Because of the extraordinary variety of structural, clinical, and therapeutic facets of hypertrophic cardiomyopathy (HCM), its diagnostic approach and comprehensive management are not uncommonly challenging. Likewise, factors such as the increasing interest in inherited cardiomyopathies, the clinical usefulness of genetic testing, and the growing use of cardiovascular magnetic resonance (CMR) have contributed to broadening the ever-expanding spectrum of HCM.
This editorial focuses on certain aspects of HCM that go beyond both the classical increased left ventricular (LV) wall thickness and the dichotomy of whether or not there is LV outflow tract (LVOT) obstruction. Thus, HCM is probably the cardiovascular condition featuring the widest phenotypic and hemodynamic variation; its associated risk of sudden cardiac death (SCD) has been and will continue to be a topic of research and discussion; finally, the invasive management of patients who are symptomatic due to intraventricular gradients should always take into account structural abnormalities involving the valvular and subvalvular apparatuses.
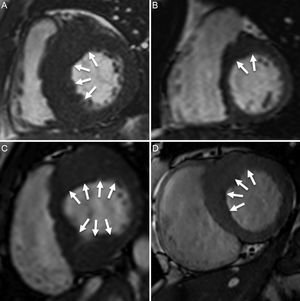
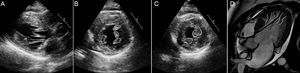
PHENOTYPE AND NOMENCLATUREAlthough phenotypic expression of HCM can be characterized by any location of hypertrophy, the major form of presentation is clustering of several contiguous hypertrophied segments. Specialists therefore tend to make the diagnosis of the disease together with the manner in which hypertrophy is distributed. Thus, the septal asymmetric phenotype is by far the leading form of HCM.1 However, there is considerable variation in the way hypertrophy involves the interventricular septum, ranging from single involvement of septal segments to–more frequently–involvement of septal segments along with other LV walls (Figure 1). From the clinical and research points of view, it is correct to report septal asymmetric HCM when there is involvement of ≥ 1 septal segment at the basal or midventricular levels, showing significant asymmetry with respect to most segments in the LV lateral wall. Nevertheless, virtually any other phenotype is also possible, ie, apical, apical with midventricular extension, concentric symmetric, and lateral asymmetric. However, regardless of the phenotypic expression, the absence of a family history of the disease and particularly the detection of cases with uncommon clinical presentation should be regarded with caution and infiltrative processes should be ruled out.
Septal asymmetric hypertrophic cardiomyopathy. A: significant septal involvement. B: mild involvement of anterior-septal segment (arrows). C: significant involvement of anterior, anterior-septal, inferior-septal, inferior segments. D: moderate involvement of anterior and anterior-septal segments.
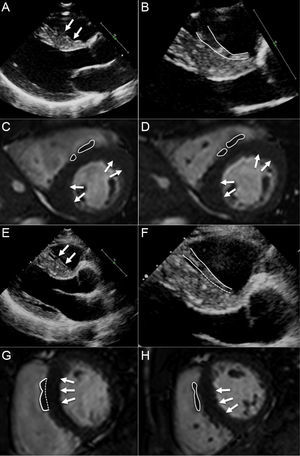
Assuming that septal asymmetric HCM is the most frequent diagnostic challenge faced by cardiologists when imaging unclear cases, it is crucial to recall concepts such as maximal LV wall thickness. Data from large cohorts have shown that the mean maximal LV wall thickness in HCM is about 21 mm to 22mm.1 Furthermore, its corresponding standard deviation (± 5 or ± 6)1 implies that most patients have at least 1 segment ≥ 15mm. A number of conditions that may lead to increased LV wall thickness, ie, systemic hypertension, aortic stenosis, athlete's heart, cardiac amyloidosis, with the corresponding concern for differential diagnosis. However, it is extremely rare that any of these conditions mimic the extent of hypertrophy usually observed in HCM, including the maximal LV wall thickness measured. This statement is based on 2 aspects that are worthy of comment. First, measurement of LV wall thickness should avoid including the right ventricular septomarginal trabeculae (Figure 2). By excluding that right-side structure, it is unusual to find an LV wall thickness of more than 15mm at the interventricular septum in athletes or patients with hypertension, aortic stenosis, or amyloidosis. Second, correct measurement of LV wall thickness in questionable cases of HCM depends on experience. In general, the learning curve for such a task may be minimized by a multimodality imaging approach to HCM, including side-by-side echocardiography and CMR analysis (Figure 2).
A: suspected septomarginal trabeculae (arrows). B: zoomed view of basal septum where septomarginal trabeculae is confirmed (delineated in white). C and D: cardiovascular magnetic resonance imaging from the same patient confirming the septomarginal trabeculae (delineated in white); hypertrophic cardiomyopathy is confirmed by detection of increased thickness at the insertion points of the right ventricle (arrows). E: suspected septomarginal trabeculae (arrows). F: zoomed view of basal septum where septomarginal trabeculae is closely related to the septum at the most basal region (delineated in white). G and H: cardiovascular magnetic resonance imaging from the same patient confirming the septomarginal trabeculae and its relationship with the anterior septum (delineated in white); hypertrophic cardiomyopathy is confirmed by detection of increased thickness at the anterior and inferior septum (arrows).
Clinical and echocardiographic medical records usually describe HCM as obstructive or nonobstructive. This dichotomy is derived from the names historically used for this disease. As demonstrated, most patients with HCM will show an intraventricular obstruction, either at rest (about one-third) or on exercise (about one-third).2 However, possible reasons for missing intraventricular gradients and for describing many cases of HCM as nonobstructive include the fact that HCM should be investigated by skilled sonographers who are not always available, the frequent impossibility of scanning patients in an attempt to search for obstruction at peak exercise (instead of using provocation maneuvers or drugs), and the nature of the obstruction itself, which varies widely in terms of the quantified gradient (dependent on hemodynamic setting, arrhythmia, volume status, and body position). This nomenclature often remains with patients for long periods of time, if not permanently. Therefore, keeping in mind the epidemiology of LV intraventricular obstruction in HCM, the possibility of obstruction should frequently be suspected and never ruled out in the routine follow-up of patients. Caution is advised when previous medical records include nonobstructive status, which refers to a single and particular moment in the disease course.
SUDDEN CARDIAC DEATH RISK ASSESSMENTThe assessment of SCD risk in HCM has been a topic of outstanding interest and discussion among experts for several decades. Proof of this is that clinicians still regularly face complicated situations in terms of risk assessment, which are hard to fit into the guidelines and experts’ recommendations. The root problem for this longstanding controversy is the low SCD rate that characterizes HCM. Thus, it is virtually impossible to develop a sufficiently large epidemiological study to assess, in terms of risk stratification, specific variables in all cardiology areas, ie, clinical evaluation, electrocardiography and arrhythmic substrate, hemodynamic factors, structural findings on imaging techniques, genetics, and so on. Although clarification about particular settings and discussion on the true value of specific factors are beyond the scope of this article, some aspects may help to put risk assessment in these patients into perspective.
As suggested above, due to its low event rate, most classical risk factors came into play by a simple association with SCD reported in the 1980s and 1990s. Subsequently, more comprehensive studies used the initially selected factors to establish the basis for SCD risk in HCM.3 Although this approach has generally been accepted, we should not forget that failure in risk stratification may come from this biased selection of potential risk factors. This is evident from the recent incorporation of new aspects in risk assessment, such as left atrial size and LVOT gradient, while other aspects have lost ground, such as blood pressure response during exercise.
A wise approach to risk assessment is to bear in mind the historical details that might have gone unnoticed. Left atrial size was already shown to play some role in SCD risk prediction in the late 1990s,4 but has only recently been formally accepted in patient management. Maximal LV wall thickness is considered a relevant parameter. Its role in risk assessment derives from a pivotal study in the early 1990s.5 In that study, both maximal LV wall thickness and wall thickness index, a parameter that quantified the overall extent of LV hypertrophy, were clearly associated with SCD in HCM patients. However, only maximal LV wall thickness remained as the parameter to be taken into account. Investigation of the extent of LV hypertrophy in risk stratification is warranted by both these historical data and current access to easier and accurate quantification by CMR.6 Finally, although measurement of maximal LV wall thickness has shown good reproducibility, caution has traditionally been recommended in patients with marked hypertrophy,7 in which a single number might have significant therapeutic implications. The aforementioned represent some examples of the importance of deep knowledge in the field when assessing SCD risk in patients with HCM.
The history of research on risk factors for SCD in HCM demonstrates the changing nature of risk stratification itself. There is still important work to be done to elucidate the underlying pathophysiology of a possible relationship between SCD and parameters showing significant variation, such as LVOT gradient; is it a risk marker or a risk factor? Equally, do gradients at the midventricular level have the same meaning? Furthermore, it is difficult not to accept tissue characterization by CMR, ie, the type and amount of myocardial fibrosis, as a crucial aspect in the risk assessment of these patients. Currently, it seems reasonable to at least consider CMR as an arbitrator in borderline cases. Probably, definition of how to quantify fibrosis and its standardization will eventually help to establish when and how CMR should be used. Moreover, a decreasing ejection fraction in HCM patients entails important prognostic implications.8 In this regard, advanced analysis of LV function, ie, the study of myocardial mechanics, might be key to understanding the overlap and the real differences between systolic dysfunction and the presence of significant myocardial fibrosis. As a final observation, HCM is an arrhythmic entity and, rather than searching for multiple possible risk markers, progress in this field is a matter of looking into the underlying substrate, namely fibrosis and disarray. Basic and translational research focussing on histology and electrophysiology are fundamental to introduce new insights in the clinical arena.
In summary, decision-making regarding primary prevention for SCD in HCM may be cumbersome. An individualized approach to risk assessment is probably the best care clinicians can provide to their patients. However, this approach needs expert recommendations, in which estimated risk will come closest to the actual risk and the true value of well- and lesser-known risk factors is included in the risk stratification process.
STRUCTURAL ABNORMALITIES BEYOND HYPERTROPHYThe definition of HCM has always been characterized by references to LV hypertrophy. Additionally, a frequent subset of words can be found when defining HCM, such as spectrum, wide array, and various, illustrating the wide diversity of this entity. Although these adjectives mostly concern the structural variety of phenotypic expressions in HCM, the wide spectrum of these findings might represent a comparable spectrum of hemodynamic abnormalities, clinical manifestations, and treatment options. Several features can be observed apart from hypertrophy, and their possible management implications are what make this condition extraordinarily fascinating.
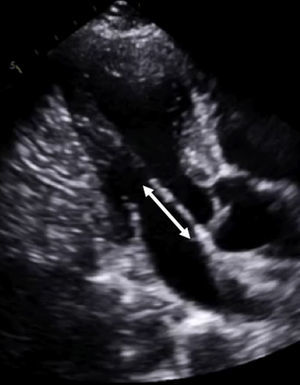
Starting with the mitral valve leaflets and proceeding to the subvalvular apparatus might be revealing in patients with presumptive or confirmed HCM. The anterior and posterior mitral leaflets often appear elongated on imaging techniques9 (Figure 3). The leaflet elongation has been suggested not to be associated with the magnitude of hypertrophy, thus representing a primary component of the disease.9 Therefore, this finding is relevant in terms of familial screening and follow-up in questionable cases. In addition, although the participation of multiple factors in LVOT obstruction is generally accepted, a significantly long anterior mitral leaflet together with a narrow LVOT should lead us to carefully search for a significant intraventricular gradient when it is not easily recorded at rest.
In the subvalvular apparatus, the anomalous attachment of most of the chordae tendineae bundle over the body and base of the mitral leaflets is sometimes striking in confirmed cases of HCM. The presence of this particular insertion into the anterior mitral leaflet is often associated with chordae tendineae anterior systolic motion. Less frequently observed is the anomalous insertion of the anterolateral papillary muscle (PM) directly into the anterior mitral leaflet10 (Figure 4A). This abnormality is invariably associated with the presence of either midventricular or significant LVOT gradients due to anterior displacement of the anterolateral PM. Other factors, such as accessory tendinous connections from the PM to the septum, may further contribute to the generation of anterior displacement and systolic gradient (Figures 4B and C). The most frequently observed abnormality pertaining to the subvalvular apparatus is likely to be apical displacement of the PM insertion point into the LV wall.11 In addition to the above-mentioned anomalies, this apical insertion eventually facilitates the proximity of the anterolateral PM to the septum all the way up to the mitral leaflets, thereby creating a suitable setting for intraventricular gradients.
A: anomalous insertion of the anterolateral papillary muscle (arrow heads) directly into the anterior mitral leaflet (arrow) in a patient diagnosed with septal asymmetric hypertrophic cardiomyopathy. B and C: anomalous tendinous connection (arrow heads) from the papillary muscle to the septum; the arrows indicate normal chordae tendineae bundle insertion into the mitral leaflets.
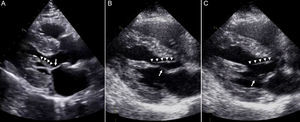
Finally, another relevant finding closely related to the subvalvular apparatus could be the presence of LV muscle bundles extending from the apex to the midventricular or basal levels of the LV anterior or septal walls12 (Figure 5). These accessory bundles are usually present in patients undergoing surgical myectomy.12 In fact, they have been shown to be frequently attached to the anterolateral PM at the apical level, which again seems to ease the anterior displacement of the anterolateral PM and its closeness to the septum.
A: suspected apical-basal muscle bundle (dashed line); continuous lines indicate normal papillary muscle tips. B: posteromedial and bifid anterolateral papillary muscle (continuous lines) and suspected apical-basal muscle bundle (dashed line). C: anterolateral papillary muscle fused with the origin of the apical-basal muscle bundle (continuous and dashed lines); a second muscle bundle anteriorly positioned (dashed line). D: cardiovascular magnetic resonance imaging from the same patient confirming a main apical-basal muscle bundle with secondary bundles (dashed lines); at the apical level the main bundle is fused with the anterolateral papillary muscle (continuous line).
The series of structural abnormalities discussed above has not been appropriately addressed in well-designed studies to demonstrate their prevalence and independent participation in symptomatic patients with HCM. It is our experience that at least one of these findings is present in most HCM cases (over 80%; data not published). Obviously, defining their role in LV midventricular and LVOT obstruction is crucial, since alcohol septal ablation is unlikely to be completely effective in its resolution. In contrast, the surgical approach seems to be more appropriate in this setting.13 Thus, long mitral leaflets with a low leaflet-septal contact point require an extended myectomy, sometimes together with a mitral valve intervention, ie, leaflet reconstruction or plication. Anomalous insertion of chordae tendineae and PMs into the leaflets raise the question of whether the resulting obstruction should be approached by also intervening in the subvalvular apparatus other than the myectomy procedure. Likewise, it seems reasonable to surgically eliminate factors that favor the anterior displacement of the anterolateral PM, namely removal of tendinous connections and muscle bundles,10,12 or partial release of the PM insertion point into the LV wall.10,12
CONCLUSIONSBecause HCM has multiple facets, its diagnostic approach and therapeutic management warrant the presence of specialized areas within cardiovascular medical centers. However, a comprehensive knowledge of the disease, including both the most significant features and lesser known aspects, is desirable in the screening and follow-up of patients with HCM outside centers of expertise. Eventually, many of these aspects are likely to relate to particular clinical settings, SCD risk stratification, and assessment of the need for certain invasive therapies.
CONFLICTS OF INTERESTNone declared.