectiveness, we estimated the frequency of adverse events over the patient's lifetime, quality-unadjusted life years, and QALYs.7 The cost analysis included direct health care costs and indirect costs. Finally, we evaluated the incremental cost-effectiveness ratio (ICER).8
The base case of the model was a 55-year-old man, with SBP 170mmHg, total cholesterol 190mg/dL, high-density lipoprotein cholesterol 35 mg/dL, and heart rhythm 79 bpm, nonsmoker, diabetic, no history of ischemic heart disease, and no left ventricular hypertrophy (). A 3% annual discount rate was used.9 The model was also estimated for a cohort of women with the same baseline characteristics as those used for men. Estimations were carried out with Stata 13.1 (StataCorp; College Station, Texas, United States).
Probabilities of Transitioning Between Health StatesThe Framingham equations adapted to the Spanish population were used to estimate the risk of ischemic heart disease and AMI (REGICOR [Registre Gironí del Cor]10) and the risk of CVA (FRESCO11). The equation from the original Framingham project12 was used to estimate the risk of heart failure, as we could not find related equations adapted to the Spanish population. The probability of each of these events occurring was reestimated in each cycle, so that the increased risk associated with aging could be taken into account. The estimated risk of end-stage renal disease was obtained from the study by Hsu et al.13
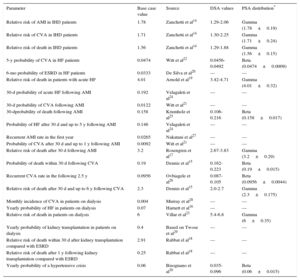
It was assumed that the probabilities of new events occurring in patients who had experienced one of the events in the model would be independent of the blood pressure level; hence, they were calculated using reported relative risk values or probability values14–28 (Table 1). In addition, it was assumed that 40% of dialysis patients per year would receive a renal transplant29 and that patients could experience a hypertensive crisis without transitioning to another state.30
Clinical Data and Probabilities of Transition
| Parameter | Base case value | Source | DSA values | PSA distribution* |
|---|---|---|---|---|
| Relative risk of AMI in IHD patients | 1.78 | Zanchetti et al14 | 1.29-2.06 | Gamma (1.78±0.19) |
| Relative risk of CVA in IHD patients | 1.71 | Zanchetti et al14 | 1.30-2.25 | Gamma (1.71±0.24) |
| Relative risk of death in IHD patients | 1.56 | Zanchetti et al14 | 1.29-1.88 | Gamma (1.56±0.15) |
| 5-y probability of CVA in HF patients | 0.0474 | Witt et al22 | 0.0456-0.0492 | Beta (0.0474±0.0009) |
| 6-mo probability of ESRD in HF patients | 0.0333 | De Silva et al20 | — | — |
| Relative risk of death in patients with acute HF | 4.01 | Arnold et al19 | 3.42-4.71 | Gamma (4.01±0.32) |
| 30-d probability of acute HF following AMI | 0.192 | Velagaleti et al24 | — | — |
| 30-d probability of CVA following AMI | 0.0122 | Witt et al21 | — | — |
| 30-dprobability of death following AMI | 0.158 | Krumholz et al25 | 0.106-0.216 | Beta (0.158±0.017) |
| Probability of HF after 30 d and up to 5 y following AMI | 0.146 | Velagaleti et al24 | — | — |
| Recurrent AMI rate in the first year | 0.0265 | Nakatani et al27 | — | — |
| Probability of CVA after 30 d and up to 1 y following AMI | 0.0092 | Witt et al21 | — | — |
| Relative risk of death after 30 d following AMI | 3.2 | Rosengren et al17 | 2.67-3.83 | Gamma (3.2±0.29) |
| Probability of death within 30 d following CVA | 0.19 | Dennis et al15 | 0.162-0.223 | Beta (0.19±0.015) |
| Recurrent CVA rate in the following 2.5 y | 0.0956 | Ovbiagele et al26 | 0.087-0.105 | Beta (0.0956±0.0044) |
| Relative risk of death after 30 d and up to 6 y following CVA | 2.3 | Dennis et al15 | 2.0-2.7 | Gamma (2.3±0.175) |
| Monthly incidence of CVA in patients on dialysis | 0.004 | Murray et al28 | — | — |
| Yearly probability of HF in patients on dialysis | 0.07 | Harnett et al16 | — | — |
| Relative risk of death in patients on dialysis | 6 | Villar et al23 | 5.4-6.8 | Gamma (6±0.35) |
| Yearly probability of kidney transplantation in patients on dialysis | 0.4 | Based on Twose et al29 | — | — |
| Relative risk of death within 30 d after kidney transplantation compared with ESRD | 2.91 | Rabbat el al18 | — | — |
| Relative risk of death after 1 y following kidney transplantation compared with ESRD | 0.25 | Rabbat el al18 | — | — |
| Yearly probability of a hypertensive crisis | 0.06 | Bisognano et al30 | 0.035-0.096 | Beta (0.06±0.015) |
AMI, acute myocardial infarction; CVA, cerebrovascular accident; DSA, deterministic sensitivity analysis; ESRD, end-stage renal disease; HF, heart failure; IHD, ischemic heart disease; PSA, probabilistic sensitivity analysis.
The risk of death according to age in patients who experienced no events was taken from the Spanish Statistics Institute (Instituto Nacional de Estadística mortality tables.31 Risk was calculated as the difference between all-cause mortality and mortality due to the causes included in the model.
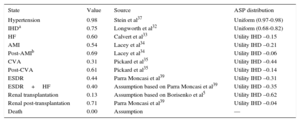
Utility EstimatesThe utility data associated with each health state were taken from the literature5,32–36 (Table 2). The utility score assigned to hypertensive status was obtained from Stein et al.37
Utility Scores According to Health State
| State | Value | Source | ASP distribution |
|---|---|---|---|
| Hypertension | 0.98 | Stein et al37 | Uniform (0.97-0.98) |
| IHDa | 0.75 | Longworth et al32 | Uniform (0.68-0.82) |
| HF | 0.60 | Calvert et al33 | Utility IHD –0.15 |
| AMI | 0.54 | Lacey et al34 | Utility IHD –0.21 |
| Post-AMIb | 0.69 | Lacey et al34 | Utility IHD –0.06 |
| CVA | 0.31 | Pickard et al35 | Utility IHD –0.44 |
| Post-CVA | 0.61 | Pickard et al35 | Utility IHD –0.14 |
| ESDR | 0.44 | Parra Moncasi et al39 | Utility IHD –0.31 |
| ESDR+HF | 0.40 | Assumption based on Parra Moncasi et al39 | Utility IHD –0.35 |
| Renal transplantation | 0.13 | Assumption based on Borisenko et al5 | Utility IHD –0.62 |
| Renal post-transplantation | 0.71 | Parra Moncasi et al39 | Utility IHD –0.04 |
| Death | 0.00 | Assumption | — |
AMI, acute myocardial infarction; CVA, cerebrovascular accident; ESRD, end-stage renal disease; HF, heart failure; IHD, ischemic heart disease; PSA, probabilistic sensitivity analysis
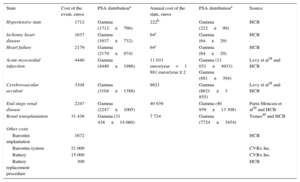
The direct health care costs, obtained from Hospital Clínic de Barcelona, were calculated as the average cost of episodes occurring during 2012 (Table 3). The post-AMI, post-CVA, and end-stage renal disease costs were taken from the literature38–40 and updated to the 2012 amounts.
Direct Health Care Costs
| State | Cost of the event, euros | PSA distributiona | Annual cost of the state, euros | PSA distributiona | Source |
|---|---|---|---|---|---|
| Hypertensive state | 1712 | Gamma (1712±766) | 222b | Gamma (222±99) | HCB |
| Ischemic heart disease | 1637 | Gamma (1637±732) | 64c | Gamma (64±29) | HCB |
| Heart failure | 2179 | Gamma (2179±974) | 64c | Gamma (64±29) | HCB |
| Acute myocardial infarction | 4440 | Gamma (4440±1986) | 11 031 euros/year=1 881 euros/year ≥ 2 | Gamma (11 031±4933) Gamma (881±394) | Levy et al38 and HCB |
| Cerebrovascular accident | 3104 | Gamma (3104±1388) | 8621 | Gamma (8621±3 855) | Levy et al38 and HCB |
| End-stage renal disease | 2247 | Gamma (2247±1005) | 40 939 | Gamma (40 939±13 308) | Parra Moncasi et al39 and HCB |
| Renal transplantation | 31 438 | Gamma (31 438±14 060) | 7 724 | Gamma (7724±3454) | Temes40 and HCB |
| Other costs | |||||
| Barostim implantation | 1672 | HCB | |||
| Barostim system | 21 000 | CVRx Inc. | |||
| Battery | 15 000 | CVRx Inc. | |||
| Battery replacement procedure | 309 | HCB | |||
HCB, Hospital Clínic de Barcelona; PSA, probabilistic sensitivity analysis.
It was assumed that the cost of the procedures for Barostim implantation and battery replacement would be the same as the cost associated with pacemaker implantation. The cost of the Barostim system was provided by the manufacturer. The drug therapy costs were obtained from the Spanish Pharmacopeia-National Formulary (Vademécum).41
Indirect CostsThe percent reduction in productivity caused by work disability or death was calculated as equal to 1 minus the utility level (). The productivity of 1 fully capacitated person was assumed to be equal to the crude mean cost of a Spanish worker in 2012 (30 905 euros/year).42 It was assumed that individuals older than 65 years would not be working and they were assigned a productivity of zero.
Effectiveness of BarostimThe decrease in SBP associated with Barostim use was based on published data.30,43 The SBP reduction observed in the study by Bakris et al43 was assumed to remain constant over the patient's lifetime (Table 4). The strategy used in the literature search on the effectiveness of Barostim is described in .
Effectiveness of Barostim (Systolic Blood Pressure Reduction)
| Parameter | Base case, mmHg | Source | DSA values | PSA distribution* |
|---|---|---|---|---|
| Reduction in the first months following implantation | 0 | Assumption based on Bisognano et al30 | — | — |
| Reduction 2-12 mo following implantation | 26 | Bisognano et al30 | 22-30 | Gamma (26±2.2) |
| Reduction 13-24 mo following implantation | 35 | Bisognano et al30 | 31-39 | Gamma (35±2.3) |
| Reduction after mo 25 following implantation | 35 | Bakris et al43 | 31-39 | Gamma (35±2.3) |
DSA, deterministic sensitivity analysis; PSA, probabilistic sensitivity analysis.
To examine the parameters having the strongest impact on the ICER, a deterministic sensitivity analysis was carried out. The baseline values for the clinical variables were substituted for extreme values, based on data from the related literature and clinical experience (Table 1 and Table 4).
A probabilistic sensitivity analysis was conducted using Monte Carlo methods. We performed 10 000 simulations of QALYs and costs, each of which was executed using random values from the parameters in the model (Table 1, Table 2, Table 3, and Table 4).
Impact on the BudgetA budgetary impact analysis was carried out, associated with the introduction of Barostim use in the Spanish Health System. The assumptions and main results are shown in .
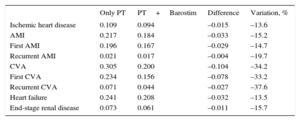
RESULTSBarostim lowered the probability of experiencing all the adverse events considered (Table 5). The probability of having a first CVA decreased by 0.078 points, representing a 33.2% drop in the number of cases compared with those occurring with optimal drug therapy. Recurrent CVAs decreased by 37.6%. The probability of having a first AMI fell by 0.029 points, implying a 14.7% reduction in the number of cases. Recurrent AMI decreased by 19.7%. Cases of ischemic heart disease and heart failure fell by approximately 13.5%. Finally, cases of end-stage renal disease decreased by 15.7%.
Frequency of Clinical Events per Individual in the Base Case
| Only PT | PT+Barostim | Difference | Variation, % | |
|---|---|---|---|---|
| Ischemic heart disease | 0.109 | 0.094 | –0.015 | –13.6 |
| AMI | 0.217 | 0.184 | –0.033 | –15.2 |
| First AMI | 0.196 | 0.167 | –0.029 | –14.7 |
| Recurrent AMI | 0.021 | 0.017 | –0.004 | –19.7 |
| CVA | 0.305 | 0.200 | –0.104 | –34.2 |
| First CVA | 0.234 | 0.156 | –0.078 | –33.2 |
| Recurrent CVA | 0.071 | 0.044 | –0.027 | –37.6 |
| Heart failure | 0.241 | 0.208 | –0.032 | –13.5 |
| End-stage renal disease | 0.073 | 0.061 | –0.011 | –15.7 |
AMI, acute myocardial infarction; CVA, cerebrovascular accident; PT, pharmacologic therapy.
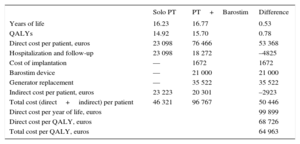
Patients treated with drug therapy alone obtained 14.92 QALYs, whereas those treated with Barostim reached 15.7 QALYs; that is, an increase of 0.78 QALY per patient (Table 6).
Effectiveness, Costs per Patient and Cost-effectiveness Ratio (Base Case)
| Solo PT | PT+Barostim | Difference | |
|---|---|---|---|
| Years of life | 16.23 | 16.77 | 0.53 |
| QALYs | 14.92 | 15.70 | 0.78 |
| Direct cost per patient, euros | 23 098 | 76 466 | 53 368 |
| Hospitalization and follow-up | 23 098 | 18 272 | –4825 |
| Cost of implantation | — | 1672 | 1672 |
| Barostim device | — | 21 000 | 21 000 |
| Generator replacement | — | 35 522 | 35 522 |
| Indirect cost per patient, euros | 23 223 | 20 301 | –2923 |
| Total cost (direct+indirect) per patient | 46 321 | 96 767 | 50 446 |
| Direct cost per year of life, euros | 99 899 | ||
| Direct cost per QALY, euros | 68 726 | ||
| Total cost per QALY, euros | 64 963 |
PT, pharmacologic therapy; QALY, quality-adjusted life year.
The average direct cost per patient receiving drug therapy was 23 098 euros, whereas the cost per patient with Barostim was 76 466 euros. The indirect costs were 23 223 euros and 20 301 euros, respectively. Considering both types of costs (direct plus indirect), the incremental cost of Barostim compared with pharmacologic therapy was 50 446 euros per patient.
The ICER of Barostim relative to drug therapy without considering indirect costs was 68 726 euros per QALY. Considering indirect costs, the ICER of Barostim vs pharmacologic therapy was 64 963 euros per QALY. The component that had the greatest weight in the incremental cost was the expenditure associated with replacement of the Barostim battery.
When the model was applied in a cohort of women, the Barostim device resulted in an increase of 0.54 QALY. The direct incremental cost was 60 242 euros and the ICER increased to 111 337 euros per QALY. Considering indirect costs, the ICER was estimated at 107 241 euros per QALY.
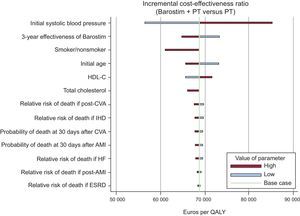
Sensitivity AnalysisIn the deterministic sensitivity analysis, the variables with the strongest impact on the ICER were the initial SBP, the effectiveness of Barostim starting from 3 years following implantation, smoker status, and the initial patient age. The deterministic sensitivity analysis showed that none of the parameters studied substantially modified the ICER (Figure 2).
Results of the deterministic sensitivity analysis. AMI, acute myocardial infarction; CVA, cerebrovascular accident; ESRD, end-stage renal disease; HF, heart failure; HDL-C, high-density lipoprotein cholesterol; IHD, ischemic heart disease; QALY, quality-adjusted life year; PT, pharmacologic therapy.
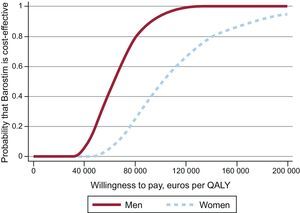
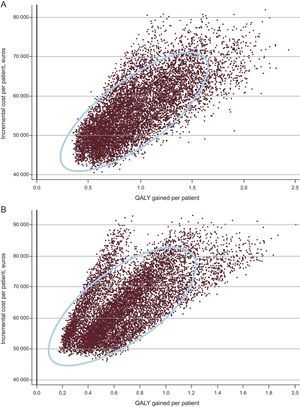
The probabilistic sensitivity analysis indicated that the probability of Barostim being cost-effective at a threshold of 30 000 euros per QALY was close to 0% (Figure 3). When the willingness to pay was raised to 100 000 euros/QALY, the probability increased to 80% in men and 25% in women. The cost-effectiveness scatter plot (Figure 4) shows that Barostim produced an increase in the number of QALYs and had an incremental cost of at least 40 000 euros per patient in all cases.
This study provides the first economic assessment of the Barostim medical device adapted to the health and social setting of Spain. The results indicate that Barostim use would contribute to a reduction in cardiovascular events in the study population. The number of AMI, CVA, and end-stage renal disease occurring in the study cohort would decrease by 15.2%, 34.2%, and 15.7%, respectively. The number of QALY would increase by 0.78% relative to treatment with drug therapy. However, this positive impact was associated with considerable expenditure: an incremental direct cost of 53 000 euros per patient receiving the device. Thus, the ICER of Barostim compared with drug therapy was close to 69 000 euros per QALY without considering indirect costs, or approximately 65 000 euros per QALY including indirect costs. In Spain, the informal cost-effectiveness threshold is estimated at 30 000 euros per QALY.44 Therefore, the cost of Barostim is high in relation to the health benefits that would be obtained in the Spanish health care setting. The ICER is also much higher than the value reported in the single published article on this topic, which analyzed the cost-effectiveness of Barostim in the German health system.5 Health care costs and indirect costs are lower in Spain than in Germany and because of this factor, Barostim use would enable smaller reductions in these costs in our country, partially explaining this difference.
The high ICER found in the present study also results from the relatively small increase in the number of QALYs associated with Barostim use. While it is true that the reduction in adverse cardiac events is considerable when measured in percent terms, the decrease is relatively moderate when viewed in absolute terms. Our estimates indicate that 3.3 AMI, 10.4 CVA, and 1.1 end-stage renal disease cases would be averted per each 100 patients receiving a Barostim device. These values are considerably lower than those reported by Borisenko et al5 in the German study, citing estimated reductions of 5.1 AMI, 12 CVA, and 2.3 end-stage renal disease cases per each 100 patients with the device. The main explanation for these differences is the lower incidence of cardiac and cerebrovascular diseases in hypertensive patients in Spain relative to their northern European counterparts. For example, according to the REGICOR10 study, an individual with the characteristics used in our study would have a 7.6% 10-year probability of experiencing coronary disease (ischemic heart disease or AMI). In the study by Borisenko et al using the Framingham equations,45 the estimated probability would be between 20% and 25%. Similarly, the risk of CVA (as a first event) in the base cohort over a 10-year horizon would be 4.4% according to the FRESCO11 study but would be 11% according to the Framingham equations for CVA.46
One factor that has a substantial impact on the cost of Barostim is the need for battery replacement throughout the patient's lifetime. Our estimates indicate that this accounts for almost 50% of the total direct cost. This finding can be explained by the current price of the battery, which requires replacement every 6 years. When this expenditure is omitted, the ICER would drop to 22 982 euros per QALY. Thus, the ICER would be decreased by future reductions in the cost of the batteries or improvements in their service life.
One of the main limitations of this study is the lack of robust information on the effectiveness of Barostim in periods longer than 3 years. The data on the long-term effectiveness of the device (ie, to the end of the patient's life) included in the analysis were based on a report showing a decrease of 35 mmHg after a mean follow-up of 2.3 years.43
Second, costs related to possible adverse effects associated with Barostim were not included in the calculations. The adverse effects identified were related to implantation of the device and included permanent nerve damage (4.8%), temporary nerve injury (4.4%), and other surgical complications (4.8%).30 If these events had been incorporated in the model, the ICER of Barostim would have been higher.
Lastly, as there are no studies on heart failure risk in the Spanish population, this factor was measured using the original Framingham equations.12 Hence, the predicted number of heart failure cases and associated adverse events may have been overestimated in our model.
CONCLUSIONSAlthough Barostim is effective in reducing SBP and progression to undesirable health states in the short term, based on the current prices and considering a willingness to pay of 30 000 euros per QALY, it is not a cost-effective option for treating the hypertensive population refractory to drug therapy in the Spanish health care setting.
FUNDINGThis study was partially funded by the SEC. M. Soto received support from the Plataforma de Innovación en Tecnologías Médicas y Sanitarias, PT13/0006/0009 project (Fundació Clínic per a la Recerca Biomèdica, PI043029).
CONFLICTS OF INTERESTJ. Brugada is a consultant on the CVRx Scientific Committee.
We thank Krzysztof Lach and Gemma Seda for the help they provided in Fundació Clínic per a la Recerca Biomèdica.