Despite the efficacy of oral anticoagulant (OAC) therapy, some patients continue to have a high residual risk and develop a stroke on OAC therapy (resistant stroke [RS]), and there is a lack of evidence on the management of these patients. The aim of this study was to analyze the safety and efficacy of left atrial appendage occlusion (LAAO) as secondary prevention in patients with nonvalvular atrial fibrillation who have experienced a stroke/transient ischemic attack despite OAC treatment.
MethodsWe analyzed data from the Amplatzer Cardiac Plug multicenter registry on 1047 consecutive patients with nonvalvular atrial fibrillation undergoing LAAO. Patientes with previous stroke on OAC therapy as indication for LAAO were identified and compared with patients with other indications.
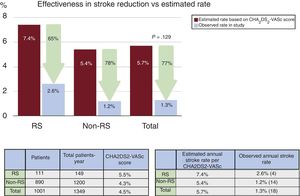
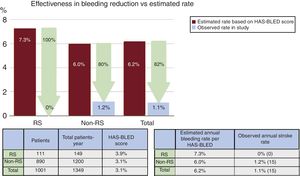
ResultsA total of 115 patients (11%) with RS were identified. The CHA2DS2-VASc and the HAS-BLED score were significantly higher in the RS group (respectively 5.5±1.5 vs 4.3±1.6; P <.001; 3.9±1.3 vs 3.1±1.2; P <.001). No significant differences were observed in periprocedural major safety events (7.8 vs 4.5%; P=.1). With a mean clinical follow-up of 16.2±12.2 months, the observed annual stroke/transient ischemic attack rate for the RS group was 2.6% (65% risk reduction) and the observed annual major bleeding rate was 0% (100% risk reduction).
ConclusionsPatients with RS undergoing LAAO showed similar safety outcomes to patients without RS, with a significant reduction in stroke/transient ischemic attack and major bleeding events during follow-up. Adequately powered controlled trials are needed to further investigate the use of LAAO in RS patients.
Keywords
Percutaneous left atrial appendage occlusion (LAAO) is an alternative treatment for stroke prevention in patients with nonvalvular atrial fibrillation.1–3 The annual rate of ischemic stroke is approximately 5% in untreated patients with nonvalvular atrial fibrillation.4 This risk is significantly reduced by vitamin K antagonists (VKA), up to 64% compared with placebo,5 and high-dose nonvitamin K antagonist oral anticoagulants (NOAC) treatment is associated with lower risk of stroke and systemic embolism (19%), relative to VKA.6 Despite the efficacy of oral anticoagulation (OAC) therapy, some patients continue to have a high residual risk and experience a stroke on OAC therapy (henceforth, “resistant stroke” [RS]). In patients with RS despite adequate VKA, after exclusion of another potential cause, current guidelines propose either increasing the target international normalized ratio (INR) to 2.5 to 3.5 in patients taking VKA or switching from VKA to NOAC.2 However, there is insufficient evidence to decide the management of patients who have experienced a stroke under treatment with NOAC. Thus, patients with stroke while on treatment with VKA and with INR 2.5 to 3.5, patients with a contraindication to switching to NOAC (for example, due to advanced renal failure) or when stroke occurs despite treatment with NOAC could be candidates for percutaneous LAAO. However, LAAO in this specific group of patients has not been analyzed before.
The objective of the present study was to investigate the procedural safety and long-term outcomes of patients with nonvalvular atrial fibrillation who underwent percutaneous LAAO with the indication of stroke despite OAC therapy.
METHODSThis is a retrospective study that included 1047 consecutive patients with nonvalvular atrial fibrillation who underwent LAAO using the Amplatzer Cardiac Plug (ACP, Abbott, Plymouth, Minnesota, United States) in 22 centers between December 2008 and November 2013 and were included in the multicenter Amplatzer Cardiac Plug registry.1 For the purpose of this study, patients with previous stroke on OAC treatment as indication for LAAO were identified and compared with patients with other indications. Procedural success was defined as successful implantation of the Amplatzer Cardiac Plug in the left atrial appendage. Periprocedural adverse events (occurring 0-7 days after the procedure or before hospital discharge, whichever occurred last) was based on the VARC criteria7 and included death, myocardial infarction, stroke, transient ischemic attack (TIA), systemic embolization, air embolization, device embolization, cardiac tamponade, and major bleeding. Adverse events during follow-up (excluding periprocedural events) included death (cardiovascular or noncardiovascular), stroke, TIA, systemic embolism, and major bleeding. Antithrombotic medication was recorded on the date of admission and at the last follow-up visit. The choice and the duration of antithrombotic therapy were individualized depending on the patient history, indication for LAAO, and physician preference. Device efficacy to prevent stroke, TIA, and systemic embolism was tested by comparing the actual event rate at follow-up with the predicted event rate by the CHA2DS2-VASc score.8,9 Similarly, bleeding reduction was assessed by comparing the actual major bleeding events to the rate predicted by the HAS-BLED score.10
Statistical analysisContinuous variables are presented as means±standard deviation and categorical variables are listed as frequencies and percentages. Continuous variables were tested using the independent samples t test and categorical variables using the chi-square test. A 2-sided P value <.05 was considered statistically significant. All statistical analyses were performed with SPSS 22.0 software (SPSS Inc, Chicago, Illinois, United States).
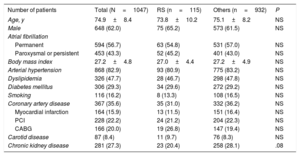
RESULTSA total of 115 patients (11%) with stroke on OAC therapy were identified (RS group). The baseline patient and procedural characteristics are shown in Table 1 and Table 2. Patients with RS had no statistically significant differences in the baseline or procedure characteristics compared with the other indications group.
Baseline patient characteristics
| Number of patients | Total (N=1047) | RS (n=115) | Others (n=932) | P |
|---|---|---|---|---|
| Age, y | 74.9±8.4 | 73.8±10.2 | 75.1±8.2 | NS |
| Male | 648 (62.0) | 75 (65.2) | 573 (61.5) | NS |
| Atrial fibrillation | ||||
| Permanent | 594 (56.7) | 63 (54.8) | 531 (57.0) | NS |
| Paroxysmal or persistent | 453 (43.3) | 52 (45.2) | 401 (43.0) | NS |
| Body mass index | 27.2±4.8 | 27.0±4.4 | 27.2±4.9 | NS |
| Arterial hypertension | 868 (82.9) | 93 (80.9) | 775 (83.2) | NS |
| Dyslipidemia | 326 (47.7) | 28 (46.7) | 298 (47.8) | NS |
| Diabetes mellitus | 306 (29.3) | 34 (29.6) | 272 (29.2) | NS |
| Smoking | 116 (16.2) | 8 (13.3) | 108 (16.5) | NS |
| Coronary artery disease | 367 (35.6) | 35 (31.0) | 332 (36.2) | NS |
| Myocardial infarction | 164 (15.9) | 13 (11.5) | 151 (16.4) | NS |
| PCI | 228 (22.2) | 24 (21.2) | 204 (22.3) | NS |
| CABG | 166 (20.0) | 19 (26.8) | 147 (19.4) | NS |
| Carotid disease | 87 (8.4) | 11 (9.7) | 76 (8.3) | NS |
| Chronic kidney disease | 281 (27.3) | 23 (20.4) | 258 (28.1) | .08 |
CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; RS, resistant stroke.
Variables are expressed as mean ± standard deviation or No. (%).
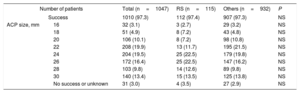
Procedural characteristics
| Number of patients | Total (n=1047) | RS (n=115) | Others (n=932) | P | |
|---|---|---|---|---|---|
| Success | 1010 (97.3) | 112 (97.4) | 907 (97.3) | NS | |
| ACP size, mm | 16 | 32 (3.1) | 3 (2.7) | 29 (3.2) | NS |
| 18 | 51 (4.9) | 8 (7.2) | 43 (4.8) | NS | |
| 20 | 106 (10.1) | 8 (7.2) | 98 (10.8) | NS | |
| 22 | 208 (19.9) | 13 (11.7) | 195 (21.5) | NS | |
| 24 | 204 (19.5) | 25 (22.5) | 179 (19.8) | NS | |
| 26 | 172 (16.4) | 25 (22.5) | 147 (16.2) | NS | |
| 28 | 103 (9.8) | 14 (12.6) | 89 (9.8) | NS | |
| 30 | 140 (13.4) | 15 (13.5) | 125 (13.8) | NS | |
| No success or unknown | 31 (3.0) | 4 (3.5) | 27 (2.9) | NS | |
ACP, Amplatzer Cardiac Plug; RS, resistant stroke.
Variables are presented as No. (%).
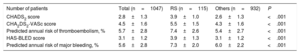
The mean CHA2DS2-VASc score was significantly higher in patients with RS (5.5±1.5 vs 4.3±1.6, P <.001), the mean HAS-BLED score was also higher in the RS group (3.9±1.3 vs 3.1±1.2, P <.001). The predicted annual risk of tromboembolism according to the CHA2DS2-VASc score was higher in the RS group (7.4±2.6 vs 5.4±2.7, P <.001) and the annual major bleeding risk according to the HAS-BLED score was also higher in patients with RS (7.3±2.0 vs 6.0±2.2, P <.001) (Table 3).
Risk scores and predicted annual risk of thromboembolism and major bleeding
| Number of patients | Total (n=1047) | RS (n=115) | Others (n=932) | P |
|---|---|---|---|---|
| CHADS2 score | 2.8±1.3 | 3.9±1.0 | 2.6±1.3 | <.001 |
| CHA2DS2-VASc score | 4.5±1.6 | 5.5±1.5 | 4.3±1.6 | <.001 |
| Predicted annual risk of thromboembolism, % | 5.7±2.8 | 7.4±2.6 | 5.4±2.7 | <.001 |
| HAS-BLED score | 3.1±1.2 | 3.9±1.3 | 3.1±1.2 | <.001 |
| Predicted annual risk of major bleeding, % | 5.6±2.8 | 7.3±2.0 | 6.0±2.2 | <.001 |
RS, resistant stroke.
Values are expressed as mean ± standard deviation.
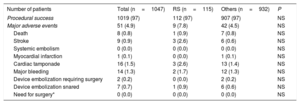
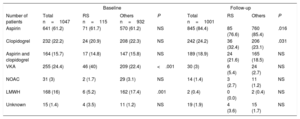
Procedural success was achieved in 1019 patients (97.3%) of the total cohort, without significant differences between both groups (Table 4). No significant differences were observed in periprocedural major safety events among 2 groups (7.8 vs 4.5%, P=.1). There were nonsignificant differences in the antithrombotic medication at baseline and at the last follow-up between the both groups (Table 5).
Procedural success and major adverse events
| Number of patients | Total (n=1047) | RS (n=115) | Others (n=932) | P |
|---|---|---|---|---|
| Procedural success | 1019 (97) | 112 (97) | 907 (97) | NS |
| Major adverse events | 51 (4.9) | 9 (7.8) | 42 (4.5) | NS |
| Death | 8 (0.8) | 1 (0.9) | 7 (0.8) | NS |
| Stroke | 9 (0.9) | 3 (2.6) | 6 (0.6) | NS |
| Systemic embolism | 0 (0.0) | 0 (0.0) | 0 (0.0) | NS |
| Myocardial infarction | 1 (0.1) | 0 (0.0) | 1 (0.1) | NS |
| Cardiac tamponade | 16 (1.5) | 3 (2.6) | 13 (1.4) | NS |
| Major bleeding | 14 (1.3) | 2 (1.7) | 12 (1.3) | NS |
| Device embolization requiring surgery | 2 (0.2) | 0 (0.0) | 2 (0.2) | NS |
| Device embolization snared | 7 (0.7) | 1 (0.9) | 6 (0.6) | NS |
| Need for surgery* | 0 (0.0) | 0 (0.0) | 0 (0.0) | NS |
RS, resistant stroke.
Values are expressed as No. (%).
Antithrombotic medication at baseline and follow-up
| Baseline | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of patients | Total n=1047 | RS n=115 | Others n=932 | P | Total n=1001 | RS | Others | P |
| Aspirin | 641 (61.2) | 71 (61.7) | 570 (61.2) | NS | 845 (84.4) | 85 (76.6) | 760 (85.4) | .016 |
| Clopidogrel | 232 (22.2) | 24 (20.9) | 208 (22.3) | NS | 242 (24.2) | 36 (32.4) | 206 (23.1) | .031 |
| Aspirin and clopidogrel | 164 (15.7) | 17 (14.8) | 147 (15.8) | NS | 189 (18.9) | 24 (21.6) | 165 (18.5) | NS |
| VKA | 255 (24.4) | 46 (40) | 209 (22.4) | <.001 | 30 (3) | 6 (5.4) | 24 (2.7) | NS |
| NOAC | 31 (3) | 2 (1.7) | 29 (3.1) | NS | 14 (1.4) | 3 (2.7) | 11 (1.2) | NS |
| LMWH | 168 (16) | 6 (5.2) | 162 (17.4) | .001 | 2 (0.4) | 0 (0.0) | 2 (0.4) | NS |
| Unknown | 15 (1.4) | 4 (3.5) | 11 (1.2) | NS | 19 (1.9) | 4 (3.6) | 15 (1.7) | NS |
LMWH, low molecular weight heparin; NOAC, nonvitamin K antagonist oral anticoagulants; RS, resistant stroke; VKA, vitamin K antagonists.
Values are presented as No. (%).
The mean clinical follow-up was 16.2±12.2 months, resulting in a total of 1349 patient years, and was complete in 1001 of 1019 of successfully implanted patients (98.2%). In all, 561 patients completed at least 1 year of follow-up. The observed annual stroke or TIA rate at follow-up for the RS group was 2.6% (65% relative reduction of thromboembolism according to the CHA2DS2-VASc score) and 1.2% for patients without RS (78% relative risk reduction according to the CHA2DS2-VASc score) (Figure 1). The observed annual major bleeding rate at follow-up for the RS group was 0% (100% relative reduction according to the HAS-BLED score) and 1.2% for those without RS (79% relative reduction) (Figure 2). A transesophageal echocardiogram at follow-up was available in 632 of 1001 (63%) of successfully implanted patients (RS group 78 of 111 patients [70%] and control group 554 of 890 [62%], P=.099) and was performed at a median of 7 (interquartile range, 3-11) months after LAAO. The rate of device thrombosis and peridevice leaks did not vary significantly between groups (device thrombosis in RS patients vs others: 5.2 vs 4.4%; P=.754; and peridevice leaks in RS patients vs others: 12.8% vs 11.4%; P=.708.
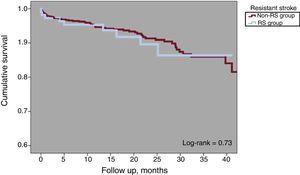
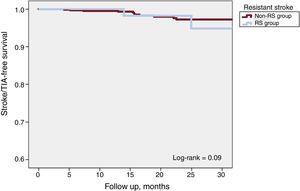
One-year all-cause mortality was 4.3% for the total population (Figure 3). A total of 63 deaths were reported at follow-up, 8 (7.2%) in the RS group and 55 (6.2%) in the non-RS group (P=.67). There were 8 periprocedural deaths (1 in the RS group [0.9%] and 7 [0.8] the non-RS group; P=.89) and none was reported as being related to the device. There were 18 strokes or TIA at follow-up, 4 (3.6%) in the RS group and 14 (1.6%) in the non-RS group (P=.12). Kaplan-Meier curves of cumulative survival free of stroke or TIA after LAAO are shown in Figure 4.
The present study evaluated the role of LAAO in patients with nonvalvular atrial fibrillation who had a stroke despite being treated with OAC. The most important findings of the study can be summarized as follows: a) the percutaneous LAAO success rate was high, above 97%, and LAAO was safely performed in patients with RS. The rate of complications was similar to that reported in other studies and to that in the non-RS group; b) percutaneous LAAO was effective for stroke reduction in both groups of patients (RS and non-RS); c) although patients with RS also had a higher risk for bleeding, there were no significant bleeding events at follow-up, which is remarkable for this patient cohort, and d) overall survival and stroke-free survival were similar in the 2 groups: RS and non-RS.
VKA have important limitations regarding their use in clinical practice.11 Regular follow-up visits are needed to keep the INR within the narrow therapeutic range which is, nonetheless, only achieved in 60% to 70% of cases according to previous studies,12 leaving a large number of patients in subtherapeutic levels and therefore at an increased risk of stroke.13 In addition, VKA may cause a number of complications, mainly bleeding. In this regard, NOAC offer some advantages not only by improving cardioembolic stroke prevention but also by reducing bleeding events. Several studies on NOAC have shown a reduction in thromboembolic events by about 20% over warfarin therapy.6 In these studies, about 20% of patients had a previous stroke or TIA, which correlates with advanced age, the most important risk factor for stroke in patients with atrial fibrillation. NOAC therapy as secondary prevention has been found to be noninferior to warfarin in the subgroup of patients with previous stroke or TIA. Despite NOAC therapy, these patients have a residual rate of stroke or systemic embolism between 2.0 to 2.8 per 100 patient-year of follow-up, which is higher than that of patients without previous stroke or TIA.14–16 Therefore, in this group of patients, with high residual risk of stroke despite OAC treatment, LAAO could play a promising role in reducing the risk of thromboembolic events.2
The management of patients with RS is challenging because, as shown in this study, they are characterized by a high thromboembolic risk by the CHA2DS2-VASc score, as well as for bleeding according to the HAS-BLED score. In this study, LAAO was associated with a reduction in the rate of stroke after comparison of observed and expected rates according to the CHA2DS2-VASc score and with reduced bleeding events in the follow-up. The reduction of stroke or TIA at follow-up compared with the expected incidence according to the CHA2DS2-VASc score (65%) was in agreement with previous studies.1,17–20 The reduction in bleeding events after LAAO was remarkable, with no major bleeding events occurring during follow-up in the RS group. Given the small number of events, this could represent a statistical bias, although as demonstrated in previous studies, LAAO significantly reduced bleeding beyond the immediate periprocedural period, and especially after discontinuation of adjuvant anticoagulant or antiplatelet treatment.21 We can speculate that when the indication for LAAO is not a contraindication for OAC, as in the RS group, the bleeding events after LAAO are really low. In contrast, in the non-RS group the indication for LAAO in many patients was a contraindication for OAC (due to previous bleeding) so the bleeding rates are higher, independently of the drug-regime used after LAAO. When deciding the strategy for stroke prevention, it is necessary to take into account the favorable effect of LAAO in reducing bleeding events in the long-term, especially if the indication for LAAO is not a contraindication for OAC or previous bleeding. On the other hand, the HAS-BLED score has been developed to predict the risk of bleeding in patients treated with OAC therapy. It would be reasonable to consider whether this score is adequate to predict bleeding events in patients with some resistance to oral anticoagulants, such as patients with RS.
In patients who experienced a stroke despite OAC therapy, it is necessary to investigate the cause of the stroke. The most frequent cause is an INR below the therapeutic range, but as described in previous studies, patients often have additional potential etiologies for stroke.22–24 In our study, we highlight the heterogeneity in the baseline treatment of patients with RS and the low use of NOAC (probably related to enrolment between 2008 and 2013).
Based on the effectiveness of LAAO in reducing stroke and bleeding, together with the low risk of periprocedural adverse events, we believe that LAAO should be considered in the secondary prevention of stroke in RS patients.
LimitationsThe present study has several limitations that should be acknowledged. This is a subanalysis of the Amplatzer Cardiac Plug registry, which was a nonrandomized, retrospective, observational study with no control group. The retrospective nature of the study is a major limitation, which may lead to underestimation of event rates, especially during follow-up. Importantly, the presence of a control group in the study, treated with OAC after the RS, would be a better comparator of the rate of ischemic and hemorrhagic events than that calculated by CHA2DS2-VASc and HAS-BLED. Another important limitation of the study is that, despite the significant reduction in the RR of stroke in patients with RS, these patients have a higher absolute risk of stroke than patients without RS. Although the study was not designed for this purpose, after LAAO, stroke reduction RR seems to be more important among patients without RS than among patients with RS.
We have no data on the possible causes for stroke despite anticoagulation (eg, INR out of range, left atrial thrombus despite anticoagulation, thrombi on an artificial heart valve, right-to-left shunt), nor about the level of INR if the patient had been treated with VKA. With respect to antithrombotic treatment at baseline, we have no data on the type of anticoagulant, only if it was VKA, NOAC, or low molecular-weight heparin. We have no transesophageal echocardiogram data before the procedure nor was transesophageal echocardiogram follow-up available for all patients.
CONCLUSIONSPatients with previous RS and indication for percutaneous LAAO had similar procedural outcomes to those of with patients without RS. According to the results of this study, LAAO could be safely performed in this group of patients, who have a high risk of thromboembolic and bleeding events. In this study, patients with RS had a significant reduction in stroke and TIA with no major bleeding events during the follow-up period, despite their overall high bleeding risk. Adequately powered controlled trials are needed to further investigate the use of LAAO in patients who experience a stroke despite OAC therapy. In this regard, robust data on the comparative efficacy of LAAO vs NOAC are of the utmost importance.
CONFLICTS OF INTERESTI. Cruz-González, X. Freixa, S. Gaafoor, H. Omran, S. Berti, G. Santoro, J. Nielsen-Kudsk, U. Landmesser, A. Aminian, M. Costa, and R. Ibrahim are consultants and proctors for Abbott. A. Tzikas, H. Sievert, P. Kanagaratnam, W. Schillinger, B. Meier, and J.W. Park are consultants, proctors, and have received research grants from Abbott. I. Cruz-González and M. Costa are proctors and consultants for Boston Scientific. I. Cruz-González is a proctor for Lifetech. S. Berti is a proctor for Edwards Lifesciences. F. Nietlispach is a consultant for Abbott, Edwards Lifesciences and Medtronic. The other authors have no conflicts of interest to declare.
- -
LAAO is an alternative to OAC therapy for the prevention of stroke in patients with nonvalvular atrial fibrillation.
- -
There have been no previous analyses of the role of LAAO as secondary prevention, among patients who have had a stroke despite OAC therapy (RS).
- -
In this study, LAAO in RS patients had a procedural succes rate of 97.0% and 4.5% rate of major periprocedural adverse events without significant differences with the non-RS group.
- -
LAAO in RS patients provided a favorable outcome with an annual risk reduction in stroke or TIA of 65% and a 100% annual risk reduction in major bleeding events during follow-up.
- -
Adequately powered controlled trials are needed to further investigate the use of LAAO in RS patients, with appropriate comparison of the classical treatment strategy vs the strategy that would add LAAO.