There are limited data on the usefulness of intravascular ultrasound (IVUS) for long coronary lesions treated with second-generation drug-eluting stents. We evaluated IVUS predictors of major adverse cardiovascular events (MACE) 12 months after implantation of everolimus-eluting stents for long coronary lesions.
MethodsA total of 804 patients who underwent both postintervention IVUS examination and long everolimus-eluting stent (≥ 28mm in length) implantation were included from 2 randomized trials. MACE was defined as a composite of cardiac death, myocardial infarction, and target-lesion revascularization.
ResultsMACE occurred in 24 patients (3.0%) over 12 months. On multivariable Cox regression analysis, independent IVUS predictors of MACE included the postintervention minimum lumen area (MLA) at the target lesion (HR = 0.623; 95%CI, 0.433-0.895; P=.010) and the ratio of MLA/distal reference segment lumen area (HR = 0.744; 95%CI, 0.572-0.969; P=.028). The MLA and MLA-to-distal reference segment lumen area ratio that best predicted patients with MACE from those without these events were 5.0 mm2 and 1.0, respectively. Patients with MLA<5.0 mm2 or a distal reference segment lumen area had a higher risk of MACE (HR = 6.231; 95%CI, 1.859-20.891; P=.003) than those without MACE.
ConclusionsPatients with a postintervention IVUS-measured MLA of<5.0 mm2 or a distal reference segment lumen area were at risk for MACE after long everolimus-eluting stent implantation.
Keywords
Contrary to bare metal stents, the clinical usefulness of intravascular ultrasound (IVUS) has not been clearly established with regard to percutaneous coronary intervention with drug-eluting stents (DES).1–8 Several meta-analyses have suggested that IVUS guidance may be associated with a lower risk of major adverse cardiovascular events (MACE) than angiographic guidance.2,4,6,7 However, randomized trials have failed to confirm the superiority of IVUS-guided DES implantation.1,3,5 Therefore, in the era of DES, the current guidelines recommend that IVUS may be considered in selected patients with complex lesions, such as left main coronary artery disease.9,10 Long-length DES implantation has increased the risk of in-stent restenosis compared with that occurring with shorter stents.11,12 Therefore, IVUS guidance may also be beneficial in patients with long coronary lesions. Recently, a randomized trial demonstrated that IVUS guidance for long DES implantation reduced target-lesion revascularization (TLR).13 Although previous IVUS studies showed that a threshold of stent expansion might predict in-stent restenosis after DES implantation,12,14 the data are still limited. This is especially true in patients with long coronary lesions. In addition, previous data were derived from retrospective observational studies or from the use of first-generation DES.12,14
The aim of the present study was to identify IVUS predictors of MACE in a large series of patients treated with long-length everolimus-eluting stents (EES) from prospective randomized trials. This type of stent is one of the most widely used second-generation DES in current clinical practice.15
METHODSStudy PopulationPatients were identified from 2 randomized trials: the RESET trial16 and the IVUS-XPL trial.13 Briefly, the RESET trial was a randomized, noninferiority trial that compared 3-months of dual antiplatelet therapy following implantation of the Endeavor sprint zotarolimus-eluting stents (Medtronic, Inc.; Santa Rosa, California, United States) with 12 months of dual antiplatelet therapy following implantation of another DES. In the prespecified long lesion subset of this study,5,16 543 patients were randomly allocated to receive either the Endeavor sprint zotarolimus-eluting stent or the EES (Xience V, Abbott Vascular; Santa Clara, California, United States). The patients were then randomly assigned to either IVUS- or angiography-guided DES implantation (2 × 2 design). In the other randomized IVUS-XPL trial, which implanted EES (Xience prime, Abbott Vascular) in patients with long coronary lesions, 1400 patients were randomly assigned to receive either IVUS- or angiography-guided EES implantation. Detailed protocols of these trials have previously been described.5,13,16 A total of 804 patients from these 2 trials who underwent both postintervention IVUS examination and long EES (≥ 28mm in length) implantation were finally included in this study, with 127 patients from the RESET trial and 677 patients from the IVUS-XPL trial. The study protocols of these trials were approved by the institutional review board of each participating institution, and written consent was obtained from all patients.
Percutaneous Coronary Intervention and Pharmacological TherapyEverolimus-eluting stent implantation was performed according to standard techniques. Overlapping stents were used if a lesion could not be covered with a single stent. The stent diameter and length were selected using online IVUS measurements. Adjunct high-pressure dilation was performed at the operators’ discretion, based on the IVUS findings.5,13 Use of IVUS was allowed at any step of EES implantation (before, during, or after implantation). The IVUS examinations before and during EES implantation were not mandatory; however, postintervention IVUS examination was mandatory. 5,13 In the IVUS-XPL trial, the postintervention IVUS criteria for stent optimization were defined as a minimal lumen cross-sectional area greater than the lumen cross-sectional area at the distal reference segments.13 One of 2 commercially available IVUS systems was used (Atlantis or I-Lab, Boston Scientific Corp./SCIMED; Minneapolis, Minnesota, United States, or Eagle Eye, Volcano Therapeutics; Rancho Cordova, California, United States).
At least 12hours prior to EES implantation, all patients received loading doses of aspirin (100mg) and clopidogrel (300mg). However, if this loading dose of clopidogrel was not administered, the patient instead received a 600-mg loading dose in the catheterization laboratory immediately prior to percutaneous coronary intervention. Unfractionated heparin was administered intraoperatively to maintain an activated clotting time longer than 250seconds. Glycoprotein IIb/IIIa inhibitors were used at the operator's discretion. After EES implantation, aspirin (100mg, daily) was prescribed indefinitely. The duration of clopidogrel administration (75mg, daily) depended on the randomized assignments of the RESET and IVUS-XPL trials. Notably, all patients from the RESET trial were allocated to 12 months of dual antiplatelet therapy.
Angiographic and Intravascular Ultrasound AnalysisAngiographic and IVUS measurements were performed by analysts who were blinded to patient and treatment assignments in an independent core laboratory at the Cardiovascular Research Center, Seoul, Korea. Before and after EES implantation, an off-line quantitative coronary angiographic system (CASS system, Pie Medical Instruments; Maastricht, The Netherlands) was used to perform quantitative coronary angiography analysis. Using the guiding catheter for magnification-calibration, the diameters of the reference vessel (the average of the proximal and distal reference lumen diameters) and the minimal luminal diameter were measured before and after EES implantation. These measurements were made from diastolic frames in a single matched view, revealing the smallest minimal luminal diameter.
Standardized planimetry of the lumen, stent, and vessel cross-sectional area was performed using planimetry software (Echoplaque, INDEC Systems; Santa Clara, California, United States) in accordance with IVUS guidelines from the American College of Cardiology.17 The target lesion and both proximal and distal reference segments were assessed quantitatively. The postintervention target lesion site was the image slice with the minimum lumen area (MLA). The proximal and distal reference segments were the most normal-looking segments within 5mm proximal and distal to the target lesion, respectively.
Follow-up and Study EndpointsAfter EES implantation, clinical assessments were performed in the hospital and at 1, 3, 6, and 12 months after discharge. The follow-up assessments were performed during a clinic visit or by telephone interview. MACE was defined as a composite of cardiac death, target lesion-related myocardial infarction, and ischemia-driven TLR.
Clinical events were defined according to the Academic Research Consortium and the expert consensus document of the third universal definition of myocardial infarction.18,19 All deaths were considered cardiac unless an unequivocal noncardiac cause could be established.18 At the 1-year follow-up, a target lesion-related myocardial infarction was defined by the following parameters: the presence of clinical symptoms, electrocardiographic changes, or abnormal imaging findings of myocardial infarction, and an increase in the creatine kinase myocardial band fraction above the upper normal limits or an increase in troponin-T/troponin-I above the 99th percentile of the upper limit of normal. The territory of the myocardial infarction was supplied by the coronary artery containing the stented lesions (implanted stent ≥ 28mm in length).13,19 Ischemia-driven TLR was defined as a repeat percutaneous coronary intervention or bypass surgery of the target lesion with either of the following: a) angiographic diameter stenosis ≥ 50% by quantitative coronary angiographic analysis with documentation of a positive stress test, or b) angiographic diameter stenosis ≥ 70% irrespective of the stress test results.18
Statistical AnalysisStatistical analysis was performed using SPSS (version 18.0.0, SPSS Inc.; Chicago, Illinois, United States). Categorical variables are reported as numbers and percentages and were compared using the chi-square test or Fisher's exact test. In contrast, continuous variables are reported as mean±standard deviation or median [interquartile range] and were compared using the Student t test or Mann-Whitney U test (if data were skewed). Multivariable Cox regression analysis was performed to determine the independent IVUS predictors of MACE during the 12 months of follow-up. Variables from univariate analysis with P values<.1 were included in the multivariable model. Receiver operator characteristic analysis was performed to determine the best cutoff values for the independent IVUS predictors of MACE. Simple linear regression analysis was also performed to evaluate the association between the size of the reference vessel and IVUS findings. All P values were 2-sided. A P value<.05 was considered statistically significant.
RESULTSA total of 24 (3%) of the 804 patients who underwent EES implantation experienced MACE during the 12 months of follow-up. Three patients died, 3 had target lesion-related myocardial infarctions, and 22 experienced ischemia-driven TLR. Of these, 2 patients died of myocardial infarction without revascularization treatment, and another patient died after a myocardial infarction, despite emergent revascularization.
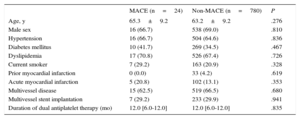
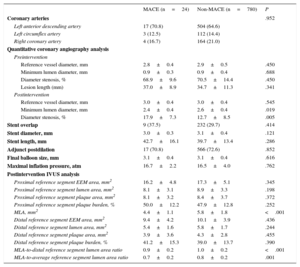
The clinical characteristics at the index procedure are listed in Table 1. There were no significant differences in clinical characteristics between patients with and without MACE. The lesional and procedural characteristics in the 2 groups are shown in Table 2. After EES implantation, as compared with the 780 patients without MACE, the 24 patients with MACE had a smaller minimum lumen diameter (2.4±0.4mm vs 2.6±0.4mm; P=.019) and larger-diameter stenoses (17.9%±7.3% vs 12.7%±8.5%; P=.005) on quantitative coronary angiographic analysis. In the postintervention IVUS analysis, the 24 patients with MACE showed smaller MLA (4.4±1.1mm2 vs 5.8±1.8mm2; P<.001), lower ratios of MLA to distal reference segment lumen area (0.9±0.2 vs 1.0±0.2; P<.001), and lower ratios of MLA to average reference segment lumen area (0.7±0.2 vs 0.8±0.2; P=.001) compared with the patients without MACE.
Baseline Clinical Characteristics
| MACE (n=24) | Non-MACE (n=780) | P | |
|---|---|---|---|
| Age, y | 65.3±9.2 | 63.2±9.2 | .276 |
| Male sex | 16 (66.7) | 538 (69.0) | .810 |
| Hypertension | 16 (66.7) | 504 (64.6) | .836 |
| Diabetes mellitus | 10 (41.7) | 269 (34.5) | .467 |
| Dyslipidemia | 17 (70.8) | 526 (67.4) | .726 |
| Current smoker | 7 (29.2) | 163 (20.9) | .328 |
| Prior myocardial infarction | 0 (0.0) | 33 (4.2) | .619 |
| Acute myocardial infarction | 5 (20.8) | 102 (13.1) | .353 |
| Multivessel disease | 15 (62.5) | 519 (66.5) | .680 |
| Multivessel stent implantation | 7 (29.2) | 233 (29.9) | .941 |
| Duration of dual antiplatelet therapy (mo) | 12.0 [6.0-12.0] | 12.0 [6.0-12.0] | .835 |
MACE, major adverse cardiovascular events.
Data are presented as No. (%), mean±standard deviation, or median [interquartile range].
Lesional and Procedural Characteristics
| MACE (n=24) | Non-MACE (n=780) | P | |
|---|---|---|---|
| Coronary arteries | .952 | ||
| Left anterior descending artery | 17 (70.8) | 504 (64.6) | |
| Left circumflex artery | 3 (12.5) | 112 (14.4) | |
| Right coronary artery | 4 (16.7) | 164 (21.0) | |
| Quantitative coronary angiography analysis | |||
| Preintervention | |||
| Reference vessel diameter, mm | 2.8±0.4 | 2.9±0.5 | .450 |
| Minimum lumen diameter, mm | 0.9±0.3 | 0.9±0.4 | .688 |
| Diameter stenosis, % | 68.9±9.6 | 70.5±14.4 | .450 |
| Lesion length (mm) | 37.0±8.9 | 34.7±11.3 | .341 |
| Postintervention | |||
| Reference vessel diameter, mm | 3.0±0.4 | 3.0±0.4 | .545 |
| Minimum lumen diameter, mm | 2.4±0.4 | 2.6±0.4 | .019 |
| Diameter stenosis, % | 17.9±7.3 | 12.7±8.5 | .005 |
| Stent overlap | 9 (37.5) | 232 (29.7) | .414 |
| Stent diameter, mm | 3.0±0.3 | 3.1±0.4 | .121 |
| Stent length, mm | 42.7±16.1 | 39.7±13.4 | .286 |
| Adjunct postdilation | 17 (70.8) | 566 (72.6) | .852 |
| Final balloon size, mm | 3.1±0.4 | 3.1±0.4 | .616 |
| Maximal inflation pressure, atm | 16.7±2.2 | 16.5±4.0 | .762 |
| Postintervention IVUS analysis | |||
| Proximal reference segment EEM area, mm2 | 16.2±4.8 | 17.3±5.1 | .345 |
| Proximal reference segment lumen area, mm2 | 8.1±3.1 | 8.9±3.3 | .198 |
| Proximal reference segment plaque area, mm2 | 8.1±3.2 | 8.4±3.7 | .372 |
| Proximal reference segment plaque burden, % | 50.0±12.2 | 47.9±12.8 | .252 |
| MLA, mm2 | 4.4±1.1 | 5.8±1.8 | <.001 |
| Distal reference segment EEM area, mm2 | 9.4±4.2 | 10.1±3.9 | .436 |
| Distal reference segment lumen area, mm2 | 5.4±1.6 | 5.8±1.7 | .244 |
| Distal reference segment plaque area, mm2 | 3.9±3.6 | 4.3±2.8 | .455 |
| Distal reference segment plaque burden, % | 41.2±15.3 | 39.0±13.7 | .390 |
| MLA-to-distal reference segment lumen area ratio | 0.9±0.2 | 1.0±0.2 | <.001 |
| MLA-to-average reference segment lumen area ratio | 0.7±0.2 | 0.8±0.2 | .001 |
EEM, external elastic membrane; IVUS, intravascular ultrasound; MACE, major adverse cardiovascular events; MLA, minimum lumen area.
Data are presented as No. (%) or mean±standard deviation.
The IVUS predictors of MACE are shown in Table 3. On multivariable Cox regression analysis, the independent predictors of MACE included postintervention MLA (hazard ratio [HR] = 0.623; 95% confidence interval [95%CI], 0.433-0.895; P=.010) and the ratio of MLA to distal reference segment lumen area (HR = 0.744; 95%CI, 0.572-0.969; P=.028). The area under the curve on the receiver operator characteristics curve of the MLA, the ratio of MLA to distal reference segment lumen area, and the combination model of MLA and the ratio of MLA-to-distal reference segment lumen area was 0.731 (95%CI, 0.652-0.811; P<.001), 0.696 (95%CI, 0.585-0.807; P=.001), and 0.766 (95%CI, 0.691-0.841; P<.001), respectively.
Intravascular Ultrasound Predictors of Major Adverse Cardiac Events at 12 Months of Follow-up
| Postpercutaneous coronary intervention | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| MLA, per 1.0mm2 | 0.520 (0.373-0.723) | <.001 | 0.623 (0.433-0.895) | .010 |
| MLA-to-distal reference lumen area ratio, per 0.1 | 0.626 (0.503-0.780) | <.001 | 0.744 (0.572-0.969) | .028 |
| MLA-to-average reference lumen area ratio, per 0.1 | 0.655 (0.508-0.845) | .001 | 0.975 (0.696-1.364) | .881 |
95%CI, 95% confidence interval; HR, hazard ratio; MLA, minimum lumen area.
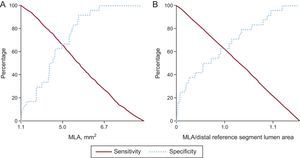
Sensitivity and specificity curves were used to identify the optimal cutoff values of MLA and the ratio of MLA-to-distal reference segment lumen area that best predicted MACE after EES implantation (Figure 1): 5.0mm2 for MLA and 1.0 for the ratio of MLA to distal reference segment lumen area. The sensitivity and specificity for MLA<5.0mm2 were 66.7% (16 of 24) and 64.6% (504 of 780), respectively. The positive and negative predictive values were 5.5% (16 of 292) and 98.4% (504 of 512), respectively. The sensitivity and specificity for MLAsmaller than thedistal reference segment lumen area to predict MACE were 62.5% (15 of 24) and 62.1% (484 of 780), respectively. The positive and negative predictive values were 4.8% (15 of 311) and 98.2% (484 of 493), respectively.
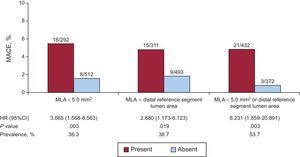
Twenty-one (87.5%) of the 24 patients with MACE had a postintervention MLA<5.0 mm2 or smaller than the distal reference segment lumen area. The MACE rate was 0.8% (3 of 372) in patients who had neither MLA<5.0mm2 nor smaller than the distal reference segment lumen area, and was 4.9% (21 of 432) in patients who had at least 1 of these variables (HR = 6.231; 95%CI, 1.859-20.891; P=.003). (Figure 2, ).
Major adverse cardiovascular events rate for patients with and without MLA <5.0mm2 or distal reference segment lumen area, at 12 months of follow-up. Patients with MLA <5.0mm2 or distal reference segment lumen area had an increased risk of MACE (HR = 6.231; 95%CI, 1.859-20.891; P=.003). 95%CI, 95% confidence interval; HR, hazard ratio; MACE, major adverse cardiovascular events; MLA, minimum lumen area.
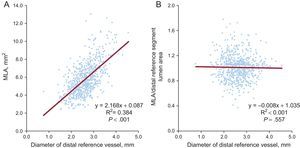
The absolute value of MLA measured by postintervention IVUS analysis was significantly associated with the diameter of the distal reference vessel measured by preintervention quantitative coronary angiographic analysis (coefficient=2.168; 95%CI, 1.965-2.370; P<.001). In contrast, the ratio of MLA to distal reference segment lumen area was not (Figure 3). In the subset of 320 patients with a distal reference vessel diameter<2.5mm, there was a lower incidence of MACE in patients with an MLA equal to or greater than the distal reference segment lumen area (1.6%, 3 of 193 patients) (HR = 0.215; 95%CI, 0.058-0.795; P=.021) compared with that in patients with an MLA smaller than distal reference segment lumen area (7.1%, 9 of 127 patients).
Association between the diameter of distal reference vessel on preintervention angiography and MLA measured by postintervention intravascular ultrasound. The size of the distal reference vessel was associated with postintervention MLA (A), but not with the ratio of MLA-to-distal reference segment lumen area (B). MLA, minimum lumen area.
In this pooled analysis of 804 patients treated with IVUS-guided long EES implantation, the overall MACE rate was 3% at 12 months. Two independent IVUS predictors of MACE were an absolute MLA value of 5.0mm2 and a relative enlargement of the MLA to the distal reference segment lumen area. In particular, these 2 predictors showed high negative predictive values.
The MACE rate in this study was lower than that of the COMPARE trial, in which a composite of cardiac death, nonfatal myocardial infarctions, and clinically justified TLR occurred in 5% of 897 patients after 12 months.20 Compared with the COMPARE trial (54%), postdilation was more frequently performed in the present study (72%), which might have resulted from IVUS guidance. Consequently, the postintervention minimum lumen diameter (2.6mm) of the present study was larger than that (2.1mm) of the COMPARE trial.20 Among excluded patients from the present study due to angiography guidance, the MACE rate was 7% in the RESET trial and 6% in the IVUS-XPL trial, respectively. These findings were consistent with the COMPARE trial.20
A previous IVUS study showed that the relative parameter (ie, ratio of MLA to reference segment lumen cross-sectional area) was significantly associated with in-stent restenosis after bare metal stent implantation.21 However, in the DES era, the absolute parameter (ie, MLA) has been considered to be more predictive for adequate stent patency or angiographic restenosis than the relative parameter.12,14,22 In the IVUS substudy of the SIRIUS trial with 72 patients,22 the optimal threshold of postintervention MLA to predict an 8-month follow-up MLA >4mm2 was 5.0mm2 for the sirolimus-eluting stent. The sensitivity, specificity, and positive predictive value with this cutoff value were 76%, 83%, and 90%, respectively. A previous angiographic follow-up study (550 patients, 670 native coronary lesions) based on real-world registry data similarly showed that an MLA of 5.5mm2 was associated with angiographic restenosis at the 6-month follow-up. However, the sensitivity, specificity, and positive predictive value were relatively low (67%, 67%, and 7%, respectively) compared with those of the SIRIUS trial substudy.12 Similarly, a recent registry study with 229 EES-treated patients suggested that the optimal postintervention MLA was 5.4mm2 to predict 9-month follow-up angiographic restenosis14 (which was consistent with the present finding of MLA of 5.0mm2). Contrary to previous studies,12,14,22 this study was derived from 2 large prospective, randomized (IVUS- vs angiography-guided) trials. Both of these trials employed second-generation DES (ie, EES).
There are limited data on the clinical usefulness of relative IVUS parameters for DES optimization. One study, which applied the IVUS criteria of DES optimization of MLA ≥ 5mm2 or>90% of distal reference segment lumen area for small vessel, failed to demonstrate the superiority of IVUS guidance (n=105 patients) over angiography guidance (n=105 patients). However, this trial was extremely underpowered, and did not include analysis of IVUS optimization.1 A recent, randomized IVUS-XPL trial reported the clinical usefulness of relative IVUS parameters for stent optimization, which included MLA greater than distal reference segment lumen area in IVUS-guided long EES-treated patients. The patients in that trial (n=315) who did not meet the IVUS criteria had a significantly higher incidence of the primary endpoint compared with those (n=363) meeting the criteria for stent optimization (4.6% vs 1.5%; P=.017, respectively).13 In this pooled analysis, there was a significant association with MACE occurrence and patients with an MLA less than the distal reference segment lumen area, even after adjustment of the absolute parameter. Even in the DES era, achieving adequate stent dimensions is still important in order to minimize restenosis and stent thrombosis. If not, stent underexpansion may cause abnormal shear stress that potentially affects neointimal hyperplasia or thrombosis formation. In long lesions, the atherosclerotic disease is diffusely distributed. Therefore, the distance from segments with the smallest lumen diameter to the distal reference segment is quite long. In addition, there is a greater difference in the reference vessel size (larger proximal reference vessel size vs smaller distal reference vessel size) in diffuse long lesions compared with that of shorter lesions.23 When longer stent implantation is considered in the treatment of diffuse long lesions, the distal margin of a long stent is frequently placed adjacent to the distal reference segment with small vessel size. This situation inevitably creates a practical dilemma. Achieving an MLA ≥ 5mm2 is mechanically impossible in the distal part of the stented segment adjacent to the small-sized distal reference segment. In this study, postintervention MLA was limited by a preintervention distal reference segment diameter (Figure 3). Therefore, another IVUS criterion of stent optimization is required when the distal margin of the stented segment is placed adjacent to a small-sized distal reference segment. In the present study, 320 (39.8%) of 804 EES-treated patients had a distal reference vessel diameter of<2.5mm. In these subgroup patients, the achievement of MLA ≥ distal reference segment lumen area was significantly associated with a lower incidence of MACE compared with patients with an MLA smaller than the distal reference segment lumen area.
Study LimitationsThe 2 trials had different study designs, and the patients included from these randomized trials might not reflect real-world clinical practice. Since the preintervention IVUS information was not available in the current dataset of this study, the impact of preintervention IVUS on MACE could not be assessed. Accordingly, the interpretations should be limited within the present findings. The associations between detailed interventional procedures and postintervention IVUS findings could not be evaluated because the analyzed IVUS data were drawn final reports written after the completion of the intervention. Long lesions of the right coronary artery were relatively rare in patients with MACE. The cost-effectiveness of IVUS evaluation was beyond the scope of this study. Finally, in practice, the achievement of these suggested IVUS criteria may be not always feasible because lesion characteristics such as heavy calcification also inhibit adequate lumen enlargement. In the present study, the frequency of patients with MLA<5.0mm2 or distal reference segment lumen area was 53.7% (432 of 804), as shown in Figure 2. The present study did not evaluate the impacts of preintervention IVUS or the IVUS findings from subsequent actions (such as adjuvant dilatation with a larger balloon) taken after IVUS examination during DES implantation. Accordingly, further investigations are required to elucidate the clinical impacts of the suggested IVUS criteria.
CONCLUSIONSThis pooled analysis of 804 patients demonstrated that the overall MACE rate was 3% during the first 12 months after IVUS-guided long EES implantation. Independent predictors of MACE were MLA and the ratio of the MLA-to-distal reference segment lumen area, as assessed by postintervention IVUS. The optimal MLA cutoff values and the ratio of MLA-to-distal reference segment lumen area that predicted MACE were 5.0mm2 and 1.0, respectively. Therefore, there is a risk of MACE in patients with a postintervention MLA<5.0mm2 or a distal reference segment lumen area after long EES implantation.
- -
Although previous IVUS studies showed that a threshold of stent expansion might predict in-stent restenosis after DES implantation, the data are still limited. This is especially true in patients with long coronary lesions. In addition, previous data were derived from retrospective observational studies or from the use of first-generation DES.
- -
Patients with a postintervention IVUS-measured MLA of less than 5.0mm2 or a distal reference segment lumen area were at risk for MACE after long EES implantation.
This study was supported by a grant from the Korea Healthcare Technology Research and Development Project, Ministry for Health and Welfare, Republic of Korea (Nos. A085136 and HI15C1277), the Mid-career Researcher Program through a National Research Foundation of Korea grant funded by the Ministry of Education, Science and Technology, Republic of Korea (No. 2015R1A2A2A01002731), and the Cardiovascular Research Center, Seoul, Korea.
CONFLICTS OF INTERESTNone declared.