Mechanical complications following a myocardial infarction are uncommon, but with dramatic consequences and high mortality. The left ventricle is the most often affected cardiac chamber and complications can be classified according to the timing in early (from days to first weeks) or late complications (from weeks to years). Despite the decrease in the incidence of these complications thank to primary percutaneous coronary intervention programs —wherever this option is available—, the mortality is still significant and these infrequent complications are an emergent scenario and one of the most important causes of mortality at short term in patients with myocardial infarction. Mechanical circulatory support devices, especially if minimally invasive implantation is used avoiding thoracotomy, have improved the prognosis of these patients by providing stability until definitive treatment can be applied. On the other hand, the growing experience in transcatheter interventions for the treatment of ventricular septal rupture or acute mitral regurgitation has been associated to an improvement in their results, even though prospective clinical evidence is still missing.
Keywords
In recent decades, advances in pharmacological, percutaneous, and surgical reperfusion have improved outcomes and prognosis in patients with acute myocardial infarction (AMI). However, patients with large AMI or who do not receive timely reperfusion are at risk of mechanical complications of AMI.1 Their incidence can be influenced by many factors. For example, there was a recent spike in this type of complication during the SARS-CoV-2 pandemic, which was mainly due to delayed first medical contact.2
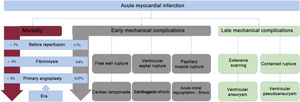
Although mechanical complications after AMI are uncommon, their consequences are dramatic and potentially lethal. The most frequently affected chamber is the left ventricle and complications are classified by time of onset after the primary event into early (days to weeks later) and late (weeks to years later).3
Early mechanical complications after AMI are ventricular septal rupture (VSR), acute mitral regurgitation (MR), and left ventricular free wall rupture (LVFWR), whereas late complications include the formation of pseudoaneurysms or true aneurysms. The common underlying mechanism is due to extensive transmural myocardial necrosis after rupture of a cardiac structure (in early complications) or extensive scarring of the affected tissue (in late complications). In AMI, there is a loss of functioning ventricular myocardium and, due to this loss, a progressive reduction in left ventricular ejection fraction that can lead to cardiogenic shock.3,4
Although the incidence of mechanical complications has decreased due to the use of percutaneous coronary intervention in the reperfusion era, mortality remains significant. While these complications are considered rare, they are an emergency scenario and are a relevant cause of short-term mortality5 (figure 1).
INCIDENCEThe actual incidence of the 3 early complications, published case series report diverse results due to underestimation or variation among the populations studied. In general, the incidence rate of these complications has been estimated to be 3/100 patients with AMI and most of them occur in patients with ST-segment elevation acute coronary syndrome (STEACS). Among such patients, the most common of the 3 complications is VSR and the least common is LVFWR.6,7
It is difficult to determine the true incidence of these complications, but in 2019 Elbadawi et al.8 analyzed 9 million hospitalizations for AMI between 2003 and 2015 in the United States. Their results were as follows: VSR in 0.21% of patients with STEACS and in 0.04% of patients with non—ST-segment elevation acute coronary syndrome (NSTEACS); papillary muscle rupture (PMR) in 0.05% of patients with STEACS and in 0.01% of patients with NSTEACS; and LVFWR in 0.01% of patients with STEACS or NSTEACS.8
GENERAL CONSIDERATIONSAlthough the incidence of these mechanical complications remains low, the associated mortality is very high (10% to >50%) (table 1). Furthermore, surgical and percutaneous treatment procedures are typically complex and require the expertise of a multidisciplinary team comprising cardiologists, interventional cardiologists, cardiac surgeons, intensivists, heart failure and heart transplant specialists, nurses, and palliative care specialists. The acuteness and lethality of these complications highlight the need for early diagnosis and immediate treatment to mitigate cardiogenic shock and avoid the risk of death.9
Summary of major early mechanical complications
| Early complication | Time course | Presumptive/established diagnosis | Treatment | Mortality |
|---|---|---|---|---|
| Free wall rupture | 3-5 d after STEACS | Tamponade/shockEchocardiogram | Emergency surgery | > 50% |
| Ventricular septal rupture | 3-5 d after STEACS | Murmur/shockEchocardiogramSpO2 in pulmonary artery> SpO2 in right atrium | Ventricular unloading (IABP, Impella*)Surgical repairPercutaneous repair | 30%-40% |
| Papillary muscle rupture | 2-7 d after STEACS | Acute pulmonary edema/shockEchocardiogram | Surgical replacement/repairPercutaneous edge-to-edge repair | 10%-40% |
IABP, intraaortic balloon counterpulsation; STEACS, ST-segment elevation acute coronary syndrome.
Over the past 2 decades, the systematic adoption of early percutaneous revascularization for patients with AMI has had a favorable impact on the overall incidence of these mechanical complications. However, despite these improvements, studies have yielded conflicting results.10 Although several studies have demonstrated favorable outcomes, most have found that the mortality rate associated with these complications has remained unchanged despite a marked increase in the use of percutaneous treatment and ventricular assist devices (VADs) and improvements in surgical techniques.
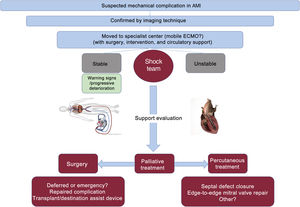
USE OF VENTRICULAR ASSIST DEVICES AND TRANSPLANTATIONPatients with mechanical complications secondary to AMI show rapidly progressive heart failure that can lead to cardiogenic shock. Therefore, these patients should be treated in hospitals able to implant VADs and with a cardiac surgery service11 (figure 2). For patients with hemodynamic instability or cardiogenic shock, the insertion of an intra-aortic balloon pump should be considered and, in cases of refractory hemodynamic collapse, hemodynamic support with extracorporeal membrane oxygenation is needed to stabilize patients until they can receive definitive treatment.11,12 Although the use of Impella-type VADs (Abiomed, United States) is more controversial, they have provided successful hemodynamic support in patients with VSR and PMR prior to surgery.13
In patients who are not candidate for surgical or percutaneous treatment, such as those with biventricular and multiorgan failure, the indication for orthotopic heart transplant or destination VADs should be assessed. Operative mortality is still high in patients undergoing heart transplant with percutaneous extracorporeal membrane oxygenation support to minimize the number of thoracotomies; nevertheless, this approach can be a successful bridging treatment in patients in whom there is no other alternative.
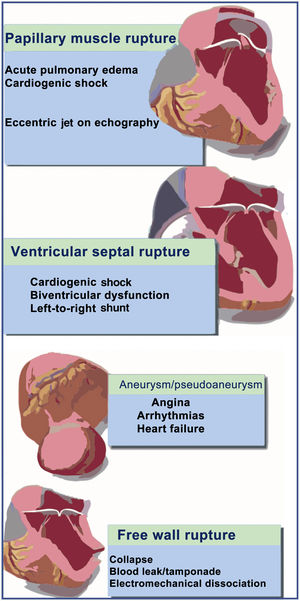
EARLY MECHANICAL COMPLICATIONSThe current treatment algorithm for these patients is shown in figure 2. The most frequent findings associated with these types of mechanical complication are shown in figure 3.
Ventricular septal ruptureVentricular septal rupture is a tear in the interventricular septum causing acute left-to-right shunt, pulmonary circulation overload, and biventricular dysfunction. Septal tear usually occurs at the margin of necrotic and nonnecrotic myocardial tissue; there is usually only 1 tear, which can vary in size and course through the interventricular septum. The course can be direct, irregular, or serpiginous.14 The size of the defect determines the magnitude of the left-to-right shunt, which in turn is directly related to prognosis and survival in these patients.15,16
Currently, the incidence of VSR after AMI is approximately 0.3%. Risk factors include older age, female sex, and nonreperfused AMI. It typically occurs 3 to 5 days after AMI and clinical presentation can range from exertional dyspnea to cardiogenic shock. Physical examination is usually characterized by a new-onset, high-intensity, holosystolic heart murmur, typically at the lower left parasternal border, accompanied by signs of venous congestion and findings related to cardiogenic shock and low cardiac output (hypotension, tachycardia, cold extremities, and oliguria).14 Initial diagnosis is based on cardiac murmur, but is confirmed by transthoracic echocardiography. In patients referred to the catheterization laboratory for coronary angiography, the diagnosis will be confirmed by left ventriculography showing the passage of contrast medium from the left to the right ventricle through the defect.15
Small defects may very rarely be asymptomatic; nevertheless, most of these patients are critically ill. Medical treatment aims to decrease left ventricular filling pressures and afterload, for which the use of inotropics, diuretics and, on many occasions, VADs should be considered. These patients should be thoroughly evaluated by a multidisciplinary team and referred for urgent surgery as a first option.17
Although surgery increases survival, its timing is controversial. Many studies have shown that operative mortality rates are lower in patients undergoing late surgery (ie, a few weeks after AMI), than in those undergoing early surgery.15 However, these results should be taken with caution; there is a possibility of patient selection bias, given that many patients who survive may undergo late surgery due their being in a less critical condition, being hemodynamically more stable, and probably having smaller defects, all of which decreases the actual surgical risk. This aspect suggests that surgery should not be delayed in an attempt to allow further septal fibrosis, because many patients do not survive such delays due to progressive cardiogenic shock, infections, and multiorgan failure. As a bridge-to-surgery treatment for patients in cardiogenic shock, hemodynamic stabilization should be preformed by the use of inotropics, vasopressors and, in some cases, VADs.18 It should be noted that coronary angiography must be performed before the surgical procedure to determine the coronary anatomy; if significant coronary artery disease is confirmed, concomitant coronary revascularization surgery will be needed.15–17Percutaneous treatment options
Surgical repair of VSR is associated with a very high mortality rate and suboptimal results due to postoperative residual shunt that can occur in up to 20% of cases. This issue has been addressed by the development of percutaneous VSR closure techniques.19
Percutaneous closure may be considered in patients who are not candidates for VSR surgical repair because of high risk. A number of studies have demonstrated the feasibility and efficacy of percutaneous closure using septal occluder devices. Although the success rate of this procedure can be close to 90% in experienced centers, in-hospital mortality is high and complications related to the procedure are common, including embolization or migration of the closure device, hemolysis, and partial or incomplete closure of the defect. Even though complete closure of small- and medium-sized ruptures has been reported, in many cases only partial closure of the defect can be achieved. However, simply reducing the size of the defect is considered to be beneficial because the degree of shunt is reduced and the patients’ hemodynamic conditions may improve until such time as myocardial tissue healing is adequate and definitive surgical closure is feasible.19,20 The approach to percutaneous closure varies, but recent series suggest that the transjugular approach may offer a better chance of success due to a more favorable sheath orientation4 (). Keys to recent improved success rates with this technique include the use of multimodal imaging techniques and implantation under external guidance to provide support and adequate sheath guidance. Given the low number of procedures addressing this complication, clinical practice guidelines indicate that percutaneous treatment is an alternative to surgery in centers with an appropriate degree of experience (without precisely clarifying what is “appropriate”); irrespective of this consideration, decisions should always be taken by a multidisciplinary team.
Ventricular assist devices should be used to stabilize patients in refractory cardiogenic shock with biventricular dysfunction that contraindicates surgical or percutaneous closure and who are otherwise good candidates, and to improve hemodynamic conditions while awaiting heart transplant.17–19Acute mitral regurgitation secondary to papillary muscle rupture
MR occurs in AMI through 2 main mechanisms: PMR and papillary muscle dysfunction secondary to ischemia. Acute MR due to papillary muscle dysfunction is much more common than that caused by PMR.
The mitral valve comprises 2 papillary muscles: the anterolateral and the posteromedial. The anterolateral muscle has a dual supply from the anterior descending and circumflex arteries, whereas the posteromedial muscle has a single supply from the posterior descending artery, which can originate from the circumflex or the right coronary artery (depending on coronary dominance). Therefore, anterolateral PMR is extremely infrequent, whereas posteromedial PMR is more common and is typically associated with inferior or lateral AMI; however, it can also be seen in NSTEACS.22
The incidence of acute MR secondary to PMR, like the other mechanical complications of AMI, has decreased in the reperfusion era, but mortality rates remain high at 10% to 40%.21
Risk factors include older age, female sex, poor collateral circulation to the affected papillary muscle, single-vessel disease, first AMI, nonreperfused AMI, and large AMI.
This mechanical complication usually occurs 2 to 7 days after AMI and muscle rupture may be partial or complete. About half of these patients have acute pulmonary edema that can rapidly progress to cardiogenic shock.23 Physical examination shows a characteristic new-onset holosystolic murmur that is heard more intensely in the apex, with a diastolic component and radiation to the axilla, although in some patients this may be absent due to rapid pressure equalization between the left atrium and ventricle. Thus, the initial diagnosis is mainly based on transthoracic echocardiography, but in many cases transesophageal echocardiography is needed given its greater diagnostic accuracy.24
If there is acute pulmonary edema, standard initial treatment is noninvasive ventilation or orotracheal intubation. The use of vasodilators and afterload reduction helps to reduce MR and increase antegrade flow. When the clinical presentation suggests cardiogenic shock, vasopressors and inotropic drugs should be prescribed and VADs may also be needed.23 The mortality rate using medical treatment alone is close to 50% and consequently early surgery is recommended.24
Emergency surgery is the treatment of choice for these patients. In contrast to surgery for VSR, surgery for PMR does not usually involve the manipulation of necrotic myocardium, and so procedural-related mortality is lower; although there is high variability, the survival of patients under medical treatment alone is very low.23 There is some debate about the optimal surgical approach because, under ideal conditions, mitral valve repair should be performed instead of replacement; however, this approach is only recommended in centers with experience in this procedure. Therefore, in general, papillary muscle-sparing mitral valve replacement is more frequently performed, since this procedure has established durability. If needed, coronary revascularization should also be performed during the same procedure.23,24
Percutaneous treatment optionsAlthough surgery remains the treatment of choice for patients with severe MR secondary to PMR, some patients may be at prohibitive surgical risk. Percutaneous edge-to-edge repair has become the treatment of choice in patients with chronic MI who are at such risk. This therapeutic option may be suitable for some patients with acute MR due to PMR in cardiogenic shock and at prohibitive surgical risk.25 One case report has described the successful use of MitraClip (Abbott, United States) in the treatment of these patients; however, this information should be treated with caution due to possible publication bias. Treatment should be individualized in all patients and each case analyzed by a multidisciplinary team.26
Other treatment options for patients at prohibitive surgical risk include medical treatment in combination with VADs as bridge therapy to stabilize and improve the patients’ clinical status and prepare them as candidates for surgical mitral repair or heart transplant.24
Although orthotopic prostheses remain untested in this setting, they offer a promising new option when the edge-to-edge option is unfeasible.
Rupture of the left ventricular free wallMyocardial rupture more often affects the left ventricle than the right. For this reason the literature typically refers to it as LVFWR. It is defined as transmural necrosis and consequent rupture of the ischemic ventricular wall, with bleeding into the pericardial space, eventually resulting in cardiac tamponade. The culprit vessels are usually deemed to be the anterior descending and circumflex arteries.27
The actual incidence of LVFWR remains unknown, because it typically occurs as sudden out-of-hospital death. However, LVFWR should be suspected when patients show hemodynamic instability or cardiogenic shock after AMI, especially in the setting of nonreperfused AMI or ineffective pharmacological reperfusion therapies.28
Previous observational studies in the reperfusion era have drawn attention to several risk factors. The frequency of LVFWR frequency is about 10 times higher in patients with the following characteristics: no history of angina or AMI; nonreperfused AMI or ineffective pharmacological reperfusion therapy; and elevated creatine kinase MB fraction concentrations (> 150 U/L).27
Clinical presentation varies and depends on whether the LVFWR is complete or partial. In the former case, clinical presentation will be cardiac tamponade and sudden death, whereas in the latter case, presentation is usually less dramatic, with angina or pericardial chest pain because the rupture is usually contained and sealed with organized thrombus and pericardium.27 LVFWR can develop within the first 5 days after AMI. However, in 90% of patients, it typically arises around 2 weeks after the initial event. Physical examination typically shows jugular venous distention, abolished or diminished heart sounds, paradoxical pulse, or evident electromechanical dissociation.28
Diagnosis is based on clinical and echocardiographic signs of cardiac tamponade. Echocardiography will typically show pericardial effusion with physiology of cardiac tamponade and evidence of cardiac chamber collapse; in some cases rupture may be seen.28
Patient survival depends on early recognition of rupture and prompt treatment. In patients with suspected LVFWR, initial medical treatment is directed at hemodynamic stabilization with fluids, vasopressors, and inotropic support. Urgent pericardiocentesis is indicated as rescue therapy.28 If the patient is stabilized and bleeding stops, conservative treatment may sometimes be warranted, but emergency surgery should be considered as paramount. Most of these patients do not survive this acute phase, experiencing cardiogenic shock leading to cardiorespiratory arrest typically with pulseless electrical activity.29
When selecting the surgical technique, direct closure techniques should be avoided given that sutures can easily tear the extremely labile necrotic tissue. The two most frequently used techniques are infarterectomy with patch reconstruction of the ventricular wall and total coverage of the affected region with an oversized patch of pericardium reinforced with glue.30
LVFWR has the worst prognosis of all mechanical complications after AMI. According to data reported in the SHOCK Trial Registry, in-hospital mortality is close to 70%.24
There are no percutaneous options for the definitive treatment of this complication, despite reports of although anecdotal cases of closure using Amplatzer-type devices (Abbott, United States); however, this approach has only been followed in cases of late presentation of pseudoaneurysm.31
LATE MECHANICAL COMPLICATIONSTrue left ventricular aneurysmTrue left ventricular aneurysm (LVA) is defined as an abnormal protrusion of the vascular wall containing all the layers of the original structure. It is characterized by a well-delineated, thin, scarred, or fibrotic wall lacking muscle tissue or containing necrotic muscle due to a healed transmural AMI. Such segments are typically akinetic or dyskinetic. Approximately 85% of true LVAs are located in the apex or anterior wall due to total occlusion of the anterior descending artery and absence of collateral circulation. Only 10% to 15% of true LVAs affect the inferior wall due to total occlusion of the right coronary artery, whereas true LVA of the lateral wall due to total occlusion of the circumflex artery is extremely rare.32
The incidence of LVA used to be as high as 35% in AMI but has fallen to between 10% and 15% with the advent of pharmacological and percutaneous reperfusion.
A number of serious complications can result from true LVA, including heart failure, ventricular arrhythmias, and embolic phenomena.32 The end result of long-term volume overload with prolonged ischemia is left ventricular dilation and failure. Two mechanisms underlie ventricular arrhythmias: myocardial tissue ischemia resulting in increased automaticity; and an aneurysm site composed of a mixture of fibrous tissue, and necrotic and inflammatory cells, which can create ectopic arrhythmogenic foci. In both cases, these ventricular arrhythmias can cause sudden death. Aneurysmal blood stasis as well as blood contact with aneurysmal procoagulant fibrous tissue lead to thrombus formation, which is present in up to 50% of patients with true LVA. This situation can lead to systemic embolism events, notably cerebral embolism, and thus anticoagulation forms part of the treatment of these patients.32,33
Small- or medium-sized aneurysms can be managed with medical treatment alone; previous studies have reported survival rates of more than 90% at 5 years. Treatment consists of the use of afterload-reducing agents, such as angiotensin-converting enzyme inhibitors, antianginal drugs, and oral anticoagulants if there is evidence of a thrombus in the aneurysm or left ventricle.33
For large aneurysms, American and European guidelines recommend aneurysmectomy combined with coronary revascularization surgery as a reasonable option for patients with intractable ventricular arrhythmias, angina refractory to medical treatment, heart failure refractory to medical treatment, or embolic events despite optimal anticoagulation. In these clinical scenarios, surgical repair is highly effective and, compared with medical treatment alone, significantly improves patient survival, symptoms, and functional class.34
Left ventricular pseudoaneurysmsLeft ventricular pseudoneurysms, also called false aneurysms, form when the cardiac rupture is contained by pericardial adhesions or scar tissue. Unlike true LVAs, they do not contain endocardium or myocardium. In addition, they more often involve the inferior or lateral wall, which is probably due to the development of pericardial adhesions in recumbent patients convalescing after AMI.35
It was previously thought that these patients showed a number of nonspecific signs and symptoms and that some patients could even be asymptomatic; however, recent studies have shown that, at some point in their clinical course, most patients have symptoms of heart failure, angina, ventricular arrhythmias, and systemic embolism, as is often the case in true LVA.36
Although the initial diagnostic test is echocardiography, its diagnostic accuracy is low. The most accurate diagnostic method is angiography, which shows a narrow orifice leading to a saccular aneurysm. Computed tomography and magnetic resonance imaging are alternatives to angiography and both are very useful in distinguishing true LVAs from left ventricular pseudoneurysms.35
The risk of rupture in untreated left ventricular pseudoneurysms is up to 45%. Consequently, it has been recommended that patients be referred for emergency surgery upon diagnosis.36 Surgery is the preferred treatment option, given that perioperative mortality is less than 10% with current techniques. The same surgical technique is typically used for both pseudoaneurysms and true aneurysms.34
Percutaneous treatment optionsIn patients at high surgical risk, percutaneous closure of left ventricular pseudoneurysms should be considered as an alternative to traditional surgery and particularly in patients not requiring concomitant coronary revascularization surgery.37 Previous studies have demonstrated the feasibility and efficacy of percutaneous closure of left ventricular pseudoneurysms using septal occlusion devices. In such patients, imaging techniques, such as computed tomography and magnetic resonance imaging, are needed to accurately determine the anatomy and plan the procedure.37,38
CHALLENGES IN THE PERCUTANEOUS TREATMENT OF MECHANICAL COMPLICATIONS OF AMIAlthough mechanical complications currently occur in less than 1% of patients admitted with AMI, patients should be treated in centers with the experience and all the technical equipment needed for their successful treatment.
Such centralization would be key to the systematic inclusion of these patients in pragmatic clinical trials, which would facilitate the development of successful care pathways and reduce the enormous variability in care that currently exists. The scarce evidence related to therapeutic options mainly comes from case series and is thus affected by publication bias. The establishment of a global initiative, perhaps within the setting of the expert group responsible for a recent article on cardiogenic shock,39 could be useful for recording and improving the care of mechanical complications and for the acquisition of the skills needed by surgical and interventional teams. Future research should address the indications for circulatory assist devices and their type, as well as the most appropriate timing for intervention based on the recent and wider availability of such aids.
CONCLUSIONSEarly revascularization is the treatment of choice for patients with AMI. As primary angioplasty has become more widespread, the incidence of mechanical complications in our setting is less than 0.1%. However, when mechanical complications occur, the clinical presentation is dramatic, with acute hemodynamic instability and a high associated mortality rate, which requires immediate recognition and urgent treatment. A multidisciplinary approach is needed to select the optimal treatment, whether percutaneous or surgical, and its timely application is essential. The percutaneous implantation of circulatory support systems, which is faster and less invasive, also opens up the possibility of keeping patients stable until they can be offered the definitive therapeutic alternative. This strategy would help to increase experience in percutaneous techniques, thus helping to produce more reproducible results and encourage well-designed prospective studies.
FUNDINGNone declared.
AUTHORS’ CONTRIBUTIONSBoth authors contributed to the design of the article and approved its final version.
CONFLICTS OF INTERESTNone declared.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.11.015