Percutaneous coronary intervention is recommended in patients with unprotected left main stenosis non suitable for coronary artery bypass graft. Long-term follow-up of those patients remains uncertain.
MethodsAll patients with de novo unprotected left main stenosis treated with stent implantation were consecutively enrolled. Percutaneous coronary intervention was indicated according to the standards of care, taking into account clinical and anatomical conditions unfavorable for coronary artery bypass graft. The primary end point was the occurrence of major adverse cardiac events, a composite of death, nonfatal acute myocardial infarction, or target lesion revascularization.
ResultsOf 226 consecutive patients included, 202 (89.4%) were treated with drug-eluting stents. Mean age was 72.1 years, 41.1% had renal dysfunction, and mean Syntax score and EuroSCORE were 28.9 and 7.4, respectively. Angiographic and procedural success was achieved in 99.6% and 92.9% of patients. At 3.0 years, the rates of major adverse cardiac events, death, nonfatal acute myocardial infarction and target lesion revascularization were 36.2%, 25.2%, 8.4%, 8.0%, respectively. Target lesion revascularization was more frequently observed when ≥2 stents were implanted rather than a single stent (18.5% vs 5.8%, P=.03); and with bare metal stents rather than drug-eluting stents (13.0% vs 7.9%, P=.24). Definite stent thrombosis was observed in 2 patients (0.9%) and probable stent thrombosis in 7 (3.1%). Female sex, impaired left ventricular function, and use of bare metal stents were significantly related with all-cause mortality.
ConclusionsHigh-risk patients with unprotected left main stenosis treated with percutaneous coronary intervention presented with a high rate of major adverse cardiac events at long-term follow-up. Female sex, impaired left ventricular function, and use of bare metal stents were predictors of poor prognosis.
Keywords
.
IntroductionUnprotected left main (ULM) stenoses are seen in 4% to 5% of patients with angina pectoris undergoing coronary angiography.1, 2 Despite medical treatment, the prognosis of ULM stenosis is very poor without revascularization, reaching a mortality rate close to 50% at 3-year follow-up.3 Coronary artery bypass graft (CABG) has been shown to significantly reduce rates of major adverse cardiac events (MACE) compared to medical treatment4, 5 and CABG is still today the standard of care for patients with ULM stenosis.6, 7 Although the number of percutaneous coronary intervention (PCI) procedures for ULM stenosis has remained stable over the past few years, it represents approximately 4% of all PCI.8 PCI is recommended in patients with favorable anatomical conditions for the procedure and in patients with clinical conditions that predict an increased risk of adverse surgical outcomes.7 Patients with ULM lesions treated with PCI because of unsuitability for CABG, either due to anatomical and/or clinical conditions, represent an extremely high-risk population and the clinical outcomes of these patients at long-term follow-up remains unknown.
The aims of our study were to evaluate the long-term follow-up of high-risk patients with ULM stenosis treated percutaneously with stent implantation and to investigate the predictive factors of long-term mortality in those patients.
Methods Study Design and PopulationThe present study is a prospective, single-cohort study. All consecutive patients with ULM disease (>50% stenosis) treated with stent implantation from 2002 to 2007 in 2 high volume PCI centers with on-site cardiac surgery were prospectively recruited. The exclusion criteria were contraindication to double antiplatelet therapy, emergency situations such as acute ST-elevation myocardial infarction or cardiogenic shock, and the presence of a protected left main stenosis, defined as ≥1 patent coronary bypass grafts to the left coronary tree.
Patients included in the present study were selected according to the standards of care of the two participating centers. All nonurgent patients were discussed jointly with cardiac surgeons, taking into account clinical and anatomical conditions unfavorable for CABG surgery; therefore, patients treated with PCI are representative of a high-risk population. The main reasons for being turned down for surgical revascularization were the presence of comorbidities (such as pulmonary disease with forced expiratory volume <1000 cc, previous neurologic vascular event or left ejection fraction <30%), advanced age (>80 years), and non suitable bypass targets in the distal coronary fields. Patients with refractory angina (urgent procedures) were not necessarily discussed with cardiac surgeons and underwent PCI according to operator criteria and current guidelines.9 Urgent procedure was considered in case of refractory angina despite full medical treatment or intra-aortic balloon pumping.
Demographic and clinical baseline characteristics were collected. Additive and logistic EuroSCORES were estimated prospectively.10 Syntax score was estimated retrospectively by reviewing the angiograms of the index procedure according to the syntax score definitions (www.syntaxscore.com/).11 Angiographic, procedural, and inhospital clinical outcomes were also collected. Clinical follow-up was assessed prospectively every 6 to 12 months in the outpatient clinics or by telephone contact for those patients transferred to other referral hospitals. Repeated coronary angiography was performed electively in 53 patients at 6 to 12 months after PCI in one of the referred centers. These patients were included in a specific protocol assessing the feasibility of multi-slice computed tomography scan to detect stent restenosis.
Percutaneous Coronary Intervention Procedure and Postintervention PracticesThe PCI procedure was performed according to standard clinical practice. All patients received ≥1 stents. Bare metal stents (BMS) were implanted according to operator discretion, taking into account the hemorrhagic risk and unsuitability for long-term dual antiplatelet therapy. Main reasons for being treated with BMS were previous life-threatening hemorrhage, noncardiac surgery planned within 6 months, and chronic anemia. Stenting technique, use of abciximab, or need for left ventricular support (intra-aortic balloon pumping or Impella Recover) were left to operator discretion. All patients received a loading dose of 250mg of acteylsalicylic acid and 600mg of clopidogrel before PCI. Unfractioned heparin was administered to maintain an activated clotting time >250s. At postprocedure discharge, patients were instructed to take 150mg of acteylsalicylic acid and 75mg of clopidogrel per day. Clopidogrel treatment was discontinued 1 month after BMS implantation and varied after drug-eluting stent (DES) implantation (6 months to lifelong). To rule out periprocedural myocardial infarction, electrocardiograms and cardiac biomarkers (troponin I and creatine kinase-MB [CK-MB]) were assessed in all patients immediately before the procedure and 18 to 24h after PCI. A routine blood sample was taken before and 24h after the procedure to assess renal function and changes in the hemoglobin value. Written informed consent was obtained from all patients.
End Points and DefinitionsThe primary end point was the occurrence of MACE, defined as the composite of all-cause mortality, acute myocardial infarction (AMI), and target lesion revascularization (TLR). Secondary end points were cardiac death and stent thrombosis according to the ARC definition.12
Mortality was defined as all causes of death. Cardiac death was classified as sudden or unexplained due to ischemic events or heart failure. In-hospital deaths after the procedure were classified as ischemic cause. Periprocedural AMI was defined as an increase in troponin and/or CK-MB 3 times the upper limit of normal, with or without symptoms, in patients without previous elevation of cardiac markers. Spontaneous AMI was defined as an increase of troponin or CK-MB values with respect to the upper limit of normality associated with electrocardiographic changes of myocardial ischemia or chest pain. A TLR was defined as any repeat revascularization with PCI or CABG due to in-stent or in-segment restenosis with ≥50% stenosis diameter (including the first 5mm of the ostial left anterior descending or circumflex arteries in case of bifurcation treatment).12
Angiographic success was defined as a residual stenosis less than 20% and TIMI flow 3 at the end of the procedure. Procedural success was defined as angiographic success without periprocedural AMI or death during the procedure.
Statistical AnalysisContinuous variables were expressed as the mean (1 standard deviation), categorical variables as count (percentage), and cumulative incidences of events as rates (percentages). Event-free survival curves were generated with Kaplan-Meier and survival curves were compared between groups using the log rank test. Predictors of all-cause mortality were investigated using univariate analysis of all clinical (age, sex, hypertension, diabetes, hyperlipidemia, smoker history, previous myocardial infarction, previous PCI or CABG, ejection fraction, renal dysfunction, EuroSCORE value, acute coronary syndrome, and urgent procedures) and angiographic characteristics (femoral approach, lesions including the left main bifurcation, syntax score, number of other coronary vessels with stenosis, use of Abciximab, use of rotablational atherectomy, use of left ventricle assist device, stent diameter, use of drug eluting-stents, and implantation of ≥2 stents). Variables with a P value <0.1 were explored in a multivariate Cox proportional hazard analysis to identify independent predictors of global mortality. Since EuroSCORE is a sum of many potential predictive factors it was not included in the multivariate analysis. Results were reported as hazard ratios (HR), together with the 95% confidence intervals (95%CI) and P values. All P values were 2-tailed, with statistical significance set at <0.05. Statistical analyses were performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, Illinois, United States).
ResultsFrom 2002 to 2007 a total of 276 consecutive patients with a ULM stenosis (>50%) were treated with PCI in 2 institutions. After excluding 37 patients with ST elevation acute coronary syndrome (ACS) and 13 patients with non-ST elevation ACS but in cardiogenic shock, the remaining 226 patients were included in the final analysis. The median follow-up was 1088 (292-1884) days, with 214 patients (94.7%) having a complete follow-up at 2 years. Angiographic follow-up was performed in 115 patients (50.9%) with a median of 8.7 months follow-up (inter-quartile range 3.9-13.4 months).
Baseline Clinical, Angiographic, and Procedural CharacteristicsBaseline clinical characteristics are shown in Table 1. Mean age was 72.1 (10.6) years (range of 30-89) and 66.8% were males. A total of 84 patients (37.2%) were diabetics; 92 (41.1%) had chronic renal failure before the procedure, defined as creatinine clearance below 60mL/min (Cockroft-Gault formula); and 85 (37.6%) had previous myocardial infarction. Additive EuroSCORE was 7.4 (8.4), and 135 patients (59.7%) had high surgical risk according to a EuroSCORE value ≥6. Acute coronary syndrome was present in 93 patients (41.2%) and an urgent revascularization procedure was performed in 30 patients (13.3%) who had refractory angina without hemodynamic instability.
Table 1. Baseline Clinical Characteristics (no.=226)
| Age, years | 72.1±10.6 |
| Age ≥80 years | 71 (31.4) |
| Male sex | 151 (66.8) |
| Hypertension | 146 (64.6) |
| Diabetes | 84 (37.2) |
| Hyperlipidemia | 130 (57.5) |
| Smoker history | 102 (45.1) |
| Previous myocardial infarction | 85 (37.6) |
| Previous percutaneous coronary intervention | 50 (22.1) |
| Previous CABG | 21 (9.3) |
| Ejection fraction, % | 56±12 |
| Ejection fraction ≤30% | 19 (8.6) |
| Additive EuroSCORE | 7.4±8.4 |
| Logistic EuroSCORE | 8.7±10.2 |
| Creatinine clearance, mL/min | 73±33 |
| Renal dysfunction (Cr Cl<60 mL/min) | 92 (41.1) |
| Acute coronary syndrome | 93 (41.2) |
| Urgent procedure | 30 (13.3) |
CABG, coronary artery bypass graft.
Data are expressed as mean ± standard deviation or no. (%).
Angiographic and procedural characteristics are summarized in Table 2. The ULM lesion was located in the distal bifurcation in 150 patients (66.4%) and almost all patients had other coronary lesions (215 patients, 95.1%). The mean Syntax score was 28.9 (11.6). Intra-aortic balloon pumping was used in 81 patients (35.8%) and a left ventricular support device (Impella Recover 2.5) in 4 patients (1.8%) who underwent a high-risk procedure due to the presence of severe left ventricular dysfunction and/or occlusion of the right coronary artery. Drug-eluting stents were implanted in 202 patients (89.4%) and use of more than 1 stent occurred in 45 patients (19.9%), all of whom were treated with DES. Angiographic success was achieved in 225 patients (99.6%). Procedural success was achieved in 210 patients (92.9%); 1 patient suffered catheter thrombosis during the procedure with slow flow and died. Fifteen patients (6.6%) presented with a nonfatal periprocedural AMI.
Table 2. Angiographic and Procedural Characteristics (no.=226)
| Femoral approach | 183 (81) |
| Left main bifurcation | 150 (66.4) |
| Syntax score | 28.9±11.6 |
| Lesions in other coronary vessels | |
| None | 11 (4.9) |
| One | 26 (11.5) |
| Two | 65 (28.8) |
| Three | 124 (54.9) |
| Use of abciximab | 64 (28.3) |
| Rotablational atherectomy | 5 (2.2) |
| Intra-aortic balloon pumping | 81 (35.8) |
| Impella Recover | 4 (1.8) |
| Stent length, mm | 17±6 |
| Stent size, mm | 3.2±0.4 |
| Drug-eluting stent | 202 (89.4) |
| Taxus | 98 (43.4) |
| Cypher | 69 (30.5) |
| Endeavor | 25 (11.1) |
| Others | 10 (4.4) |
| Stenting technique | |
| Provisional Stent | 119 (52.7) |
| Provisional Stent + kissing balloon | 70 (31) |
| Crush | 22 (9.7) |
| T Stenting | 10 (4.4) |
| Kissing Stenting | 5 (2.2) |
| More 1 than stent in the left main | 45 (19.9) |
| Intravascular ultraound guided procedure | 40 (17.7) |
Data are expressed as mean±standard deviation or no. (%).
Table 3 shows the clinical outcomes at 30 days, 1 year, 2 years, and long-term follow-up. Global mortality at 30 days occurred in 19 patients (8.4%), 11 of them in-hospital. All deaths were caused by cardiac mortality. Urgent indication was significantly associated with global mortality (23% of patients with urgent procedure died vs only 6% of patients without urgent procedure; P=.01). Two patients presented with definite stent thromboses during the first 30 days, one of them a fatal in-hospital sub-acute thrombosis and the other a sub-acute thrombosis on day 9, which was treated with PCI but the patient died 2 days later. Six probable stent thromboses were documented by sudden or unexplained death after hospital discharge. The rate of nonfatal AMI was 7%; all cases were determined by a periprocedural elevation of cardiac biomarkers. A TLR was needed in two patients (0.9%) who were treated with PCI. The indication of TLR was definite stent thrombosis at day 9 in one patient; the other presented with angina at day 15 due to stenosis of the circumflex ostium after provisional stent technique. In 29 patients (12.8%), MACE occurred and was more frequent in patients having urgent procedures than in elective patients (30% vs 10%, respectively; P=.001).
Table 3. Incidence of Adverse Events at Short- and Long-term Follow-up (no.=226)
| 30 days | 1 year | 2 year | Long-term follow-up | |
| All cause mortality | 19 (8.4) | 34 (15) | 45 (19.9) | 57 (25.2) |
| Cardiac mortality | 19 (8.4) | 29 (12.8) | 36 (16) | 44 (19.5) |
| Sudden or unexplained death | 8 (3.5) | 14 (6.2) | 18 (8) | 25 (11.1) |
| Ischemic cause | 9 (4) | 13 (5.7) | 14 (6.2) | 15 (6.6) |
| Heart failure | 2 (0.9) | 2 (0.9) | 4 (1.8) | 4 (1.8) |
| Nonfatal acute myocardial infarction | 15 (6.6) | 18 (8) | 18 (8) | 19 (8.4) |
| Stent thrombosis | ||||
| Definite | 2 (0.9) | 2 (0.9) | 2 (0.9) | 2 (0.9) |
| Probable | 6 (2.7) | 7 (3.1) | 7 (3.1) | 7 (3.1) |
| Possible | 0 | 9 (4) | 16 (7.1) | 36 (15.9) |
| Target Lesion Revascularization | 2 (0.9) | 14 (6.2) * | 14 (6.2) * | 18 (8) * |
| Re-percutaneous coronary intervention | 2 (0.9) | 11 (4.9) | 11 (4.9) | 13 (5.6) |
| CABG | 0 | 4 (1.8) | 4 (1.8) | 6 (2.7) |
| MACE | 29 (12.8) | 56 (24.8) | 65 (28.8) | 82 (36.3) |
CABG, Coronary artery bypass graft; MACE, major adverse cardiac events.
Data are expressed as no. (%).
* One patient suffered stent restenosis twice during the first year, the first treated percutaneously and the second by coronary artery bypass graft.
Clinical outcomes are summarized in Table 3. At 2-year follow-up, 45 patients died (19.9%), 36 (16.0%) of them suffering cardiac mortality. Causes for cardiac death were sudden or unexplained death (50%), ischemic events (39%), and heart failure (11%). A TLR was performed in 14 patients (6.2%), 11 (5%) of them treated with repeat PCI and 4 (2%) with CABG. One patient suffered a second in-stent restenosis and was finally treated with CABG. Overall, MACE was observed in 65 patients (28.8%).
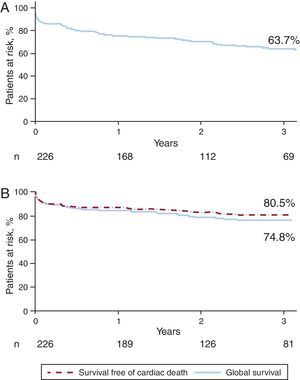
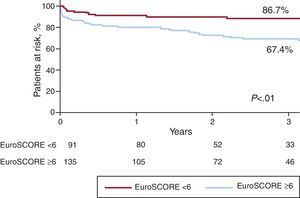
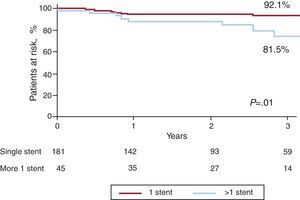
Long-term Follow-upSurvival curves of death and MACE at a median follow-up of 3 years (inter-quartile range 0.8-5.2 years) are presented in Figure 1. At this time, 57 patients had died (25.2%) and 82 (36.2%) had experienced a MACE event. Global survival was closely related to the EuroSCORE. The survival rates in patients with low surgical risk (additive EuroSCORE <6) and high surgical risk (additive EuroSCORE ≥6) were 87% and 67%, respectively; P<.01 (Figure 2). Definite or probable stent thrombosis occurred in 9 patients (4.0%) between 2 and 108 days after stent implantation (mean 20.5 days). All patients affected by stent thrombosis died. No definite stent thrombosis was detected after the first 30 days. Probable stent thrombosis occurred in 7 patients (3.1%), 6 of them within the first 30 days after the procedure. The other patient suffered a probable stent thrombosis at day 108 post-procedure. Definite or probable stent thrombosis was more frequently associated with BMS than with DES (13% vs 3%; P=.02) and tended to be more frequent in patients with acute coronary syndrome (7%) than in stable patients (2%); P=.11. AMI was detected in 19 patients (8.4%), 15 of the cases occurring in the first 30 days. Restenosis was detected in 18 patients (8.0%) and was more frequently associated with BMS than with DES (13% vs 8%, P=.24) and with complex techniques that involved implantation of more than one stent rather than a single stent technique (19% vs 6%, P=.01). None of the patients treated with complex techniques received BMS (Figure 3).
Figure 1. Major adverse cardiac events and global survival curves. A) Major adverse cardiac event-free survival; B) Global and cardiac death-free survival.
Figure 2. All-cause death-free survival curves according to the additive EuroSCORE.
Figure 3. Target lesion revascularization survival curves according to the number of stents used for left main treatment.
Predictors of Long-term MortalityTable 4 shows the results of the univariate and multivariate Cox regression analysis. Univariate analysis showed that age, female sex, previous renal failure, left ventricular ejection fraction, acute coronary syndrome at time of the index procedure, BMS, and EuroSCORE value were related with overall survival. The syntax score was not related with global mortality (HR=0.99, P=.58). Multivariate Cox-regression analysis (without the additive EuroSCORE) identified only 3 independent predictors of all-cause mortality at long term follow-up: female sex, left ventricular dysfunction (defined as ejection fraction <50%) and use of BMS for the treatment of ULM. Previous renal failure (defined as creatinine clearance <60mL/min) was almost significant (P=.06).
Table 4. Independent Predictors of Global Mortality at Long-term Follow-up
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.05 (1.02-1.08) | <.01 | 1,03 (0,99-1,07) | .14 |
| Female sex | 1.57 (0.93-2.66) | .09 | 2,01 (1,14-3,54) | .02 |
| Renal dysfunction | 2.48 (1.44-4.30) | <.01 | 1,83 (0,94-3,58) | .06 |
| Ejection fraction | 0.97 (0.95-0.99) | <.01 | 0,97 (0,05-0,99) | <.01 |
| Acute coronary syndrome | 1.69 (1.00-2.85) | .05 | 1,51 (0,85-2,68) | .16 |
| Bare metal stent | 2.24 (1.17-4.30) | .02 | 2,14 (1,08-4,25) | .03 |
| Additive EuroSCORE | 1.03 (1.01-1.05) | <.01 | - | - |
HR, hazard ratio; 95%CI, 95% confidence interval.
The main findings of our study are: a) high-risk populations with ULM stenosis experience a high rate of MACE and death at long-term follow-up; b) at 30 days, this high risk population has similar mortality to that expected by the logistic EuroSCORE; c) independent predictors of all cause mortality at long-term follow-up are women, impaired ejection fraction and use of BMS.
Our study reports one of the highest risk series of patients treated with PCI for ULM stenosis. According to the ACC/AHA Guidelines CABG is a class I indication for the treatment of ULM stenosis. 6 Nevertheless in the updated guidelines of 2009, PCI has become a class IIB in patients “with anatomic conditions associated with low risk of PCI-procedural complications and clinical conditions that predict an increased risk of adverse surgical outcomes”.7 Following these recommendations, PCI was performed in those patients at high surgical risk or in those not having suitable anatomy for bypass grafting. Only 30 patients (13%) were not discussed with cardiac surgeons due to urgent procedure in case of refractory angina. Compared with other studies,13, 14 our population is older (mean age of 72 years), had a higher proportion of patients with diabetes (37%), renal dysfunction (41%) and had high additive EuroSCORE values (mean EuroSCORE of 7.4% and 60% of patients had an additive EuroSCORE ≥6). Chieffo et al.,13 in a long term follow-up registry, and Morice et al.,14 in the subset of patients with ULM disease included in the Syntax trial, reported a very low risk profile of patients with similar baseline clinical and angiographic characteristics to CABG patients. In these studies, mean age was 64 and 65 years old, 19% and 24% of patients were diabetics, 2% and 2% had renal dysfunction and the mean additive EuroSCORE was 4.4 and 3.9; respectively. Clinical outcomes of these studies were dramatically different with respect to our results. At 1 year follow-up, all-cause of death was 2.8% and 4.2%; respectively.13, 14
Pavei et al.15 and Vaquerizo et al.16 reported two PCI registries with similar inclusion criteria to our study. Nevertheless, even though the similarity of the inclusion criteria, the study population of these studies had a better profile than our patients: mean age was 71 and 69 years-old, 27% and 29% were diabetics, renal dysfunction was 42% and 28% and 46.6% and 38% of patients had an EuroSCORE value ≥6; respectively. Accordingly, clinical outcomes were better than our results. In these studies, all cause death rates were 10.1% and 9.3% at 2 year follow-up; respectively.15, 16
Similarly as other studies,17 the high rate of MACE observed in our registry was initiated during the first 30 days after the procedure. Despite procedural success was achieved in 93% of patients, all-cause of death and MACE were 8.4% and 12.8% at 30 days; respectively. Death causes were related with definite or probable stent thrombosis, heart failure and/ or with complications due to the acute coronary syndrome treatment. Deaths were documented mainly in old patients (mean age 76 years-old), with renal dysfunction (63.2%), with acute coronary syndrome (79%) and in patients that underwent to PCI due to urgent procedures (42%). At 30-days, Pavei et al.15 reported all-cause of death and MACE of 1.4% and 1.4% respectively, whilst Buszman et al.18 reported rates of 1.4% and 4.8% respectively in a cohort who also had better risk profile. We attribute these worse short-term outcomes to the high risk population of our registry. Moreover, the observed rate of all-cause of death at that point of time is non-statistically different than that estimated by the logistic EuroSCORE (8.4% vs 8.7%, respectively; P=.91).
The stent thrombosis rate reported in our study at 3 years (1% definite stent thrombosis and 3% of probable stent thrombosis) is slightly higher than in other registries with low-risk profile of patients,19 but similar as other registries with high risk population.20 All cases of stent thrombosis were observed during the first year follow-up and were more frequently related with BMS. Six patients with probable stent thrombosis presented with sudden or unexplained deaths during the first month after stent implantation and were not submitted to necropsy confirmation of stent thrombosis. It is uncertain if intravascular ultrasound (IVUS) guidance of left main stenting can reduce the risk of stent thrombosis at short-term follow-up. In our study, IVUS-guidance was performed only in 17.7% of patients. Park et al. reported lower rates of all-cause of death and myocardial infarction using IVUS-guidance for ULM stenting with DES compared with angiographic-guidance at 3 year follow-up.21
Our registry found that the independent predictor factors of all-cause mortality were the gender, impaired ejection fraction and use of BMS. Renal dysfunction was almost statistically significant. The inclusion of the additive EuroSCORE in the Cox model did not modify the 4 independent predictor factors found in our model. Women have showed higher risk of MACE, including mortality, after CABG.22 PCI has showed inconsistent results with similar or slightly higher mortality in women.23 This fact is caused because usually the women treated with PCI are older and present with higher frequency of cardiovascular risk factors.23, 24 However, in our population, the analysis of the differences between men and women failed to be statistically significant in age, ejection fraction, additive EuroSCORE value, cardiovascular risk factors, clinical indication and use of BMS. Until our knowledge, there are no reports of worse cardiovascular outcomes in women treated with stent due to ULM stenosis. Low ejection fraction has been found in many registries to be one of the most important predictors of mortality in patients with ULM stenosis treated with PCI.15, 18, 25, 26 Renal dysfunction has also been found in a few studies to be an independent predictive factor of mortality in those patients.14, 27 The treatment of ULM stenosis with bare metal stents have been linked with an increased risk of left main restenosis in several trials but only Palmerini et al. confirmed a significant relationship between this and subsequent mortality.28 This supports the AHA recommendation to use DES in the percutaneous treatment of ULM stenosis when the patient's co-morbidities allow use of double antiplatelet therapy.29
Finally, in our study the syntax score was not related with global mortality. This result is congruent with a recent report that suggested that syntax score had a poor to moderate accuracy to predict all-cause of death at 6 months in patients with ULM stenosis treated with stent (area under curve of 0.71; 95%CI of 0.60 to 0.82).30 The same study concluded that other score test with clinical parameters, such as the NERS score, was more accurate to assess global mortality and major cardiac events.30
LimitationsThe present study is a multi-center registry. PCI was selected in front of CABG according to the clinical practice of the participating centers. Patients with ULM stenosis underwent CABG surgery and therefore the results reported in the present study are conditioned to a high-risk population. The second limitation is the small sample size included in the present study. Predictors of all-cause mortality could be influenced by the relatively few events included in the statistical models. Moreover, few patients were treated with BMS are few and they had more comorbidities than those treated with DES. Third, duration of dual antiplatelet therapy differed between patients and this treatment status was not assessed at the time of each clinical event. Fourth, angiographic follow-up was performed in 51% of patients. Although we tried to perform TLR only in cases with a clinically driven indication, some could be considered angiographically driven. Finally, IVUS was rarely used in the present registry and in some cases was used to optimize stent implantation.
ConclusionsPercutaneous treatment of high-risk patients with ULM stenosis presented a moderate-high rate of death and MACE at short- and long-term follow-up. Renal dysfunction, low ejection fraction, and use of BMS were independent predictors of global mortality.
Conflicts of interestNone declared.
Acknowledgements
We thank the Institut d’Investigació Biomèdica de Bellvitge (IDIBELL) for the grant awarded to Josep Gomez-Lara.
Received 20 June 2011
Accepted 20 December 2011
Corresponding author: Departamento de Cardiología Intervencionista, Hospital Universitari de Bellvitge, Feixa Llarga s/n, 08970 L’Hospitalet de Llobregat, Barcelona, Spain. 26587jgh@comb.cat