To evaluate the effect of iron deficiency and anemia on submaximal exercise capacity in patients with chronic heart failure.
MethodsWe undertook a single-center cross-sectional study in a group of stable patients with chronic heart failure. At recruitment, patients provided baseline information and completed a 6-minute walk test to evaluate submaximal exercise capacity and exercise-induced symptoms. At the same time, blood samples were taken for serological evaluation. Iron deficiency was defined as ferritin < 100 ng/mL or transferrin saturation < 20% when ferritin is < 800 ng/mL. Additional markers of iron status were also measured.
ResultsA total of 538 heart failure patients were eligible for inclusion, with an average age of 71 years and 33% were in New York Heart Association class III/IV. The mean distance walked in the test was 285±101 meters among those with impaired iron status, vs 322±113 meters (P=.002). Symptoms during the test were more frequent in iron deficiency patients (35% vs 27%; P=.028) and the most common symptom reported was fatigue. Multivariate logistic regression analyses showed that increased levels of soluble transferrin receptor indicating abnormal iron status were independently associated with advanced New York Heart Association class (P < .05). Multivariable analysis using generalized additive models, soluble transferrin receptor and ferritin index, both biomarkers measuring iron status, showed a significant, independent and linear association with submaximal exercise capacity (P=.03 for both). In contrast, hemoglobin levels were not significantly associated with 6-minute walk test distance in the multivariable analysis.
ConclusionsIn patients with chronic heart failure, iron deficiency but not anemia was associated with impaired submaximal exercise capacity and symptomatic functional limitation.
Keywords
Despite the introduction of new therapies and recent developments in the management of chronic heart failure (CHF), functional limitation and the presence of limiting symptoms are common in optimally treated patients. Dyspnea and fatigue are the main symptoms, leading to impaired exercise capacity.1 These factors promote the inability to perform daily activities and impact patients’ self-perceived health status. Thus, impaired health-related quality of life (HRQoL) and the occurrence of symptoms such as dyspnea or fatigue leading to advanced New York Heart Association (NYHA) functional class have become important patient-centered outcomes that need to be addressed.2
The objective assessment of functional status has been commonly undertaken by measuring peak exercise capacity using the cardiopulmonary gas exchange exercise test.3 However, functional limitations in performing daily activities imposed by CHF may also be estimated when submaximal exercise capacity is studied. As an alternative to the cardiopulmonary gas exchange exercise test, the 6-minute walk test (6MWT) or corridor test is an easy method to objectively measure patients’ submaximal functional capacity and thus gain a more realistic insight into patients’ ability to perform daily activities.4,5
The underlying mechanisms of impaired exercise capacity in CHF are numerous and not fully understood. This may explain why therapeutic options with good impact on prognosis, such as beta-blockers, have failed to show benefits in terms of functional capacity.6 Recent evidence suggests that iron deficiency (ID) is a common comorbidity7 and may be associated with impaired peak exercise capacity,8 worse HRQoL,9,10 and poorer outcomes11 in CHF patients, regardless of the presence of anemia. Furthermore, several trials have shown that intravenous iron administration can improve functional capacity and symptoms of patients with CHF and ID.12,13 Data from these studies suggest that iron status may play a role in submaximal exercise capacity. However, these studies focused only on selected patients with ID and thus had a limited power to assess the influence of iron status and/or the presence of anemia on submaximal exercise capacity and the occurrence of symptoms in a broader spectrum of patients. Therefore, we aimed to investigate the impact of iron status on submaximal exercise capacity estimated with the distance walked in the 6MWT. We also wanted to explore the influence of iron status on NYHA functional class and the occurrence of symptoms in a cohort of CFH patients seen in daily clinical practice.14
METHODSStudy Population and RecruitmentFor the purpose of this study, we analyzed the association of submaximal exercise capacity and iron/anemia status in a cohort of stable euvolemic patients with CHF. The methodology of this study has been previously reported.9 The study was conducted in accordance with the Declaration of Helsinki, the study protocol was approved by the local clinical research ethics committee, and all patients gave written informed consent after recruitment. For inclusion in the study, patients had to be in a stable condition and diagnosed with CHF with either reduced or preserved (≥ 45%) LVEF (left ventricular ejection fraction), according to the European Society of Cardiology diagnostic criteria.15 Exclusion criteria for the study were: inability to perform the 6MWT, significant primary valvular disease, hemoglobin levels < 8.5g/dL, clinical signs of fluid overload, pericardial disease, restrictive cardiomyopathy, hypertrophic cardiomyopathy, active malignancy, and chronic liver disease. Patients without iron status evaluation or 6MWT available at screening were also excluded. According to these criteria, the final cohort consisted of 538 patients. At recruitment, all patients provided peripheral blood samples and relevant clinical and demographic information, including NYHA functional class, current medical therapy, and the most recent LVEF evaluation.
Medical and nursing personnel involved in recruitment and data collection were blinded to patients’ ID and anemia status.
Iron Status and Other Laboratory MeasurementsIron deficiency was defined using the Kidney Disease Outcomes Quality Initiative guidelines criteria: ferritin < 100 ng/mL or transferrin saturation (TSAT) < 20% when ferritin is < 800 ng/mL.16 Serum iron was measured using spectrophotometry; serum ferritin and transferrin were measured using immunoturbidimetry. The TSAT was estimated using the formula: TSAT serum iron (μg/dL)/[serum transferrin (mg/dL)×1.25].17 Additional measures of iron status were: red cell distribution width,18 where values > 15% are indicative of ID anisocytosis, serum soluble transferrin receptor (sTfR) (measured using an enzyme immunoassay),19 and ferritin index. Ferritin index has been proposed as a useful tool in the diagnosis of ID states, where ratios > 2 suggest ID. The ferritin index is calculated by dividing sTfR (expressed in nmol/L or mg/L) by log10 ferritin (measured in ng/mL) and is thus increased whenever sTfR is raised and or ferritin is reduced.20 Anemia was defined as hemoglobin level < 12g/dL in women and < 13g/dL in men.21 Hemoglobin was measured using impedance laser colorimetry. None of the patients were receiving blood transfusions, erythropoietin therapy, or intravenous iron therapy at the time of inclusion.
Submaximal Exercise CapacitySubmaximal exercise capacity was assessed using the 6MWT. Each patient performed a single 6MWT using a standardized protocol as described in previous studies and guidelines.22 The test was performed by a trained nurse who would ask the patient to walk the longest distance possible in an interval of 6minutes across a 24-meter corridor. The patient could stop or slow down at any time and then resume walking, depending on the degree of fatigue. The exercise test was stopped at the patient's request. The total distance covered in 6minutes, early interruption of the test, and the presence of symptoms during the test were reported. Symptoms including dyspnea, fatigue, angina, intermittent claudication, and low output symptoms (defined as fainting, weakness, and reduced blood pressure) were evaluated according to a questionnaire and reported at the end of the test. If several symptoms were reported, the most limiting was chosen. To classify patients into 2 exercise capacity categories (impaired or preserved), we chose a cutoff point of 300 meters. This threshold has been reported previously as a predictor of mortality and morbidity in CHF with left ventricular dysfunction.23
Statistical AnalysesDemographic and other background data were summarized with basic descriptive statistics for iron status groups. For quantitative variables, arithmetic mean±standard deviation or median [interquartile range] were calculated as appropriate, and p-values were derived from a 2-sample Student t test (the Mann-Whitney U-test was used for skewed data). For qualitative variables, percentages within specified groups were calculated and P-values were derived using the chi-square test.
The preliminary analysis of functional capacity on impaired or nonimpaired iron status groups was performed using descriptive methods stated above.
The associations between submaximal exercise capacity (preserved, ≥ 300 meters and impaired < 300 meters) in the 6MWT and baseline characteristics were evaluated with simple (univariate) unadjusted logistic regression analysis.
To explore the interactions between iron status and anemia on submaximal exercise capacity, several models were developed. These models were adjusted for covariates that showed a significant statistical association with functional capacity in the univariate analyses including age, sex, LVEF, heart rate, iron status, hemoglobin, hypertension, diabetes, chronic kidney disease, use of angiotensin converting-enzyme inhibitor or angiotensin II receptor antagonists, N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels and C-reactive protein. The NYHA functional class is directly and strongly related to exercise intolerance in CHF patients and may be conceived as an alternative measure of submaximal exercise capacity.8 Thus, we prospectively decided to exclude NYHA from these models in which the distance walked in the 6MWT was the dependent variable.
We used generalized additive models to regress anemia and ID biomarkers on distance walked in 6MWT. Generalized additive models are a more flexible modelling approach which allow for non-linearity in the relationship and contribute to a more accurate exploration of continuous variables, providing a pattern which reflects the shape and trend of the association. The P-value for non-parametric effect (non-linearity test) and P-value for the association were measured for all of them. The models were adjusted for the abovementioned covariates.
A logistic regression model (adjusted for the abovementioned covariates, including hemoglobin) was performed to explore the interaction of iron status and NYHA functional class.
All statistical tests and intervals of confidence were constructed with a type I error (alpha) level of 5% with no adjustments for multiplicity, and P-values ≤ .05 were considered statistically significant. Analyses were done using R 2.15.1.
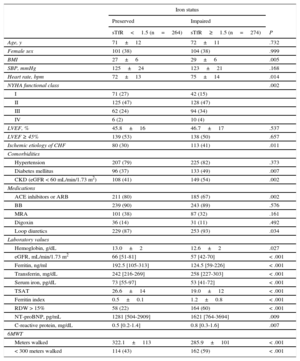
RESULTSUp to 538 stable CHF patients were included and enrolled in the study. Table 1 displays the baseline clinical characteristics of the entire cohort according to iron status. Up to 45% of the patients were anemic. The ID was present in 329 (61%) patients in the total cohort. Advanced NYHA functional class (III or IV) was more common in patients with ID and anemia. The mean distance walked in the total cohort was 304±109 meters; 288±103 meters for preserved LVEF vs 319±112 meters for reduced LVEF, P=.001. The percentage of patients with impairment on submaximal exercise capacity (distance walked in 6MWT < 300 meters) was more prominent in those with abnormal iron status (Table 1). Up to 505 patients (94%) completed the test, and 125 (23%) stopped during the test and then resumed walking.
Baseline Characteristics According to Iron Status
| Iron status | |||
|---|---|---|---|
| Preserved | Impaired | ||
| sTfR<1.5 (n=264) | sTfR≥1.5 (n=274) | P | |
| Age, y | 71±12 | 72±11 | .732 |
| Female sex | 101 (38) | 104 (38) | .999 |
| BMI | 27±6 | 29±6 | .005 |
| SBP, mmHg | 125±24 | 123±21 | .168 |
| Heart rate, bpm | 72±13 | 75±14 | .014 |
| NYHA functional class | .002 | ||
| I | 71 (27) | 42 (15) | |
| II | 125 (47) | 128 (47) | |
| III | 62 (24) | 94 (34) | |
| IV | 6 (2) | 10 (4) | |
| LVEF, % | 45.8±16 | 46.7±17 | .537 |
| LVEF ≥ 45% | 139 (53) | 138 (50) | .657 |
| Ischemic etiology of CHF | 80 (30) | 113 (41) | .011 |
| Comorbidities | |||
| Hypertension | 207 (79) | 225 (82) | .373 |
| Diabetes mellitus | 96 (37) | 133 (49) | .007 |
| CKD (eGFR < 60 mL/min/1.73 m2) | 108 (41) | 149 (54) | .002 |
| Medications | |||
| ACE inhibitors or ARB | 211 (80) | 185 (67) | .002 |
| BB | 239 (90) | 243 (89) | .576 |
| MRA | 101 (38) | 87 (32) | .161 |
| Digoxin | 36 (14) | 31 (11) | .492 |
| Loop diuretics | 229 (87) | 253 (93) | .034 |
| Laboratory values | |||
| Hemoglobin, g/dL | 13.0±2 | 12.6±2 | .027 |
| eGFR, mL/min/1.73 m2 | 66 [51-81] | 57 [42-70] | < .001 |
| Ferritin, ng/ml | 192.5 [105-313] | 124.5 [59-226] | < .001 |
| Transferrin, mg/dL | 242 [216-269] | 258 [227-303] | < .001 |
| Serum iron, pg/dL | 73 [55-97] | 53 [41-72] | < .001 |
| TSAT | 26.6±14 | 19.0±12 | < .001 |
| Ferritin index | 0.5±0.1 | 1.2±0.8 | < .001 |
| RDW > 15% | 58 (22) | 164 (60) | < .001 |
| NT-proBNP, pg/mL | 1281 [504-2909] | 1621 [764-3694] | .009 |
| C-reactive protein, mg/dL | 0.5 [0.2-1.4] | 0.8 [0.3-1.6] | .007 |
| 6MWT | |||
| Meters walked | 322.1±113 | 285.9±101 | < .001 |
| < 300 meters walked | 114 (43) | 162 (59) | < .001 |
6MWT: 6-minute walk test; ACE, angiotensin converting-enzyme; ARBs, angiotensin receptor blockers; BB, beta blockers; BMI, body mass index; CHF, chronic heart failure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricle ejection fraction; MRAs, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal pro-B type natriuretic peptide; NYHA, New York Heart Association; RDW, cell distribution width, SBP, systolic blood pressure; sTfR, serum soluble transferrin receptor; TSAT: transferrin saturation.
Data are expressed as No. (%), mean ± standard deviation or median [interquartile range].
Values of estimated glomerular filtration rate, ferritin, transferrin, serum iron, N-terminal pro-B type natriuretic peptide and C-reactive protein are compared using the Mann-Whitney test.
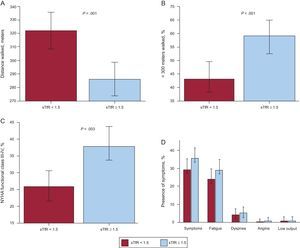
Patients with impaired iron status had worse submaximal exercise capacity in the unadjusted analysis (Figure 1 A). The mean distance covered during the 6-minute period was 285±101 meters in the ID group compared with 322±113 meters in patients with normal iron status (P<0.005). Moreover, the percentage of individuals with impaired exercise capacity was higher in the abnormal iron status group (59% vs 43%; P<.001, respectively) (Figure 1 B) and advanced NYHA functional class was more prevalent in the ID group (26% vs 36%; P<.05, respectively) (Figure 1 C). Impaired iron status was significantly associated with the presence of symptoms during the 6MWT. Symptoms were more common in the abnormal iron status group than in non-ID patients (35% vs 27%; P<0.05, respectively) (Figure 1 D). The most frequent symptom experienced was fatigue, which was significantly associated with iron status. No significant differences were observed in the other symptoms, including dyspnea.
Associations between submaximal exercise capacity, New York Heart Association functional class and symptoms according to iron status. A: Distance walked in the 6-minute walk test. B: Percentage of patients with impaired submaximal exercise capacity. C: Percentage of patients with advanced New York Heart Association functional class (III-IV). D: Patients’ reported symptoms during the 6-minute walk test. 6MWT, 6-minute walk test; NYHA, New York Heart Association; sTfR, serum soluble transferrin receptor.
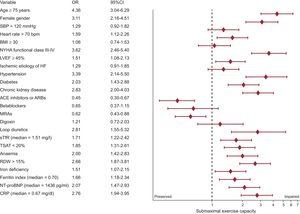
To explore the association between submaximal exercise capacity and baseline characteristics, a univariate binary logistic regression analysis was used (Figure 2). Several factors were associated with worse submaximal exercise capacity including higher age, female sex, and comorbidities such as hypertension, diabetes, and chronic kidney disease. Anemia and ID such as high sTfR, low TSAT, high ferritin index, and red cell distribution width > 15% were also associated with impaired exercise capacity in the unadjusted analysis.
Clinical factors associated with impaired submaximal exercise capacity defined as 6-minute walk test distance < 300 meters (univariate logistic regression analyses). 6MWT, 6-minute walk test; 95%CI, 95% confidence interval; ACE, angiontensin converting-enzyme; ARB, angiotensin receptor blockers; BMI, body mass index; CRP: C-reactive protein; HF, heart failure; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal pro-B type natriuretic peptide; NYHA, New York Heart Association; RDW, red cell distribution width; SBP, systolic blood pressure; sTfR, serum, soluble transferrin receptor; TSAT, transferrin saturation.
In multivariable generalized additive models, adjusted for the significant factors identified in the univariate analysis, abnormal iron status assessed by sTfR (P=.023) and ferritin index (P=.019) was significantly associated with worse submaximal exercise capacity, whereas hemoglobin was not (P=.61) (Table 2). Interestingly, when we analyzed the impact on exercise capacity of the elements of ID definition (TSAT and ferritin) separately, none of these factors showed significant association.
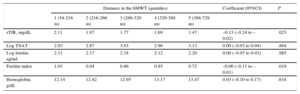
Estimated Values of Different Iron Biomarkers and Hemoglobin Distributed According to the Distance Walked in the 6-minute Walk Test in Quintiles (Multivariate Analyses)
| Distance in the 6MWT (quintiles) | Coefficient (95%CI) | P | |||||
|---|---|---|---|---|---|---|---|
| 1 (54-216 m) | 2 (216-266 m) | 3 (266-329 m) | 4 (329-388 m) | 5 (388-729 m) | |||
| sTfR, mg/dL | 2.11 | 1.87 | 1.77 | 1.69 | 1.47 | –0.13 (–0.24 to –0.02) | .023 |
| Log TSAT | 2.93 | 2.87 | 3.03 | 2.96 | 3.13 | 0.00 (–0.03 to 0.04) | .864 |
| Log ferritin, ng/ml | 2.11 | 2.17 | 2.18 | 2.12 | 2.20 | 0.00 (–0.03 to 0.03) | .985 |
| Ferritin index | 1.03 | 0.94 | 0.86 | 0.85 | 0.72 | –0.06 (–0.11 to –0.01) | .019 |
| Hemoglobin, g/dL | 12.14 | 12.42 | 12.95 | 13.17 | 13.47 | 0.03 (–0.10 to 0.17) | .618 |
6MWT, 6-minute walk test; 95%CI, 95% confidence interval; sTfR, serum soluble transferrin receptor; TSAT, transferrin saturation.
All models were adjusted for age, sex, heart rate, left ventricular ejection fraction, hypertension, diabetes, chronic kidney disease, treatment with angiotensin converting-enzyme inhibitors or angiotensin receptor blockers, logarithm of N-terminal pro-B type natriuretic peptide and logarithm of C-reactive protein.
Iron status biomarkers have been adjusted as well as for hemoglobin, whereas the fifth model (hemoglobin) has been adjusted for serum soluble transferrin receptor as a measure of iron status.
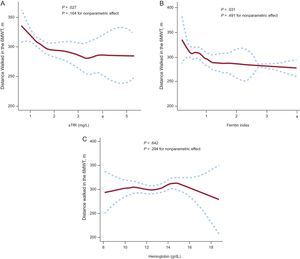
All generalized additive models fitted to explore the association of ID biomarkers (sTfR and ferritin index) and hemoglobin did not reveal a nonlinear component of the relationships with distance walked in the 6MWT (P-value for non-parametric effect: P=.16, P=.49 and P=.29, respectively), which means we cannot assume nonlinearity. However, the important issue of these models is the visual inspection of the plots. The pattern and the shape of the line reflect the trend of the association. This model also confirmed the strong association between iron biomarkers and submaximal exercise capacity (P=.027 and P=.031 respectively) whereas hemoglobin was not associated (P=.642). As represented in model 1 and 2 (Figure 3), lower exercise capacity was related to higher values of ID biomarkers (reflecting more ID).
Multivariate analysis using generalized additive models to evaluate the relationship between iron deficiency biomarkers (A and B), hemoglobin (C) and the distance walked in the 6-minute walk test in meters. 6MWT: 6-minute walk test; sTfR, serum soluble transferrin receptor. Dotted curves indicate 95% confidence interval for the smoothed hazard.
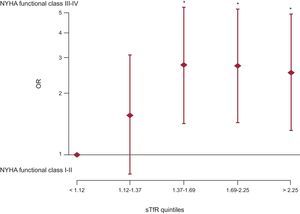
In further multivariable modelling (Figure 4), we investigated the influence of sTfR divided in quintiles as a measure of iron status for advanced heart failure functional class. In this adjusted analysis, more pronounced iron depletion, as represented by higher levels of sTfR, was associated with advanced NYHA class, and this association was independent of anemia.
Multivariate binary logistic regression model to evaluate the influence of iron status (estimated with levels of serum soluble transferrin receptor) on advanced New York Heart Association functional class (III or IV). NYHA, New York Heart Association; OR: odds ratio; sTfR, serum soluble transferrin receptor. *P < 0.05.
The main novel finding of this descriptive study is that impairment of submaximal exercise capacity in CHF patients is associated with abnormal indices of iron metabolism, and this finding is independent of anemia status or hemoglobin. Impaired maximal exercise capacity in ID patients with CHF has been previously described,8 using the cardiopulmonary gas exchange exercise test (measured with peak oxygen consumption); however, we evaluated a parameter which measures submaximal exercise capacity directly related to the patient's ability to perform daily activities and with implications for quality of life.
Furthermore, we have shown that ID patients report symptoms more frequently during submaximal exercise and that the predominant symptom is fatigue. Moreover, abnormal iron status was associated with advanced NYHA functional class and this association was independent of the presence of anemia on multivariable analysis.
Patients with CHF, despite optimal treatment,24 have functional limitation, especially due to exercise intolerance. Cardinal symptoms in CHF are dyspnea and fatigue, but little is known about the exact mechanisms leading to them. In this regard, we observed that fatigue was the most frequent exercise-induced symptom in these patients and was more commonly reported in patients with abnormal iron status.
Iron deficiency has been recently noted as a new primary comorbidity in CHF patients with unfavorable effects in terms of prognosis,11 HRQoL,9,10 and peak exercise capacity.8 Our data add further evidence of the primary role of iron on functional measures of performance in patients with CHF. The assessment of the clinical condition in CHF patients can be easily done with the 6MWT, which also provides information on the ability to perform daily activities.
Our results showed a lineal association between ID biomarkers and submaximal exercise capacity. This type of association has been previously reported between iron deficiency and quality of life.9 Exercise intolerance is directly related to HRQoL in CHF patients.25,26 Recent evidence shows that ID is a key determinant of HRQoL in CHF patients9,10 and this association is well established with overall and physical dimension scores, irrespective of anemia status. In support of this association, our study provides the first evidence that ID itself predicts submaximal exercise capacity, reflecting inability to perform daily activities. Therefore, we can hypothesize that ID would impair submaximal exercise capacity and promote the presence of symptoms and secondarily affect the performance of daily activities, one of the major determining factors in HRQoL. However, it would be interesting to design new studies to confirm that the impact of ID on submaximal exercise capacity is the key element that connects ID with impaired HRQoL.
The definition of ID in CHF has not been totally validated and therefore we used a multiple biomarker strategy to better characterize iron status in CHF patients, as reported in previous studies.9 We additionally used other parameters to evaluate iron status such as sTfR and ferritin index. These 2 biomarkers allow an accurate description of iron status in patients with chronic inflammatory conditions such as CHF. In adjusted analysis, we found that impaired submaximal exercise capacity, but not hemoglobin, correlated strongly with sTfR and ferritin index (P<.05 for both) (Figure 3), suggesting that iron depletion and not anemia is a key determinant of submaximal exercise performance. Interestingly, when we analyzed the impact on exercise capacity of the elements of ID definition separately (TSAT and ferritin), none of these factors showed a significant association. These results confirm the importance of using different parameters simultaneously to asses the effect of ID on CHF and of using more sophisticated ID parameters such as sTfR to reflect iron status as a spectrum of severity of iron depletion.
Measurement of sTfR has been shown to be a good marker of ID; however a cutoff point has not been well established in CHF patients. In this regard, when we analyzed the curve of the interaction between sTfR and exercise capacity in meters (generalized additive models), we interestingly observed that the turning point of 300 meters corresponded to a value of 1.6mg/L for sTfR, which is close to some values previously described27 in advanced CHF patients (assessed by NYHA functional class) with ID.
One hypothesis to explain this datum, which has been previously reported,9,10 is that anemia is merely an indicator of greater ID. Iron plays an essential role in the oxygen transport, oxygen storage, oxidative enzymes, and respiratory chain proteins involved in skeletal and heart muscle energy metabolism.28 The effects of ID are not only due to the role of iron on erythropoiesis.29 To understand the role of iron on the presence of symptoms and functional impairment, tissue ID and oxygen transport deficiency should be considered separately. Experimental evidence suggests that iron itself improves muscle function and exercise capacity in animals,30 and these findings emphasize the role of iron as a cofactor in skeletal and cardiac muscle function. Some authors support the hypothesis that ID may be behind mitochondrial dysfunction.29
Additionally, and in support of this hypothesis, there is evidence on the effect of intravenous iron supplementation on ID anemic and nonanemic patients with CHF, increasing 6MWT distance, and improving functional capacity (NYHA functional class) and overall quality of life.12,25 There are also new data from an interventional study evaluating the benefits of intravenous iron therapy on ID patients,13 showing an improvement on functional capacity, symptoms and HRQoL, which supports our hypothesis and results. However, the effects of iron supplementation directly on CHF specific symptoms such as fatigue or dyspnea have not yet been investigated.
LimitationsThis study has several limitations that need to be mentioned. First of all, the distance walked in the 6MWT may vary according to the patient's motivation or other individual factors. This is a cross-sectional study and thus iron status and submaximal exercise capacity were only measured at a single moment and information about changes in time cannot be provided, so that a causal relationship cannot be established. Since this is a single center study, the applicability of our finding to other countries and ethnicities is unclear. Patients who were unable to perform the 6MWT were not included in the study; therefore, the association of ID and severe functional impairment will merit further evaluation.
A more detailed understanding of the integrated pathophysiology underlying the complex interaction between exercise response and ID in CHF patients is needed. However, the contribution of ID to the generation of symptoms in CHF establishes iron as a potential treatment for patients, so that clinical evaluation with the 6MWT should be performed and could help in the decision to treat patients with proven submaximal impaired exercise capacity.
CONCLUSIONSPatients with CHF and abnormal iron status have impaired submaximal exercise capacity irrespective of hemoglobin. This could explain the link between impaired HRQoL and ID in CHF patients. Consequently, assessment of iron status in patients with CHF may complete information about clinical status and may help to define the therapeutic plan.
FUNDINGStatistical support was funded by Vifor Pharma Ltd, Switzerland.
CONFLICTS OF INTERESTJ. Comin-Colet was a member of the FAIR-HF (Ferinject® Assessment in patients with Iron deficiency and chronic Heart Failure) and CONFIRM-HF (Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure) steering committees and has received honoraria for speaking for Vifor Pharma Ltd.