Stroke etiology remains undetermined in up to 30% of cases. Paroxysmal atrial fibrillation is found in 20% to 28% of patients with stroke initially classified as being of undetermined etiology. The aim of our study was to analyze left atrial function in ischemic stroke patients to identify patterns associated with cardioembolic etiology and to determine whether the patterns identified can be found in individuals initially classified as having a stroke of undetermined etiology.
MethodsWe studied a cohort of in-hospital ischemic stroke patients referred for transthoracic echocardiography. Treating neurologists determined stroke etiology based on the TOAST classification. Left atrial contractile function was assessed using 2-dimensional echocardiography to determine their ejection fraction and speckle tracking to measure left atrial strain rate: a-wave. Left atrial function was compared between stroke etiology subgroups and healthy controls.
ResultsNinety-seven patients (aged 67±15 years) with ischemic stroke (16.5% large-artery atherosclerosis, 15.5% small-vessel occlusion, 11.3% cardioembolic, 5.1% other determined etiology, 51.1% undetermined etiology) and 10 healthy volunteers (aged 63±7 years) were included. Left atrial ejection fraction was significantly decreased only in patients with stroke of cardioembolic and undetermined etiology compared with the control group (31.5±17.2%, 40.2±17.1%, and 59.1±8.4%, respectively; P=.004). The left atrial strain rate was significantly lower in patients with stroke caused by cardioembolic or undetermined etiology, or large-artery atherosclerosis compared with controls (−0.86±0.49, −1.31±0.56, −1.5±0.47, −2.37±1.18, respectively; P<.001).
ConclusionsPatients with stroke of undetermined etiology with left atrial function (ejection fraction and strain) similar to that of cardioembolic stroke patients may be misclassified and could potentially benefit from prolonged electrocardiography monitoring. Left atrial function analysis (ejection fraction and strain) might help to identify potential cardioembolic sources in patients with stroke of undetermined etiology.
Keywords
Stroke is a major cause of mortality and disability in adults, especially cardioembolic stroke, but the etiology can remain undetermined even after comprehensive study.1–3 Other causes of stroke4 include large-artery atherosclerosis, small-vessel occlusion, or other determined etiology. When echocardiography shows significant left ventricle dysfunction masses, cardioembolic stroke is relatively easy to diagnose. Nevertheless, most cardioembolic strokes are secondary to atrial fibrillation (AF),5 which is a well-established independent risk factor for this etiology.6
Approximately 10% of patients with acute ischemic stroke or transient ischemic attack will have newly diagnosed AF during their hospital admission for stroke7; an additional 12% of patients may be found to have AF when screened by continuous long-term electrocardiographic monitoring,8,9 which has been recently introduced in the recommendations for the management of patients with an acute ischemic stroke or transient ischemic attack without other apparent cause.10 Given the major burden of AF, it is possible that some cardioembolic strokes could be misclassified as being of undetermined etiology; if a cardioembolic source could be confirmed, this group of patients would benefit from anticoagulation therapy. Previous studies have suggested that unknown AF could be present in 20% to 28% of patients with stroke of undetermined etiology.11–15
It is well known that left atrial (LA) size predicts AF and stroke16,17 and is also related to stroke in patients without previous AF.18,19 Left atrial function, assessed by volumetric changes (left atrial ejection fraction [LAEF]) or by deformation imaging (LA strain [LAS] and LAS rate [LASR])20–23 has also been linked to paroxysmal AF.15,24–26 In patients with stroke of undetermined etiology, LAS (s-wave) has been related to paroxysmal AF.27 Additionally, in patients with persistent AF, LAS was a predictor of stroke28 even in patients with a low risk score for stroke.29 More recently, diagnosis of paroxysmal AF with continuous Holter monitoring has been related to LA function changes in patients with stroke of undetermined etiology15; patients with AF showed a more reduced LA function than those without AF.
The CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes, previous stroke [doubled], vascular disease, age 65–74, and sex [female]) score30 includes clinical parameters to establish stroke risk in patients with AF. It is also a strong predictor of ischemic stroke in patients with coronary artery disease without AF.31,32
Considering the potential burden of AF in stroke patients and the large number of patients with stroke of undetermined etiology, we hypothesized that some strokes classified as being of undetermined etiology might have a cardioembolic source, with silent, undiagnosed, underlying AF; in this sense, the evaluation of surrogates of atrial disease such as LA size and function could depict a subgroup of patients at risk of having AF and a potential reclassification to cardioembolic stroke. Therefore, the aim of our study was to characterize LA function in patients admitted to hospital with ischemic stroke in order to identify LA function patterns that could identify potentially misclassified stroke of undetermined etiology that actually has cardioembolic source. Long-term monitoring could be useful in these patients, and the introduction of anticoagulation therapy, as appropriate, could potentially reduce their risk of stroke recurrence.
METHODSWe included a retrospective cohort of in-hospital patients with ischemic stroke referred for transthoracic echocardiography after hospital admission between 2010 and 2013. The final diagnosis, based on the TOAST classification,4 was established by the treating neurologist.
PatientsThe retrospective cohort included patients admitted to the stroke unit of our center due to ischemic stroke who had undergone transthoracic echocardiography to assess the etiology. For etiological work-up, echocardiography is mainly performed in patients with large-vessel infarction and no significant (< 50%) extracranial or intracranial stenosis. The exclusion criteria were hemorrhagic stroke, severe reduction of left ventricular ejection fraction (< 35%), severe valvular heart disease, the presence of prosthetic heart valves, or active endocarditis. Stroke risk was assessed using the CHA2DS2-VASc score.30 Healthy volunteers without previous known cardiovascular disease were recruited and age-matched with patients in order to have reference values of normal LA function. All participants signed an informed written consent form and the study was approved by our ethics committee.
Stroke DiagnosisStroke diagnosis was established by the treating neurologists; only patients with ischemic stroke were included. Patients were admitted to our stroke unit and had 72-hour continuous electrocardiogram monitoring as well as extracranial and intracranial artery assessment by ultrasound or magnetic resonance angiography. Using the TOAST classification,4 stroke etiology was subdivided as cardioembolic, large-artery atherosclerosis, small-vessel occlusion, other determined etiology, or undetermined.
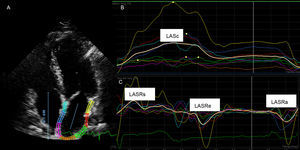
Echocardiography Acquisition and AnalysisA comprehensive 2-dimensional transthoracic echocardiography study with conventional Doppler and tissue Doppler imaging was performed using the commercially available system Philips IE33 (Andover, Massachusetts, United States). All patients were in sinus rhythm during echocardiography acquisition. Two-dimensional echocardiographic images were taken at a rate above 50 frames/s. Images were digitally stored for off-line analysis in a workstation by 2 echocardiographers blinded to the final diagnosis of the stroke subtype. Left atrial and left ventricle dimensions were determined according to current recommendations.33 Left atrial volume was measured at the end of LA diastole and systole on apical 4-chamber views. The LAEF was calculated as [(LA diastole-LA systole)/LA diastole – LA systole) × 100. Left atrial longitudinal deformation was analyzed off-line from 2-dimensional echocardiography, using speckle tracking derived strain and strain rate, with a commercially available software package (Qlab version 7.1, Philips). From the apical 4-chamber view, 3 points in the LA (2 in the mitral annulus and 1 in the LA roof) were indicated and the endocardial border was manually traced using a point-and-click technique. The software divided the LA wall into 6 segments and the average was used for analysis. The following LA parameters were determined: LAS during ventricular systole, LASR during ventricular systole, LASR during early ventricular filling phase, and LASR during late diastole/atrial contraction (LASRa). The onset of the QRS complex on the surface electrocardiogram21–23 was selected as the reference point for strain analysis (Figure 1). The LASRa was chosen as the strain reference value because of its stronger relationship with AF onset in the general population compared with LASR during ventricular systole.26
Left atrial strain profiles measured using Qlab (Philips). A: 2-dimensional echocardiographic apical 4-chamber view; the left atrial endocardial border was manually traced using a point-and-click technique; B: left atrial global longitudinal strain; C: left atrial longitudinal strain rate waves. LASRa, left atrial strain rate during late diastole/atrial contraction; LASRe, atrial strain rate during early ventricular filling phase; LASRs, left atrial strain rate during ventricular systole; LASs, left atrial strain during left ventricular systole.
The variables are shown as mean±standard deviation, frequency distribution, or proportions, as appropriate. Normal distribution of quantitative variables was assessed using the Kolmogorov-Smirnov test. A descriptive and comparative analysis was performed between the different diagnostic groups. The chi-square or Fisher test was used to compare categorical variables, and Student's t test was used for independent samples for quantitative variables. Analysis of variance and Bonferroni statistical tests were used to compare quantitative variables between more than 2 groups. A P-value < .05 (2-sided) was considered statistically significant. Intraobserver and interobserver intraclass correlations (Cronbach alpha) were performed in 10 patients for LAS analysis and were, respectively, as follows: LAS, 0.984, 0.978; LASR during ventricular systole, 0.979, 0.953; LASRa, 0.963, 0.960; LASR during early ventricular filling phase, 0.961, 0.943. Data were processed with SPSS version 19 (IBM, Armonk, New York, United States).
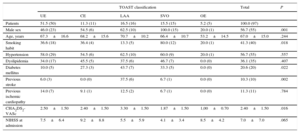
RESULTSDemographics and Clinical DataSeven patients were excluded due to poor echocardiography quality. Finally, 97 patients with ischemic stroke and 10 healthy controls were included (67±15 vs 63±8 years; P=.36). The baseline characteristics of stroke patients are shown in Table 1. Patients were mostly men with hypertension and a smoking habit, with a CHA2DS2-VASc score of 2.4±1.5. The most prevalent stroke etiology was undetermined etiology, followed by large-artery atherosclerosis and small-vessel occlusion. Only 5 patients had previous known AF; all of them were finally classified as having a cardioembolic stroke. Patients with stroke of other determined etiology were younger and had a low prevalence of cardiovascular risk factors. In contrast, small-vessel occlusion and large-artery atherosclerosis groups had the highest prevalence of cardiovascular risk factors. The highest CHA2DS2-VASc score was observed in the group of patients with large-artery atherosclerosis stroke, attributable to a higher prevalence of diabetes and previous stroke. Despite a lower CHA2DS2-VASc score in patients with stroke due to small-vessel occlusion, this group also had a higher proportion of men and smokers. With no previous known cardiovascular disease or stroke history in the healthy controls, their mean CHA2DS2-VASc score was 0.6±0.8.
Baseline Characteristics of Stroke Patients
| TOAST classification | Total | P | |||||
|---|---|---|---|---|---|---|---|
| UE | CE | LAA | SVO | OE | |||
| Patients | 51.5 (50) | 11.3 (11) | 16.5 (16) | 15.5 (15) | 5.2 (5) | 100.0 (97) | |
| Male sex | 46.0 (23) | 54.5 (6) | 62.5 (10) | 100.0 (15) | 20.0 (1) | 56.7 (55) | .001 |
| Age, years | 67.3±16.6 | 68.2±15.6 | 70.7±10.2 | 66.4±10.7 | 53.2±14.5 | 67.0±15.0 | .244 |
| Smoking habit | 36.6 (18) | 36.4 (4) | 13.3 (5) | 80.0 (12) | 20.0 (1) | 41.3 (40) | .018 |
| Hypertension | 58.0 (29) | 54.5 (6) | 62.5 (10) | 60.0 (9) | 20.0 (1) | 56.7 (55) | .557 |
| Dyslipidemia | 34.0 (17) | 45.5 (5) | 37.5 (6) | 46.7 (7) | 0.0 (0) | 36.1 (35) | .407 |
| Diabetes mellitus | 10.0 (5) | 27.3 (3) | 43.7 (7) | 33.3 (5) | 0.0 (0) | 20.6 (20) | .022 |
| Previous stroke | 6.0 (3) | 0.0 (0) | 37.5 (6) | 6.7 (1) | 0.0 (0) | 10.3 (10) | .002 |
| Previous ischemic cardiopathy | 14.0 (7) | 9.1 (1) | 12.5 (2) | 6.7 (1) | 0.0 (0) | 11.3 (11) | .784 |
| CHA2DS2-VASc | 2.50±1.50 | 2.40±1.50 | 3.30±1.50 | 1.87±1.50 | 1,00±0.70 | 2.40±1.50 | .016 |
| NIHSS at admission | 7.5±6.4 | 9.2±8.8 | 5.5±5.9 | 4.1±3.4 | 8.5±4.2 | 7.0±7.0 | .065 |
CE, cardioembolic stroke; CHA2DS2-VASc: congestive heart failure, hypertension, age ≥ 75 (doubled), diabetes, stroke (doubled), vascular disease, age 65-74, and sex (female); LAA, large-artery atherosclerosis; NIHSS, National Institute of Health Stroke Scale; OE, other known etiology; SVO, small-vessel occlusion; TOAST, Trial of Org 10172 in Acute Stroke Treatment; UE, undetermined etiology.
Data are expressed as % (no.) or mean±standard deviation.
Table 2 shows the echocardiographic parameters in the different stroke etiology groups and in the control group. In the analysis of variance among stroke subtypes, we found no differences in left ventricle dimensions, left ventricular ejection fraction, LA volume or LAEF. Despite a trend toward larger LA volumes and lower LAEF in patients with cardioembolic stroke, differences were not statistically significant between the different groups. When LA function was analyzed using LA deformation, patients with stroke of other determined etiology showed similar values to healthy controls. All the remaining patients with other stroke subtypes showed significantly lower LA deformation parameters (LAS during ventricular systole, LASR during ventricular systole, LASR during early ventricular filling phase) with no significant differences among them. The LASRa was decreased in all stroke subtypes, and the lowest values were found in patients with stroke of undetermined etiology and cardioembolic stroke with no statistically significant differences among them, but both showed significantly lower LASRa values than patients with small-vessel occlusion stroke. These findings indicate the presence of impaired LA function in stroke patients, especially in the cardioembolic stroke group, which showed the lowest LAS values.
Echocardiographic Parameters
| TOAST classification (stroke patients) | Omnibus P-value (stroke patients) | Controls | |||||
|---|---|---|---|---|---|---|---|
| UE | CE | LAA | SVO | OE | |||
| LVEF, % | 57,8±10,2 | 53,3±11,5 | 57,6±7,6 | 61,4±4,1 | 59,6±3,6 | .265 | 63.9±4.2 |
| LV diameter, mm | 50,0±6,1 | 50,3±6,4 | 49,1±5,0 | 51,2±4,3 | 50,8±4,9 | .898 | 51.3±5.5 |
| LA volume, mL | 62,9±24,6 | 82,8±40,2 | 58,1±26,5 | 61,0±27,1 | 67,2±31,1 | .203 | 65.2±25.4 |
| LAEF, % | 40,2±17,1 | 31,5±17,2 | 41,4±18,0 | 48,7±19,4 | 55,7±27,7 | .070 | 59.1±8.4 |
| LASs, % | 16,2±7,4a | 10,4±5,9b | 16,9±4,3c | 16,7±6,9d | 28,6±12,7a,b,c,d | <.001 | 28.3±5.2 |
| LASRs, s−1 | 0,98±0,47a | 0,65±0,38b | 1,00±0,30c | 1,02±0,49d | 2,02±1,39a,b,c,d | <.001 | 1.81±0.42 |
| LASRa, s−1 | –1,31±0,56e | –0,86±0,49f | –1,50±0,47 | –1,91±0,77e,f | –1,70±0,81 | <.001 | −2.37±1.18 |
| LASRe, s−1 | –0,86±0,61a | –0,54±0,40b | –0,85±0,38 | –0,74±0,42d | –1,76±1,15a,b,d | .004 | −1.63±1.02 |
CE, cardioembolic; LA, left atrial; LAA, large-artery atherosclerosis; LASs, left atrial strain during left ventricular systole; LASRa, left atrial strain rate during late diastole/atrial contraction; LASRe, left atrial strain rate during early ventricular filling phase; LASRs, left atrial strain rate during ventricular systole; LV, left ventricle; LAEF, left atrial ejection fraction; LVEF, left ventricular ejection fraction; OE, other known etiology; SVO, small-vessel occlusion; TOAST, Trial of Org 10172 in Acute Stroke Treatment; UE, undetermined etiology.
Statistically significant differences in Bonferroni post hoc analysis:
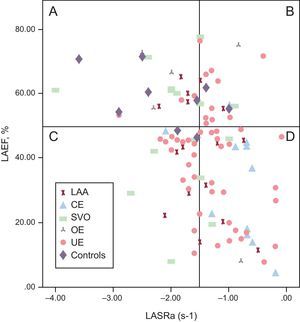
To identify potential patterns of LA function, we classified patients according to their normal or abnormal LA function. To define “normality,” an LAEF cut-off of 50% was chosen, according to previous reports.15,24 Additionally, the “normality” of LA booster function was determined with LASRa values obtained in the control group: the mean value in this group was −2.37±1.18 s−1 (95% confidence interval, −3.21 s−1 to –1.52 s−1). The lower value of the confidence interval (–1.52 s−1) was assumed as the limit of normality of LASRa. Figure 2 shows the distribution of the entire cohort of stroke patients according to their LAEF and LASRa. Most patients with cardioembolic stroke (82%) had consistent impairment of LA function (reduced LAEF and reduced LASRa) and were located in the “d” quadrant. In our population, 44% of patients with stroke of undetermined etiology were also located in the “d” quadrant. The remaining stroke subtypes were diffusely distributed in all quadrants.
Patient distribution by left atrial ejection fraction and left atrial strain rate. “a” quadrant: normal left atrial ejection fraction and left atrial strain rate (a wave); “b” quadrant: normal left atrial ejection fraction and low left atrial strain rate (a wave); “c” quadrant: low left atrial ejection fraction and normal left atrial strain rate (a wave); “d” quadrant: low left atrial ejection fraction and left atrial strain rate (a wave). CE, cardioembolic stroke; LAA, large-artery atherosclerosis stroke; LAEF, left atrial ejection fraction; LASRa, ate (a wave); OE, other determined etiology of stroke; SVO, small-vessel occlusion stroke; UE, stroke of undetermined etiology.
We studied LA function in patients with different subtypes of ischemic stroke as defined by the TOAST classification. Patients with cardioembolic stroke had significant impairment of LA function (LAEF and LAS). None of the patients with stroke of undetermined etiology had previously known AF but almost half of them showed impaired LA function similar to patients with cardioembolic stroke, suggesting major LA disease that could have been the source of the ischemic stroke. Patients with other stroke subtypes did not have a definite pattern of LA function, although patients with stroke due to large-artery atherosclerosis also showed a slightly impaired LA function.
Previous studies12-15,17 have reported that up to 28% of patients with stroke of undetermined etiology develop AF during follow-up. These patients showed an increased risk of recurrent stroke unless they were administered appropriate treatment. Longer term Holter monitoring (30 days) is a new recommendation of recent guidelines.10 Like other authors,27 we have shown that the analysis of LA function could also detect or predict AF on admission and is more comfortable for patients, as images are already acquired for a routine echocardiographic examination and only additional post-processing analysis is needed. Long-term monitoring is expensive and has limited availability. Therefore, echocardiography is a cost-effective study to select patients who could benefit from long-term monitoring.
During the cardiac cycle, the LA acts as a reservoir, receiving pulmonary venous return during left ventricular systole; as a conduit, passively transferring blood to the left ventricle during early diastole; and as a pump, actively filling the left ventricle in late diastole. All of these functions participate in the atrial contribution to stroke volume.34 Left atrial ejection fraction is a marker of overall LA function and has been related to AF onset.15,24 In our study, LAEF was decreased in patients with cardioembolic stroke, as previously reported, as well as in patients with stroke of undetermined etiology.15 Left atrial ejection fraction < below 50% has been proposed as a cut-off point to rule out or suspect cardioembolic stroke,15 as well as to identify paroxysmal AF.20 In our population, only 1 patient with cardioembolic stroke had an LAEF>50%. Based on previous studies that related LAS to paroxysmal AF onset,16,26 we proposed the addition of LAS analysis to LAEF not only to rule out the diagnosis of cardioembolic stroke, but also to potentially identify patients with stroke of undetermined etiology that might be misclassified as cardioembolic stroke in order to extend the etiologic study with long-term monitoring and start anticoagulation therapy.
Of note, most patients had impaired reservoir function (depicted by LAS and LASR during ventricular systole), which was likely related to LA fibrosis35,36 and left ventricle function. In patients with stroke of undetermined etiology, LA strain during ventricular systole has recently been proposed as a useful tool to detect patients with occult paroxysmal AF.27 In our study, impairment of LAS during ventricular systole was similar in the groups with strokes due to undetermined etiology, large-artery atherosclerosis, and small-vessel occlusion and was more reduced in the group with cardioembolic stroke. The LASRa was selected as the LA function marker of occult AF because of evidence that it is superior to LAS during ventricular systole for the detection of paroxysmal AF in the general population.26 The LA booster function (LASRa) was lowest in the subgroups with cardioembolic stroke (−0.86±0.49 s-1) and stroke of undetermined etiology (−1.31±0.56 s-1), but was much less reduced in the large-artery atherosclerosis group (−1.5±0.47 s-1). The classification of patients according to LAEF and LASRa (Figure 2) showed that patients with cardioembolic stroke were clearly confined in the quadrant of low LAEF and low LASRa, suggesting a strong relationship between LA dysfunction and cardioembolic etiology. Previous studies have also described the association of decreased LAS and stroke among patients with persistent chronic AF28,29; our findings confirm this previously reported association and raise the question of a potential role of the analysis of LA function with LAS in patients with cardioembolic stroke to improve their risk stratification for recurrent stroke.
Patients with stroke of undetermined etiology had impairment of LAEF or LASRa and almost half of them had low values for both parameters. Patients with stroke of other determined etiology and the control group had a similar distribution of these values; finding this could be explained by the fact that these “other” etiologies of stroke (nonatherosclerotic vasculopathies, hypercoagulable states, hematologic disorders) are less related to systemic atherosclerosis. Regarding the remaining patients with other stroke subtypes, they were diffusely distributed in all the quadrants, suggesting an absence of relationship with LA function. Patients with small-vessel occlusion stroke did not show significant differences in LASRa compared with the control group. The finding that patients with stroke of undetermined etiology had significant impairment of LA function that differed significantly with the other subtypes (except cardioembolic stroke) indicates an association between LA dysfunction and stroke of undetermined etiology. Patients with stroke of undetermined etiology and impaired LA function (both LAEF and LASRa) could potentially have paroxysmal AF and, therefore, there may indeed have been a cardioembolic source of stroke.
Left atrial volume has been related to AF onset,16 as well as to the incidence of stroke in patients without known AF.18,19 In our cohort, LA volume did not show significant differences between groups, despite a trend to larger LA volumes in patients with cardioembolic stroke; in fact, this trend could be secondary to the presence of a proportion of patients with cardioembolic stroke who had previous known paroxysmal AF (36.4%). The similar LA volumes in patients with stroke of undetermined etiology and in healthy controls suggest that this parameter is less useful to reclassify the subgroup of patients with stroke of undetermined etiology with a misclassified cardioembolic stroke.
Clinical ImplicationsWe identified a subgroup of patients with stroke of undetermined etiology with LA function (ejection fraction and strain) similar to that of patients with cardioembolic stroke. These patients may correspond to patients with a misclassified cardioembolic stroke. The analysis of LA function using transthoracic echocardiography (LAS and LAEF) is a noninvasive, inexpensive, and feasible way to identify patients with LA dysfunction20-23 and with a high risk of paroxysmal AF25,26; these patients could benefit from an extended etiologic study with long-term monitoring of heart rhythm. Currently, implantable loop recorders are the most effective tool to detect occult AF, but because of their invasive nature, they would not be appropriate for all patients and should be only implanted in selected patients.37-38 If further prospective studies (with 30-day Holter or implantable loop recorder) confirm the usefulness of study of LA function by speckle tracking strain to determine which patients have a strong probability of having cardioembolic stroke, anticoagulation may be started after the echocardiographic study. The development of tools to identify patients with stroke of undetermined etiology who have a high risk of an underlying cardioembolic etiology and the initiation of anticoagulation therapy could reduce stroke recurrence in these patients.
LimitationsOur single-center study is based on a retrospective cohort of a limited number of ischemic stroke patients. We only included those patients with ischemic stroke in whom the etiology study included echocardiography. Patients with a clear etiologic diagnosis of stroke (eg, severe carotid stenosis) are not usually referred for echocardiographic analysis leading to a small size of the group of patients with stroke due to large-artery atherosclerosis. In addition, the size of the control group was limited; however, data are increasingly available in the current literature with similar values to those reported in the present study. Left atrial volume was not indexed by body surface area, and therefore we cannot exclude a potential bias by the size of the patients. Finally, there was no systematic follow-up of patients to detect and confirm any potential paroxysmal AF. However, our findings are hypothesis-generating; further study in a larger prospective cohort of patients with long-term follow-up is certainly warranted.
CONCLUSIONSStudy of LA function with transthoracic echocardiography is feasible and potentially helpful to discriminate those patients with ischemic stroke of undetermined etiology and significant LA dysfunction that might be misclassified as having cardioembolic stroke. Long-term monitoring could be appropriate in these patients, who could potentially benefit from anticoagulant therapy.
CONFLICTS OF INTERESTNone declared.