Despite advances in drug therapy, pulmonary hypertension—particularly arterial hypertension (PAH)—remains a fatal disease. Untreatable right heart failure (RHF) from PAH eventually ensues and remains a significant cause of death in these patients. Lowering pulmonary input impedance with different PAH-specific drugs is the obvious therapeutic target in RHF due to chronically increased afterload. However, potential clinical gain can also be expected from attempts to unload the right heart and increase systemic output. Atrial septostomy, Potts anastomosis, and pulmonary artery denervation are interventional procedures serving this purpose. Percutaneous balloon pulmonary angioplasty, another interventional therapy, has re-emerged in the last few years as a clear alternative for the management of patients with distal, inoperable, chronic thromboembolic pulmonary hypertension. The current review discusses the physiological background, experimental evidence, and potential clinical and hemodynamic benefits of all these interventional therapies regarding their use in the setting of RHF due to severe pulmonary hypertension.
Keywords
Pulmonary hypertension (PH) is defined by a mean pulmonary artery pressure (PAP) > 25mmHg at rest, measured during right heart catheterization.1 The term pulmonary arterial hypertension (PAH) describes a subpopulation of patients with PH (group 1 of the current clinical classification), characterized by the presence of precapillary PH including a pulmonary artery wedge pressure < 15mmHg and a pulmonary vascular resistance > 3 Wood units.1,2 PAH is a progressive and fatal disorder that affects the pulmonary vasculature and the heart and has no cure. Progressive right heart failure (RHF) remains the main cause of death in this population.
In the last 2 decades, there has been an extraordinary advance in the treatment of PAH. The use of PAH-specific drugs targeting dysfunctional pathways that lead to the characteristic vascular remodeling in this condition have improved both quality of life and survival.1–3 However, PAH-specific drugs are not always available and, most importantly, not all patients respond.1,4,5 Furthermore, many patients still deteriorate over time despite treatment and therefore other therapeutic alternatives should be considered. In this regard, dedicated interventions have been applied to selected patients with PAH.1,3,6–15 These include established and more widespread procedures such as atrial septostomy (AS) and attractive emerging strategies, including a Potts shunt (anastomosis),1,3,8–11 pulmonary artery denervation (PADN),12–14 and balloon pulmonary angioplasty (BPA) in patients with inoperable chronic thromboembolic PH (CTEPH).1,6,7,15 This review highlights current understanding and the potential role of all these interventions in the management of RHF in PH.
BALLOON ATRIAL SEPTOSTOMYThere is clinical and experimental evidence suggesting that an interatrial shunt may be of benefit in the setting of PAH. From a clinical standpoint, we know that primary PH patients with a patent foramen ovale live longer than those without a shunt.16 In addition, we have recognized that patients with Eisenmenger's syndrome with a comparable degree of PH live longer and have less RHF than patients with primary PH.17 Several experimental studies have shown the potential benefit of a right-to-left shunt in the setting of PH.18,19 Years ago, several techniques to perform AS without thoracotomy were developed20,21 and in 1983 Rich and Lam22 performed the first AS in a patient with PAH. However, the precise role of AS in the management of PAH remains uncertain because most of our knowledge comes from small, noncontrolled series or case reports, and a somewhat generalized perception that the procedure carries an unacceptably high risk. Interestingly, experience with AS has increased considerably in the last few years (Figure 1).
Worldwide experience with atrial septostomy in the treatment of pulmonary arterial hypertension. The left panel shows the number of procedures over the years. The right panel shows that the immediate procedure-related mortality has decreased as a result of experience and modifications to the technique.
Since the last review,23 5 more series10,24–27 and case reports have been added.28–31 To date, 461 procedures have been performed in 364 patients, as reported in 28 series () and 26 case reports. Atrial septostomy has been performed both in child and adult populations (mostly women [68%]) with idiopathic PAH (76%) in advanced stages of the disease (functional classes III and IV) and failed medical treatment.
Persistent RHF alone (53.4%) or in combination with syncope (21.4%) remains the most common indication for the procedure (). Balloon dilatation AS (BDAS) accounts for by far the most widely used technique (83%). Reported series have shown beneficial hemodynamic effects, including a decrease of ∼20% in right atrial pressure (RAP), an increase of about 30% in cardiac index and, as expected, a decrease of ∼10% in arterial oxygen saturation (SaO2%). Furthermore, mortality associated with the procedure has decreased significantly over the last few years (Figure 1).
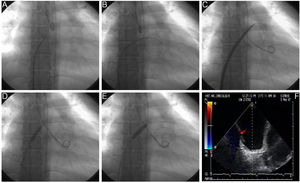
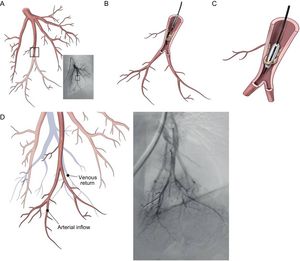
Procedural Details of Technical AspectsBalloon dilation AS involves a standard right-left heart catheterization. Septal puncture is performed in most instances using a standard Brockenbrough needle and a long sheath and dilator (ie, Mullins-type sheath) to cross the atrial septum. Sequential static atrial septoplasty is gradually performed using different sized noncompliant peripheral balloons in a careful and controlled (step-by-step) approach32,33 (Figure 2). A decision on the final diameter of the septostomy is reached when the following hemodynamic changes are met: a drop in baseline SaO2% of no more than 10%, and/or an increase in left ventricular end-diastolic pressure. We advocate maintaining the latter below 18mmHg. Altogether, these recommendations are important to avoid refractory hypoxemia and/or pulmonary edema, 2 life-threatening complications of the procedure. In the worldwide experience, the mean size of the atrial shunt is 11mm (range, 8-18). Reintervention has been repeated on 71 occasions due to spontaneous closure of the defect during follow-up.
Balloon dilation atrial septostomy technique. The procedure involves a standard right and left heart catheterization. A: View of the Mullins dilator with the Brockenbrough needle at its tip. A small contrast injection is done to map the intended puncture site, which can also be done by using intracardiac echocardiography. B: Once perforation of the septum is done, an Inoue circular-end guidewire is positioned in the left atrium. C: A 4-mm initial dilation of the atrial septum is done with the Inoue dilator, and concluded with an 8mm balloon (D and E). Dilation of the atrial septum can occasionally offer moderate resistance but it has been always opened successfully with hand inflation, which has been measured to reach 4 to 6 atmospheres. Inflation is increased just enough to eliminate the balloon waist (D) and is repeated at least twice to counter elastic recoil. F: Transesophageal echocardiography view of the created defect. Modified from Sandoval et al.32 with permission.
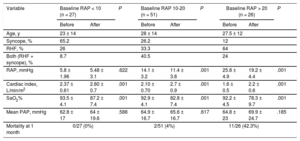
Hemodynamic changes after septostomy depend on baseline RAP23 (Table); at higher baseline RAP, the more pronounced hemodynamic effect, especially in patients with RAP > 20mmHg. However, it is this subset of patients who are deemed at high risk for complications including death during the procedure as a result of refractory hypoxemia. RAP > 20mmHg has been associated with more than 10 times the risk of death in these patients.23 We do not perform AS in this population. It may be considered that the best risk-benefit ratio is therefore for patients with an RAP between 10 and 20mmHg. Finally, most published data concerning hemodynamic impact following BDAS has been in the resting condition; however, it is during exercise when septostomy could prove to be even more useful, as a pop-off safety valve.18 This is highlighted by data showing improvement in exercise tolerance after creation of the atrial shunt.33–35
Hemodynamic Effects of Atrial Septostomy According to Baseline Right Atrial Pressure
| Variable | Baseline RAP < 10 (n = 27) | P | Baseline RAP 10-20 (n = 51) | P | Baseline RAP > 20 (n = 26) | P | |||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||||
| Age, y | 23 ± 14 | 28 ± 14 | 27.5 ± 12 | ||||||
| Syncope, % | 65.2 | 26.2 | 12 | ||||||
| RHF, % | 26 | 33.3 | 64 | ||||||
| Both (RHF + syncope), % | 8.7 | 40.5 | 24 | ||||||
| RAP, mmHg | 5.8 ± 1.96 | 5.48 ± 3.1 | .622 | 14.1 ± 3.2 | 11.4 ± 3.8 | .001 | 25.8 ± 4.9 | 19.2 ± 4.4 | .001 |
| Cardiac index, L/min/m2 | 2.37 ± 0.61 | 2.80 ± 0.7 | .001 | 2.10 ± 0.70 | 2.7 ± 0.9 | .001 | 1.6 ± 0.5 | 2.2 ± 0.6 | .001 |
| SaO2% | 93.5 ± 4.1 | 87.2 ± 7.4 | .001 | 92.9 ± 4.1 | 82.8 ± 7.4 | .001 | 92.2 ± 4.5 | 78.3 ± 9.7 | .001 |
| Mean PAP, mmHg | 62.8 ± 17 | 64 ± 19.6 | .588 | 64.9 ± 16.7 | 65.6 ± 16.7 | .617 | 64.8 ± 23 | 69.9 ± 24.7 | .185 |
| Mortality at 1 month | 0/27 (0%) | 2/51 (4%) | 11/26 (42.3%) | ||||||
PAP, pulmonary artery pressure; RAP, right atrial pressure; RHF, right heart failure; SaO2%, arterial oxygen saturation.
Reproduced from Sandoval et al.8 with permission.
Recent decreases in procedure-related mortality may be attributed to several factors. First and foremost, interventionists have followed the recommendations suggested in 1998 to minimize the risk of death during the procedure,36 in particular avoiding the procedure in patients with impending death and performing it only in centers with experience in both PAH and AS. Second, technical modifications have been established to improve safety during septostomy. The technique evolved from the time when blade-balloon AS was first described,22 when controlling the size of the shunt was uncertain in contrast to a recent trend to switch to BDAS, originally described by Hausknecht et al.37 and Rothman et al.,38 and soon adopted by our group in 1998.33 As previously mentioned, performing a step-by-step procedure has resulted in lower procedure-related mortality. Another relevant aspect concerning procedural safety has been the introduction of intracardiac echocardiography to guide transeptal puncture, first described by Moscussi et al.39 and quickly adopted by many others.27,40–44
Preserving Shunt PatencySpontaneous atrial shunt closure after septostomy is frequently encountered during mid-term follow-up. Various approaches have been used to address this problem. One has been the implantation of custom-made fenestrated occluders after septostomy (Figure 3).44–50 Another approach is the use of a fenestrated stent during septostomy as described by Stumper in 2003 with the so-called diabolo-fenestrated stent technique,24 which has been used in some recent series,27,35 or a “butterfly stent” described by Prieto et al.40 and modified by Roy et al.42 Recently, Guerrero et al.28 reported the use of cryoplasty to freeze the border of the newly created atrial defect in order to maintain patency. Although reported successful in the short-term, it remains to be determined whether these devices and interventions are useful and last in the long-term. This is certainly an interesting and promising area of research and development in the near future50 (see also PROPHET trial, NCT03022851).
Outcome After Septostomy and Impact of Septostomy on Long-term SurvivalIn most series, an immediate improvement in functional class, symptoms such as syncope, and/or RHF is reported for most patients who survive the procedure. However, there is no formal analysis regarding mid-term outcomes. In a recent publication, Chiu et al.25 have reported that for patients in whom the indication was refractory RHF (n = 21), half improved and only 5 died from RHF. For those who underwent septostomy for syncope (n = 19), most improved and only 2 patients died during the first year. The impact of septostomy on survival of these patients is difficult to establish as long-term controlled studies are lacking, but reports from Kerstein et al.,51 Sandoval et al.,33 Law et al.,52 and Troost et al.35 and more recently by Chiu et al.25 and Sandoval et al.9 have shown a beneficial effect on survival at 1, 2, and 3 years, when survival is compared with that of historic controls or predicted survival. In all of these studies, however, there is a decrease in the survival curves over time, reflecting the palliative nature of the procedure.
In summary, based on a review of the collective worldwide experience, BDAS stands as an additional strategy in the treatment of severe RHF in the setting of PAH; it improves hemodynamic variables that correlate with clinical improvement and survival. Although performed in an advanced stage of the disease, AS results in clinical and hemodynamic benefit and a trend toward improvement in survival. Procedure-related mortality is decreasing as emerging imaging (ie, intracardiac echocardiography) and septal puncture techniques (ie, radiofrequency) have simplified the procedure.39,43,53 Atrial septostomy is useful for patients with failed combination drug therapy,27 as a bridging strategy to transplant,38 or when no other treatment option is available. All these represent the indications for the procedure.1,3,8,23,32
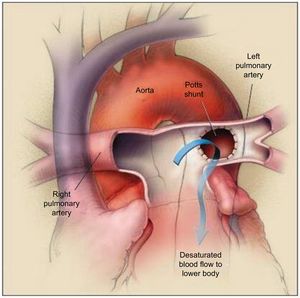
POTTS ANASTOMOSIS (SHUNT)The Potts shunt consists of the creation of an anastomosis between the left pulmonary artery and the descending aorta. Originally, this shunt was offered to alleviate cyanosis in several forms of congenital heart disease (ie, tetralogy of Fallot, pulmonary atresia).54 Interest in its implementation has been revisited for the treatment of PAH, as an alternative to AS using the same concept and rationale: creation of a right-to-left shunt to increase systemic output55 (Figure 4). In theory, the advantage of a Potts shunt over AS is that it avoids oxygen desaturation in the upper part of the body, including the brain and the coronary arteries.56
The Potts shunt procedure. The left pulmonary artery is anastomosed to the descending aorta, allowing the desaturated blood to go from the left pulmonary artery to the lower part of the body (arrow). The right pulmonary artery passes in front of the ascending aorta because an arterial-switch procedure has been performed. Reproduced with permission from Blanc et al.55
The first description of the use of a Potts shunt in the setting of PH was reported by Blanc et al.55 From within the same group, Baruteau et al.56 described the long-term experience with procedures performed in 8 children who had severe PAH refractory to medical PAH treatment. Potts was performed via a left thoracotomy without cardiopulmonary bypass. Two procedure-related deaths occurred due to low cardiac output attributed to discontinued preoperative medical treatment or to a restrictive anastomosis. The long-term outcome of the 6 survivors was excellent in terms of functional class and exercise capacity. An alternative transcatheter Potts shunt (TPS) was described by Esch et al.57 In that report, 4 patients with severe PAH underwent TPS under general anesthesia. Vascular perforation was performed under fluoroscopic guidance and a covered stent was placed between the pulmonary artery and the aorta. Although considered successful from a technical standpoint, 1 patient died during the procedure due to massive hemothorax and another died from ventilator-associated pneumonia. The 2 survivors had significant symptomatic improvement and no late complications at follow-up.
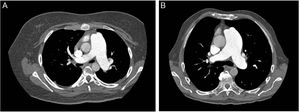
Since bleeding is the most feared complication of TPS, performing a chest computed tomography prior to the procedure could help select the ideal candidates, as proposed by Guo et al.58 There are 2 types of relationships between the left lower pulmonary artery and descending aorta (Figure 5); in type 1, there is practically no distance between the vessels (ideal candidate) while type 2 has a considerable gap between the structures, thus increasing the risk of bleeding. The radiofrequency-assisted perforation approach is another refinement to improve the safety profile in TPS creation.59
Computed tomography of the thorax prior to the procedure could help select the ideal candidates for Potts anastomosis. Two types of relationships exist between the left lower pulmonary artery and descending aorta. In type 1 (A), the distance between the vessels is minimal and in type 2 (B) the distance is greater, thus increasing the risk of bleeding. Patients with a type 1 relationship are better candidates for Potts anastomosis.
Performing a Potts shunt in a PAH center with solid surgical and interventional teams is highly desirable. Baruteau et al.11 have recently published a compendium of their experience in 24 children including both surgical anastomosis (n = 19) and TPS (n = 5) in the setting of PAH. After a median follow-up of 2.1 years, functional class and exercise capacity dramatically improved in the 21 survivors. Furthermore, this physiologic rationale has been extended to newborn and infant populations with suprasystemic PH of diverse etiologies by stenting the ductus arteriosus (residual or probe-patent).60
Potts anastomosis (surgical or TPS) is indeed an innovative approach for the management of PAH and an attractive alternative to AS. However, more experience and technical refinements are needed to decrease procedure-associated risks with the surgical approach and to establish TPS as an accepted therapeutic modality for advanced PAH.
PULMONARY ARTERY DENERVATIONPulmonary artery denervation is another innovative and very provocative interventional approach for the treatment of PH. It attempts to abolish the sympathetic nerve supply to the main pulmonary arteries, thus reducing increased sympathetic stimulation of the pulmonary circulation.61–72
Increased Sympathetic Activity in PAHSeveral investigations have demonstrated increased sympathetic activity in patients with PAH.61–63 The cause is incompletely understood.66,71 Increased filling pressures, decreased cardiac output, and an abnormal baroreflex control all appear to play a role in the increased sympathetic activation. Interestingly, increased sympathetic activity has been significantly reduced after BDAS in PAH patients.64 Excessive adrenergic stimulation in PAH, through β1-adrenergic receptor pathway desensitization, as seen in other forms of heart failure, may also impair RV function.65 Accordingly, excessive sympathetic overdrive may be an important mechanism in the pathophysiology of RHF in the setting of PAH.
Experimental Studies on the Effects of PADNPrevious publications have demonstrated that distension and occlusion of one branch of the pulmonary artery results in an increase in pulmonary vascular pressure and resistance that appears to be induced by a pulmo-pulmonary reflex.66,67 It has been postulated that baroreceptors are situated close to the bifurcation of the main pulmonary artery and are involved in facilitating a neural reflex as a result of activation by stretch receptors and also that this reflex could be prevented.67 Based on these findings, the first in vivo attempts with PADN were performed in a dog model of PH induced by balloon inflation of the pulmonary artery,68 demonstrating that catheter radiofrequency ablation performed proximal to the main bifurcation of the pulmonary artery was the most effective area for PADN. Subsequent experimental studies have confirmed the pattern of nerve injury after PADN,12 the relatively persistent hemodynamic benefits,69 and the potential downregulation of the activity of the renin-angiotensin-aldosterone system in local tissue after the procedure.70 Although these experimental models are of short duration and do not necessarily resemble human PAH,71 they provide evidence of the importance of the sympathetic nervous system in the pathogenesis of PAH and also that PADN, by interrupting the sympathetic activation, may play a role in the management of PAH.
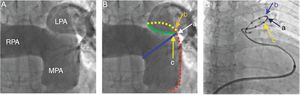
Human Experience With PADN in PAHBased on the experimental evidence and borrowing the concept of renal artery denervation in the treatment of refractory systemic hypertension, there has been recent interest in PADN as a therapeutic intervention in PAH.6,7,13,14 The experience with this intervention in human PAH is limited to a couple of studies performed by the same group of investigators.13,14 In the pilot first-in-human PADN-1 single-center study performed by Chen et al.,13 21 patients with PAH who were nonresponders to medical treatment were enrolled. Thirteen patients underwent PADN at the bifurcation of the main pulmonary artery and at the ostium of the right and left main pulmonary arteries with the use of a dedicated radiofrequency ablation catheter (Figure 6). Pulmonary artery denervation success, defined as a reduction in PAP > 10mmHg without complication, occurred in 12 of the 13 patients. At a mean follow-up of 3 months, mean PAP decreased (from 55 ± 5mmHg to 36 ± 5mmHg, P < .01) and the 6-minute walk test results also improved. This initial report was met with skepticism due to the nonrandomized nature of the study, the low-risk stratification of the cohort, and the short-term follow-up.
Pulmonary arterial angiography, position of electrodes, and pulmonary artery denervation procedure. A: Anterior-posterior and cranial (200) view of the pulmonary arterial angiograph. B: The red line represents the lateral wall of the main pulmonary artery (MPA), the blue line represents the anterior wall of the left pulmonary artery (LPA), and the point where the 2 lines intersect is point a; the intersection of the yellow (posterior wall of the LPA) and red lines is point b, which is 1 to 2mm posterior to point a; the green line starts from the inferior wall of the right pulmonary artery (RPA) and ends at point a, and point c localizes at this level and 1 to 2mm anterior to point a. C: A pulmonary artery denervation catheter with 10 electrodes is positioned at the distal MPA, with electrodes a, b, and c at points a, b, and c, respectively. Reproduced with permission from Chen et al.14
In a subsequent publication (phase II) from the Pulmonary Artery Denervation-1 study, Chen et al.14 expanded on their experience and reported the hemodynamic, functional, and clinical responses to PADN in 66 patients with PH of different etiologies. There were no complications during the procedure, although chest pain was a significant adverse effect.14 At 6 months, there was a reduction in the mean PAP from 53 ± 19 to 44.8 ± 16.4mmHg (P < .001), and this effect persisted at 1-year, which did not appear to be related to concomitant medical treatment because PAH-specific drugs were discontinued after the performance of PADN. Improvements in the 6-minute walk test results, functional class, and cardiac function were documented. During the course of the 1-year follow-up, there were considerable PH-related events (including 6 deaths), reflecting disease progression. Furthermore, some authors argue that although unproven, some of these deaths might have been directly related to withdrawing effective drug therapies.72
Although experimental evidence and clinical experience in favor of PADN as a novel and targeted approach for the treatment of PAH is slowly increasing, I believe, as do other authors,6,7,71 that due to the small and heterogeneous nature of the studied populations, the open-label, noncontrolled design of the studies, and the isolated experience of only 1 center, it is not yet possible to draw firm conclusions or recommendations regarding the efficacy and optimal timing of PADN in the setting of human PAH. The safety and efficacy of the PADN procedure, however, deserves further evaluation and remains to be proven in larger, randomized placebo-controlled clinical trials such as the DENERV’AP study (NCT02525926).
PERCUTANEOUS BALLOON PULMONARY ANGIOPLASTYPercutaneous BPA is another interventional technique that is emerging as a therapeutic option for the management of inoperable CTEPH. Chronic thromboembolic PH is a devastating, lethal, and distinct form of PH, included as group 4 in the clinical classification of PH.1,73,74 It is believed to be due to obstruction of the pulmonary vascular bed as a result of incomplete resolution of acute pulmonary thromboemboli that organizes into fibrous tissue within the main pulmonary arteries. In the nonoccluded vessels, remodeling of the microvasculature leads to a pulmonary arteriopathy similar to that of PAH, contributing significantly to the pathology of CTEPH.1,73,75 This in turn increases pulmonary vascular resistance, which leads to progressive PH, RHF, and death. Early diagnosis, appropriate evaluation, and treatment by an expert multidisciplinary team are mandatory as CTEPH represents a potentially curable form of PH.1,15,73,76–78
The treatment of choice for CTEPH is surgical pulmonary endarterectomy (PEA). At present, periprocedural mortality rates have diminished considerably in experienced centers in the United States and Europe. For survivors, substantial improvements have been demonstrated in pulmonary hemodynamics, quality of life, and survival.1,15,73,76 However, not all CTEPH patients are suitable candidates for PEA. According to the results of the International Prospective Registry on CTEPH,15,74,79 a substantial proportion of patients with CTEPH were considered inoperable due to distal, surgically inaccessible disease and/or significant comorbidities that precluded surgery. It has also been established that up to 10% to 15% of operated patients will have persistent PH.15,73,76,77,80 Patients deemed inoperable or those who have persistent or recurrent PH after PEA represent a population in which either PAH-specific therapy (ie, riociguat) and/or percutaneous BPA may be beneficial.1,15,73,77,81–101
BackgroundBalloon pulmonary angioplasty in the setting of CTEPH was explored for the first time by Feinstein et al.81 in a series of 18 patients with inoperable CTEPH in 2001. In that study, improvements in hemodynamics (PAP decreased from 43 ± 12 to 37.7 ± 10.7mmHg), functional class, and exercise capacity were shown after a mean 3-year follow-up. However, significant complications were reported, including reperfusion pulmonary edema in 11 of 18 patients, perforation of the PA, and death (1 each). Despite disappointing initial results, interest in percutaneous BPA has increased over the last 5 years as an effective treatment for distal, inoperable forms of CTEPH, mainly due to technical modifications and the better results shown in numerous reports mainly from Japanese groups.1,15,82–85
Current Experience With BPA in CTEPHKataoka et al.82 and Sugimura et al.83 revisited BPA by demonstrating successful results in terms of efficacy and a relatively good safety margin. This successful experience with percutaneous BPA in Japan extended to many other centers in Europe.86–90 In a recent and thorough review regarding worldwide experience, Lang et al.15 made a careful analysis of the 3568 procedures performed in 885 CTEPH patients reported in 21 series published to date. Much can be learned from this review about the indications, technique and diagnostic imaging refinements, as well as the safety and efficacy, long-term outcome, and future prospects of BPA in the management of CTEPH. More recent reports have followed this review.89,90
Percutaneous BPA should be performed only in experienced centers with appropriate facilities and sound interventional programs for CTEPH. A PEA multidisciplinary team is not only necessary but should be considered mandatory, because the first consideration for BPA should be defining the feasibility of PEA.1,15,74,76,77 Balloon pulmonary angioplasty may be considered for patients with distal, inoperable disease and for those with an elevated risk-benefit ratio for PEA. The criteria for technical surgical inaccessibility should be established by at least a second opinion of an additional expert surgical team.1,15,73,76 Inoperable disease based on an elevated risk due to comorbidities is less well defined.15 Other indications for the procedure may include patients with persistent PH and/or residual lesions after PEA who are unresponsive to the new specific-PAH drugs.15,77,80,91–93 What is clear, however, is that BPA should not be attempted in patients with large central thrombi or unilateral total occlusion.15 Also, when considering BPA, it is important to acknowledge that BPA is a demanding procedure and definitely not complication-free.15,90,94
The technique for BPA has evolved over the years as a result of technical advances and growing experience. The aim of the procedure is to open obstructed distal vessels or widen stenotic lesions to improve pulmonary perfusion and hemodynamics in an attempt to improve RV function or to prevent RHF, which represents the main cause of death in these patients. In brief, once the vascular lesions to be treated have been identified, a guide wire is passed across the lesion or occlusion, and dilation with an undersized (low balloon/artery ratio) balloon is used to open the lesion by crushing the fibrous material against the vessel wall15 (Figure 7); unlike PEA, no fibrous obstructive material is removed during BPA. Balloon pulmonary angioplasty involves repeat catheterizations with multiple dilations during each session to achieve optimal results. In a review of worldwide experience,16 the mean number of procedures per patient was 4 (3568 procedures/885 patients) and the number of sessions per patient ranged from 3 to 10. The number of sessions seems to relate to operator experience and the extent of the disease (localization and type of lesion). With proper candidate selection, results after BPA are rewarding. In most reported series there have been significant improvements in hemodynamic parameters, functional class, and exercise capacity.15 Impact on long-term survival has also been documented.82,83,90
Percutaneous balloon pulmonary angioplasty procedure. A: Pulmonary angiography, showing a stenosis in the subsegment of the 10th segmental artery (anterior view). B: The catheter is introduced into a web stenosis. C: The wire is introduced between the fibrotic material and the balloon is inflated, leading to rupture of the web. D: Angiography after balloon pulmonary angioplasty shows an improvement of blood flow with better perfusion of the parenchyma and quick venous return. In contrast to pulmonary endarterectomy, the fibrous material is not removed from the arteries, but is crushed against the vessel wall. Reprinted with permission from Lang et al.15
In expert hands, BPA is a relatively safe procedure, with only 13 procedure-related deaths reported among 885 patients (1.46%).15 Operators should, however, be familiar with and prepared to manage complications such as pulmonary vascular injury (wire perforation of the pulmonary artery, dissection), hemoptysis, and the most common and feared complication of reperfusion pulmonary edema, which may be sufficiently severe to require mechanical ventilation.15,94 In this context, the PEPSI (Pulmonary Edema Predictive Scoring Index), which evaluates flow in the target vessel before and after BPA, may be useful.84 Radiation exposure and contrast media-induced renal failure are additionally important considerations in BPA.15,95
Role of ImagingPulmonary vascular imaging techniques have made an important contribution in the development and refinement of the BPA technique and their role is critical for the appropriate definition and approach of vascular lesions.15,95–99 According to expert opinion, digital-subtraction pulmonary angiography is the most common and useful method.15,96,97 Other imaging techniques such as contrast-enhanced tomography, cone-beam computed tomography, optical coherence tomography, and magnetic resonance imaging may be complimentary techniques for patient evaluation.15,95,98,99 In addition, dual-energy computed tomography might be useful to document and evaluate the increase in pulmonary perfusion after BPA.15,100
Future HorizonsSome questions remain regarding the current and future role of BPA in the management of CTEPH. For many patients with proximal CTEPH, PEA remains the procedure of choice.1,73,76 When there is clear evidence of distal, inoperable disease, either BPA or PH-specific drug therapy should be considered.1,15,73,77 Comparisons between them are lacking but the answer at least in terms of efficacy may come from the current RACE (Riociguat Versus Balloon Pulmonary Angioplasty in Non-operable Chronic thromboEmbolic Pulmonary Hypertension) clinical trial (NCT02634203). In this particular group, perhaps the combination of both BPA and PAH-specific drugs may be the appropriate form of treatment. Finally, patients with borderline proximal-distal disease need to be considered. There is still no clear-cut approach for these patients, but a combined PEA and BPA strategy has been successfully attempted.101 As stated by Lang et al.,15 future CTEPH management may consist of PEA, BPA, and targeted medical therapy combined either simultaneously or sequentially according to individual patient needs.
FINAL REMARKSDespite significant advances in pharmacological treatments, PH and in particular PAH, remains an incurable disease. Interventional and surgical interventions have been used as palliative treatments late in the course of the disease. A clear demonstration of the safety and efficacy of any of the interventional therapies available has been limited by the lack of a prospective and controlled study, the relative success of PAH-specific treatments in recent years, and mainly by the lack of training/expertise in these procedures worldwide. However, the fact that these strategies improve hemodynamics, quality of life, and prolong survival in a high-risk population of PAH patients should not be overlooked. As we wait for the release of new and effective targeted therapies, we should consider these additional strategies in an attempt to provide a more comprehensive support for the failing RV. Percutaneous BPA, on the other hand, by demonstrating efficacy and safety, is rapidly gaining a definitive place in the treatment algorithm of inoperable CTEPH. I strongly suggest that these surgical or interventional strategies should be available in PH-referral centers.
CONFLICTS OF INTERESTNone declared.
The author wants to thank Juan Pablo Sandoval for his invaluable help in the editing of the manuscript.