Transcatheter aortic valve implantation is used as an alternative to surgical valve replacement in patients with severe aortic stenosis who are considered high-surgical-risk or inoperable. Two of the main areas of uncertainty in this field are valve durability and long-term survival.
MethodsThis prospective single-center registry study from a tertiary hospital included all consecutive patients who underwent percutaneous aortic valve implantation between 2008 and 2012. Clinical follow-up lasted a minimum of 2.5 years and a maximum of 6.5 years. Valve Academic Research Consortium-2 definitions were used.
ResultsSeventy-nine patients were included, with an immediate success rate of 94.9%. The median survival was 47.6 months (95% confidence intervals, 37.4-57.9 months), ie, 4 years. One quarter of deaths occurred in the first month, and most were of cardiovascular cause. After the first month, most deaths were due to noncardiovascular causes. The mean values of valve gradients did not increase during follow-up. The cumulative rate of prosthetic valve dysfunction was 15.3%, with no cases of repeat valve replacement.
ConclusionsHalf of the patients with aortic stenosis who underwent transcatheter aortic valve implantation were alive 4 years after the procedure. There was a 15.3% prosthetic valve dysfunction rate in cumulative follow-up, with no cases of repeat valve replacement.
Keywords
Aortic stenosis (AS) is the most common acquired valvular heart disease, with a prevalence of up to 4.6% in patients older than 75 years, and is the primary reason for valve surgery in adults.1 In developed countries, the most common cause is degenerative AS.2 The natural history of the disease begins with a long subclinical period, which cannot be modified by medical treatment.3 Symptoms appear when AS is hemodynamically severe, and from that point the survival rate rapidly falls if the valve is not replaced. Survival in patients with severe AS only began to improve with the introduction of surgical valve replacement.4 However, despite 60 years of experience, it has been estimated that more than a third of eligible candidates do not undergo surgical valve replacement.2,5 The main reason is their high surgical risk, assessed with scores such as EuroSCORE or the STS (Society of Thoracic Surgeons) score, although there are other limiting factors: advanced age, liver disease, porcelain aorta, coronary artery bypass graft, pulmonary hypertension, right ventricular dysfunction, and the condition known as hostile chest.6
It was with these circumstances in mind that transcatheter aortic valve implantation (TAVI) was developed, a procedure that has grown exponentially since its introduction little more than a decade ago. The current indication for TAVI is symptomatic severe AS in patients considered inoperable by a multidisciplinary team due to high surgical risk (class I-B recommendation). In patients who are operable but high-risk, the decision to operate should be made on an individual basis (class IIa-B recommendation).7 These indications are primarily based on 2 randomized clinical trials, in which TAVI was shown to have similar outcomes to surgical valve replacement in patients with high surgical risk (PARTNER A),8 and to improve survival and functional class more than medical treatment (including valvuloplasty) in inoperable patients (PARTNER B).9
Transcatheter aortic valve implantation is successfully performed in approximately 90% to 98% of patients.10–13 Several registries have reported 30-day mortality of around 5% to 15%,8,13,14 1-year mortality of 15% to 30.7%,9,14,15 and 2-year mortality of 26.3% to 43%.12,16,17 However, data beyond 2 years postprocedure are scarce, especially in Spain. Among the areas of uncertainty relating to TAVI are long-term patient survival and valve durability.
METHODSAimsThe primary aim of this study was to analyze long-term all-cause death-free survival in a cohort of consecutive patients with severe AS, indication for valve replacement, and high surgical risk who underwent TAVI. The secondary aims were to describe the cause and timing of deaths, adverse events, and valve function at follow-up.
Design and Sample SelectionThis was a prospective observational study with follow-up of all consecutive patients (N = 79) who had a TAVI procedure in our center between June 2008 and June 2012.
All patients had a diagnosis of severe AS and indication for valve replacement according to the European Society of Cardiology guidelines on the management of valvular heart disease.7 Patients were considered high-surgical-risk if predicted mortality was ≥ 15% on EuroSCORE, or ≥ 10% on the STS score and if they were considered to be inoperable based on Heart Team assessment of comorbidities and other factors.6
ProcedureClinical assessment and diagnostic testing of patients with severe AS and high surgical risk were similar to published recommendations and have been previously described.6,18–20 Informed consent was obtained from all patients. The procedures were performed in a cardiac catheterization laboratory under sterile conditions, according to the manufacturer's established protocols, under general anesthetic, and with continuous transesophageal echocardiographic monitoring.19,20 If significant coronary artery disease was found, the patients underwent revascularization and TAVI was postponed for 1 month. Vascular access was obtained via surgical femoral cutdown, with the exception of the first 10 patients (percutaneous closure). Post-TAVI medical treatment consisted of acetylsalicylic acid 100mg (indefinitely) and clopidogrel 75mg (6 months). The implanted prosthesis was Edwards SAPIEN or the subsequent Edwards SAPIEN XT (from 2010), both from Edwards Lifesciences. In patients with adequate vascular access (iliofemoral diameter < 7mm, or < 6mm in XT model), transfemoral access was used, otherwise transapical access was used.
Study ParametersThe variables were entered in a specially-dedicated database. In October 2011, the first European consensus document on TAVI, called Valve Academic Research Consortium (VARC), was published and subsequently revised in the VARC-2 recommendations.6 For our study, all variables were adapted to VARC-2 definitions, with the exception of postprocedure acute kidney injury (24-h diuresis not recorded) and the combined early safety endpoint (which included acute kidney injury). The following are definitions of the most relevant variables (definitions of all variables are available in the appended ):
- •
Mortality: all-cause mortality (primary endpoint), subclassified as cardiovascular or noncardiovascular (secondary endpoint); deaths of unknown cause were attributed to cardiovascular causes.
- •
Major adverse event: all-cause mortality, stroke, readmission for valve-related symptoms or for worsening heart failure, deterioration in functional class to class III-IV, or prosthetic valve dysfunction; equivalent to the VARC-2 composite endpoint of clinical efficacy after 30 days.
- •
Acute kidney injury postprocedure, not requiring hemodialysis: creatinine raised by > 0.5mg/dL or > 50% of baseline value.
- •
Device success (VARC-2): post-procedure survival, correct positioning of a single prosthetic heart valve in the proper anatomical location and intended performance of the prosthetic heart valve (absence of mismatch, mean gradient < 20mmHg or peak velocity < 3 m/s and absence of moderate or severe aortic regurgitation [AR]).
- •
Early safety at 30 days (modified from VARC-2): all-cause death, stroke, life-threatening bleeding, acute kidney injury post-procedure, coronary artery obstruction requiring intervention, major vascular complication, or prosthetic valve dysfunction requiring intervention (surgical valve replacement, repeat TAVI, or valvuloplasty).
- •
Prosthetic valve dysfunction (VARC-2): mean prosthetic gradient > 20mmHg, effective orifice area < 0.9cm2 to 1.1cm2, Doppler velocity index < 0.35, or moderate or severe AR.
Prospective follow-up of adverse events was carried out during clinic visits or via telephone at 1 month, 6 months, and annually thereafter. The inclusion period was from June 2008 until June 2012. Clinical follow-up ended in January 2015, giving a minimum follow-up of 2.5 years and maximum of 6.5 years. No patients were lost to clinical follow-up and contact was maintained with all patients until their death or until end of follow-up. Four patients were lost to echocardiographic follow-up, because they moved out of the Community of Madrid (clinical follow-up was completed, but without echocardiographic data).
Statistical AnalysisThe descriptive analysis used count and percentage for categorical variables, and mean ± standard deviation or median [interquartile range] for qualitative variables. Echocardiographic data were compared using a paired Student t test. Survival data was analyzed using the Kaplan-Meier method. In the adverse events analysis, only the first recorded event was included. A bilateral P value < 0.05 was considered significant. The programs SPSS 20 (SPSS Inc.) and Stata 11.1 (StataCorp LP) were used.
RESULTSPopulation CharacteristicsIn total, 79 procedures were performed, all with approved indications, except for 7 cases with off-label indications: 2 cases with an aortic ring < 1.8cm, 2 with a prosthetic mitral valve, 2 valve-in-valve implants in degenerative bioprotheses, and 1 with a bicuspid aortic valve.
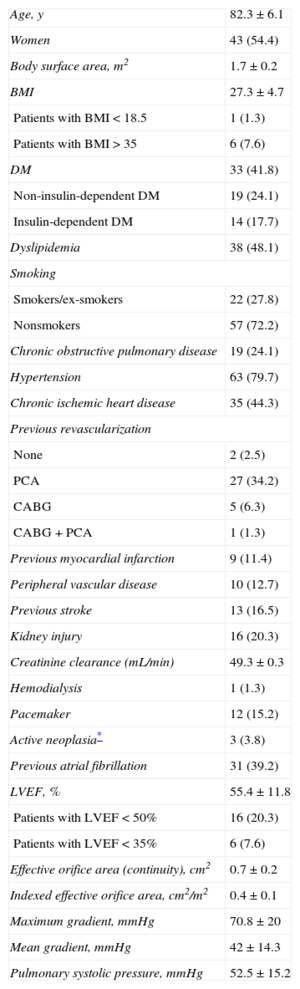
The baseline clinical characteristics and echocardiographic data are shown in Table 1. All patients had symptomatic severe AS, indication for valve replacement, and high surgical risk, or were inoperable. Access was transfemoral in 64 procedures (81%) and transapical in 15 (19%). The Edwards SAPIEN valve was implanted in 14 patients (17.7%) and the Edwards SAPIEN XT was implanted in 65 patients (82.3%). The mean surgical risk scores were as follows: logistic EuroSCORE, 16.9% ± 9.1% (1.8% to 46.9%); EuroSCORE-2, 5.7% ± 3.8% (0.7% to 18.1%); STS score, 5.9% ± 2.9% (1.1% to 13.4%).
Baseline Clinical Characteristics and Echocardiographic Parameters of the Study Population (N = 79)
| Age, y | 82.3 ± 6.1 |
| Women | 43 (54.4) |
| Body surface area, m2 | 1.7 ± 0.2 |
| BMI | 27.3 ± 4.7 |
| Patients with BMI < 18.5 | 1 (1.3) |
| Patients with BMI > 35 | 6 (7.6) |
| DM | 33 (41.8) |
| Non-insulin-dependent DM | 19 (24.1) |
| Insulin-dependent DM | 14 (17.7) |
| Dyslipidemia | 38 (48.1) |
| Smoking | |
| Smokers/ex-smokers | 22 (27.8) |
| Nonsmokers | 57 (72.2) |
| Chronic obstructive pulmonary disease | 19 (24.1) |
| Hypertension | 63 (79.7) |
| Chronic ischemic heart disease | 35 (44.3) |
| Previous revascularization | |
| None | 2 (2.5) |
| PCA | 27 (34.2) |
| CABG | 5 (6.3) |
| CABG + PCA | 1 (1.3) |
| Previous myocardial infarction | 9 (11.4) |
| Peripheral vascular disease | 10 (12.7) |
| Previous stroke | 13 (16.5) |
| Kidney injury | 16 (20.3) |
| Creatinine clearance (mL/min) | 49.3 ± 0.3 |
| Hemodialysis | 1 (1.3) |
| Pacemaker | 12 (15.2) |
| Active neoplasia* | 3 (3.8) |
| Previous atrial fibrillation | 31 (39.2) |
| LVEF, % | 55.4 ± 11.8 |
| Patients with LVEF < 50% | 16 (20.3) |
| Patients with LVEF < 35% | 6 (7.6) |
| Effective orifice area (continuity), cm2 | 0.7 ± 0.2 |
| Indexed effective orifice area, cm2/m2 | 0.4 ± 0.1 |
| Maximum gradient, mmHg | 70.8 ± 20 |
| Mean gradient, mmHg | 42 ± 14.3 |
| Pulmonary systolic pressure, mmHg | 52.5 ± 15.2 |
BMI, body mass index; CABG, coronary artery bypass graft; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; PCA, percutaneous coronary angioplasty.
Data are expressed as No. (%) or mean ± standard deviation.
The prosthesis was successfully implanted in 75 patients (94.9%). There were 4 implant failures: 2 cases were cancelled because of major vascular complications during percutaneous access, and there were 2 cases of valve embolization. The VARC-2 composite endpoint of device success was achieved in 69 patients (87.3%). The patients without device success were the 4 implant failures, 5 patients with a mean gradient of > 20mmHg and/or moderate AR after the procedure, and 1 death during the procedure; 6 of the 7 off-label procedures had device success according to VARC-2.
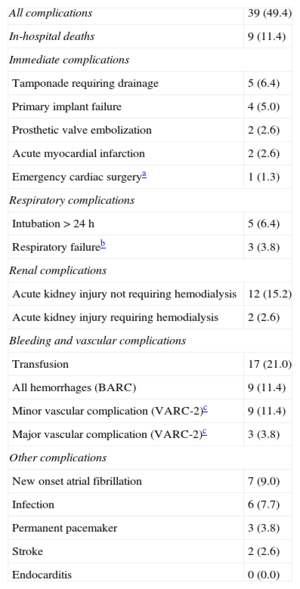
One patient died during the procedure (coronary occlusion with refractory shock). In-hospital mortality was 11.4% (9 patients, 2 of them with implant failure) and 30-day mortality was 12.7%. In-hospital complications are shown in Table 2. Of the 79 patients who underwent the procedure, 55 (69.6%) achieved the 30-day event-free modified early safety composite endpoint.
In-hospital Complications
| All complications | 39 (49.4) |
| In-hospital deaths | 9 (11.4) |
| Immediate complications | |
| Tamponade requiring drainage | 5 (6.4) |
| Primary implant failure | 4 (5.0) |
| Prosthetic valve embolization | 2 (2.6) |
| Acute myocardial infarction | 2 (2.6) |
| Emergency cardiac surgerya | 1 (1.3) |
| Respiratory complications | |
| Intubation > 24 h | 5 (6.4) |
| Respiratory failureb | 3 (3.8) |
| Renal complications | |
| Acute kidney injury not requiring hemodialysis | 12 (15.2) |
| Acute kidney injury requiring hemodialysis | 2 (2.6) |
| Bleeding and vascular complications | |
| Transfusion | 17 (21.0) |
| All hemorrhages (BARC) | 9 (11.4) |
| Minor vascular complication (VARC-2)c | 9 (11.4) |
| Major vascular complication (VARC-2)c | 3 (3.8) |
| Other complications | |
| New onset atrial fibrillation | 7 (9.0) |
| Infection | 6 (7.7) |
| Permanent pacemaker | 3 (3.8) |
| Stroke | 2 (2.6) |
| Endocarditis | 0 (0.0) |
BARC, Bleeding Academic Research Consortium; VARC, Valve Academic Research Consortium.
Values are expressed as No. (%).
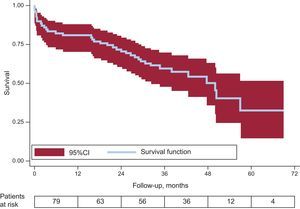
The primary endpoint of the study was all-cause death-free survival at follow-up, which was analyzed in all 79 patients who underwent TAVI, independent of procedural success. Mean follow-up was 34 months (18.3-42.4 months), ie, 2.8 years, with a cumulative follow-up of 2416.7 patient-months. Median survival was 47.6 months (95% confidence interval, 37.4-57.9 months) (almost 4 years). The Kaplan-Meier survival curve is shown in the Figure.
Cumulative survival at the end of years 1 to 5 was 79.7%, 70.9%, 58.9%, 49.5%, and 32%, respectively. If we selected only those patients who survived to hospital discharge (n = 70), the mean survival would be 50.3 months (95% confidence interval, 41.8-58.8 months), and survival at 1, 2, 3, 4, and 5 years would have been 90%, 80%, 66.5%, 55.9%, and 36.1%.
Stratified analysis by 50% and by quartiles of patient inclusion was performed, as well as by access via the transfemoral or transapical route, with no significant differences found.
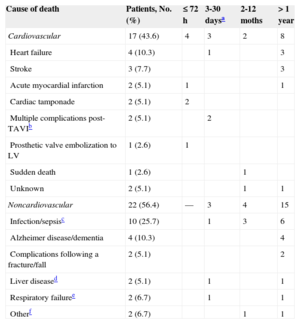
Causes of DeathOf the 79 patients who underwent TAVI, 39 (49.4%) died during follow-up (Table 3). Slightly less than half of the deaths (43.6%) were of cardiovascular cause; the most common cardiovascular causes were heart failure and stroke. Infection was the most common noncardiovascular cause and also the most common absolute cause. A quarter of deaths (10 of 39) occurred within 1 month, when death was most commonly due to cardiovascular cause (70%). After the first month, deaths were distributed more evenly in time and were predominantly of nonvascular cause (65.5%).
Causes and Timing of all Deaths in the Series (n = 39)
| Cause of death | Patients, No. (%) | ≤ 72 h | 3-30 daysa | 2-12 moths | > 1 year |
|---|---|---|---|---|---|
| Cardiovascular | 17 (43.6) | 4 | 3 | 2 | 8 |
| Heart failure | 4 (10.3) | 1 | 3 | ||
| Stroke | 3 (7.7) | 3 | |||
| Acute myocardial infarction | 2 (5.1) | 1 | 1 | ||
| Cardiac tamponade | 2 (5.1) | 2 | |||
| Multiple complications post-TAVIb | 2 (5.1) | 2 | |||
| Prosthetic valve embolization to LV | 1 (2.6) | 1 | |||
| Sudden death | 1 (2.6) | 1 | |||
| Unknown | 2 (5.1) | 1 | 1 | ||
| Noncardiovascular | 22 (56.4) | — | 3 | 4 | 15 |
| Infection/sepsisc | 10 (25.7) | 1 | 3 | 6 | |
| Alzheimer disease/dementia | 4 (10.3) | 4 | |||
| Complications following a fracture/fall | 2 (5.1) | 2 | |||
| Liver diseased | 2 (5.1) | 1 | 1 | ||
| Respiratory failuree | 2 (6.7) | 1 | 1 | ||
| Otherf | 2 (6.7) | 1 | 1 |
TAVI, transcatheter aortic valve implantation; LV, left ventricle; VARC, Valve Academic Research Consortium.
Until 30 days or hospital discharge; as in the Valve Academic Research Consortium-2 definition of procedural mortality.
One patient had a femoral artery dissection that required urgent vascular surgery and afterward had multiple complications; the other patient had multiple complications peri-transcatheter aortic valve implantation and post-transcatheter aortic valve implantation (coronary artery occlusion requiring urgent angioplasty, cardiac tamponade requiring pericardiocentesis and kidney injury requiring hemodialysis). Both patients had a long hospital stay post-transcatheter aortic valve implantation and died before discharge.
The origin of infection/sepsis was respiratory in 7 patients; urinary in 1; gastrointestinal in 1; and of unknown origin in 1 after endocarditis was ruled out (negative blood cultures and imaging).
In the study population, 52 patients (65.8%) had a major adverse event during follow-up (VARC-2 composite endpoint of clinical efficacy). A quarter (25%) of adverse events occurred in the first month and approximately half (51.9%) in the first year. The median adverse event-free survival was 25.4 months (95% confidence interval, 13.9-36.9 months) (2.1 years).
Of 52 adverse events, 30 (57.7%) were fatal and 22 (42.3%) were nonfatal. The main cause of adverse events was worsening functional class or worsening heart failure (in 16 patients). These 16 patients had a concomitant diagnosis of respiratory tract infection and/or exacerbation of chronic obstructive pulmonary disease. An echocardiogram was performed in all 16, and none had evidence of new prosthetic valve dysfunction or deterioration in ejection fraction. Of the other 6 patients who had nonfatal adverse events, 4 patients had a stroke (2 strokes, 2 transient ischemic attacks), 1 required a pacemaker, and 1 required hospital admission for an unknown cause in another center (which was counted as an adverse event). The cumulative rate of stroke during follow-up was 11.4% (6.3% for stroke and 5.1% for transient ischaemic attack). The median time from procedure to stroke was 489 days.
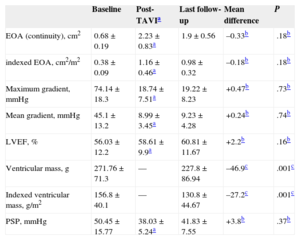
Prosthesis Function at Follow-upThe following patients were excluded from echocardiographic follow-up: patients who died before discharge from hospital (n = 9) or before 12 months follow-up (n = 7), those with implant failure who were discharged (n = 2), and 2 patients who were lost to echocardiographic follow-up. Table 4 shows the parameters at baseline, postprocedure, and at the last available follow-up (n = 59). All parameters improved significantly after the procedure. The mean values obtained at the last echocardiographic follow-up were not significantly different from the mean postprocedure values. This highlights the stability of gradient and ejection fraction at follow-up. The ventricular mass and ventricular mass index were significantly reduced at follow-up compared with baseline values.
Mean Echocardiographic Data at Baseline, Postprocedure, and at the Last Available Follow-up
| Baseline | Post-TAVIa | Last follow-up | Mean difference | P | |
|---|---|---|---|---|---|
| EOA (continuity), cm2 | 0.68 ± 0.19 | 2.23 ± 0.83a | 1.9 ± 0.56 | –0.33b | .18b |
| indexed EOA, cm2/m2 | 0.38 ± 0.09 | 1.16 ± 0.46a | 0.98 ± 0.32 | –0.18b | .18b |
| Maximum gradient, mmHg | 74.14 ± 18.3 | 18.74 ± 7.51a | 19.22 ± 8.23 | +0.47b | .73b |
| Mean gradient, mmHg | 45.1 ± 13.2 | 8.99 ± 3.45a | 9.23 ± 4.28 | +0.24b | .74b |
| LVEF, % | 56.03 ± 12.2 | 58.61 ± 9.9a | 60.81 ± 11.67 | +2.2b | .16b |
| Ventricular mass, g | 271.76 ± 71.3 | — | 227.8 ± 86.94 | –46.9c | .001c |
| Indexed ventricular mass, g/m2 | 156.8 ± 40.1 | — | 130.8 ± 44.67 | –27.2c | .001c |
| PSP, mmHg | 50.45 ± 15.77 | 38.03 ± 5.24a | 41.83 ± 7.55 | +3.8b | .37b |
EOA, effective orifice area; LVEF, left ventricular ejection fraction; PSP, pulmonary systolic pressure; TAVI, transcatheter aortic valve implantation.
Values at the last follow-up were compared with values on discharge, except for ventricular mass values, which were compared with baseline values. Data are expressed as mean ± standard deviation.
Post-transcatheter aortic valve implantation echocardiograms were done between 24hours post-procedure and discharge from hospital. The statistical comparison of parameters on discharge against baseline values was statistically significant for all variables (P < 0.05).
On post-TAVI echocardiogram, 94.9% of patients had mild or undetectable AR, and only 3 patients (5.1%) had moderate AR. At follow-up, 3 more patients (cumulative rate, 10.2%) had moderate AR. No cases of severe AR were detected. Regarding prosthetic valve stenosis, no patients had a post-TAVI gradient > 25mmHg; 3 patients (5.1%) had gradients of 20mmHg to 25mmHg, which is defined in VARC-2 as “possible stenosis”. This percentage did not increase throughout follow-up. There were no cases of “significant stenosis” (mean gradient ¿ 35mmHg).
The VARC-2 composite variable of prosthetic valve dysfunction increased from 10.2% post-procedure to 15.3% at follow-up (10.2% because of moderate AR and 5.1% because of a mean gradient > 20mmHg). None of those patients needed repeat valve replacement. There were no documented cases of aortic complication, mitral valve lesions, endocarditis, or prosthetic valve thrombosis.
DISCUSSIONStudy Population and Procedure OutcomesIn this study we have described the long-term follow-up and results of a prospective single-center registry that included consecutive patients who underwent TAVI. The study population included patients of advanced age (mean 82.3 years), with significant comorbidities and moderately elevated surgical risk scores (EuroSCORE, 16.9%; STS score, 5.9%). The risk profile was similar to those in other series published in Spain, but was slightly lower than those in other international series.12,14,21,22
Our short-term study results were within the figures published in contemporary national and international series, which had success rates between 90% and 98%10–13,21 and 30-day mortality between 7.4% and 14.5%.12–14,22,23 More recent studies have reported 30-day survival rates > 95%,24,25 which may be explained by the increased experience in those centers and technical advances in the devices.
In our study, the VARC-2 composite endpoint of device success was not achieved in 10 of the 79 patients (12.7%), a figure which was similar to or better than contemporary studies that used VARC-1 definitions (12.9%-20.0).23,26 However, in the recent CHOICE study, the Edwards SAPIEN arm had an excellent device failure rate of 4.1% (according to VARC-1).25
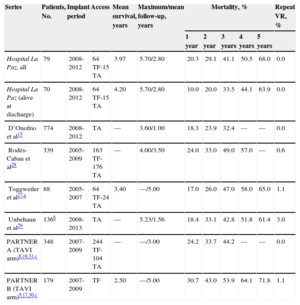
Long-term SurvivalThere are few series on Edwards SAPIEN valve with follow-up ≥ 3 years, and none of them are national studies. Their results are summarized in Table 5. The single-center series by Toggweiler et al27 describes 5 complete years of follow-up for 88 selected patients (implant failure and deaths before 30 days were excluded), in which the first generations of valve and delivery systems were used. These authors obtained results similar to those of our unselected series. In an exhaustive study, Rodés-Cabau et al28 published a follow-up of up to 4 years in a multicenter Canadian study with 339 consecutive patients (which probably included those of Toggweiler et al27); their results were also comparable to ours.
Long-term Mortality Data for Edwards Sapien Valve in This Study, Other International Studies, and 2 Clinical Trials
| Series | Patients, No. | Implant period | Access | Mean survival, years | Maximum/mean follow-up, years | Mortality, % | Repeat VR, % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 year | 2 year | 3 years | 4 years | 5 years | |||||||
| Hospital La Paz, all | 79 | 2008-2012 | 64 TF-15 TA | 3.97 | 5.70/2.80 | 20.3 | 29.1 | 41.1 | 50.5 | 68.0 | 0.0 |
| Hospital La Paz (alive at discharge) | 70 | 2008-2012 | 64 TF-15 TA | 4.20 | 5.70/2.80 | 10.0 | 20.0 | 33.5 | 44.1 | 63.9 | 0.0 |
| D’Onofrio et al15 | 774 | 2008-2012 | TA | — | 3.60/1.00 | 18.3 | 23.9 | 32.4 | — | — | 0.0 |
| Rodés-Cabau et al28 | 339 | 2005-2009 | 163 TF-176 TA | — | 4.00/3.50 | 24.0 | 33.0 | 49.0 | 57.0 | — | 0.6 |
| Toggweiler et al27,a | 88 | 2005-2007 | 64 TF-24 TA | 3.40 | —/5.00 | 17.0 | 26.0 | 47.0 | 58.0 | 65.0 | 1.1 |
| Unbehaun et al29 | 136b | 2008-2013 | TA | — | 5.23/1.56 | 18.4 | 33.1 | 42.8 | 51.8 | 61.4 | 3.0 |
| PARTNER A (TAVI arm)8,16,31,c | 348 | 2007-2009 | 244 TF-104 TA | — | —/3.00 | 24.2 | 33.7 | 44.2 | — | — | 0.0 |
| PARTNER B (TAVI arm)9,17,30,c | 179 | 2007-2009 | TF | 2.50 | —/5.00 | 30.7 | 43.0 | 53.9 | 64.1 | 71.8 | 1.1 |
TA, transapical; TAVI, transcatheter aortic valve implantation; TF, transfemoral; VR, valve replacement.
Patients from this single-center series are probably included in the series of Rodés-Cabau et al28; however, data are given by it being the only study with complete 5-year data. Toggweiler et al27 reported a selected population from which they excluded patients with implant failure and deaths before 30 days. Five-year follow-up was obtained in 84 of 88 patients.
Two other series offer some data on 5 year mortality: Unbehaun et al29 reported a mortality of 61.4% of 136 selected (alive at 30 days) patients from an administrative data source; the TAVI arm of PARTNER B recently reported a 5-year mortality of 71.8%.30 D’Onofrio et al. reported a 3-year mortality of 32.4%15; in the TAVI arm of PARTNER A, 3-year mortality was 44.2%.31
In general, 5-year mortality after a TAVI procedure with the Edwards SAPIEN valve is around 60% to 70%, and is limited by a very old population with significant comorbidities (as a reference, the estimated 5-year mortality of American controls with no comorbidities would be 40.5%30). Currently, the reduction in peri-TAVI mortality (30-day survival > 95%24,25) and the shift toward lower-risk candidate characteristics enables us to predict that the long-term mortality of patients who undergo TAVI today will probably decrease further.
Causes of Death and Major Adverse EventsRegarding causes of death, the findings are in line with those of a high risk procedure, with high mortality in the first month (a third of all deaths), of predominantly cardiovascular cause, and mortality of predominantly noncardiovascular causes in the medium- and long-term. Among specific causes of death, infection was prominent, particularly when associated with respiratory disease, which, independently of TAVI, is a common cause of death in elderly patients.32 The next most common cause of death was advanced dementia, requiring us to consider the relevance of cognitive function in patient prognosis. Importantly, residual cardiovascular mortality after the first year was 34.5%, similar to that of other series.28,30
Major adverse events were concentrated in the first year post-TAVI and occurred in two thirds of patients in the long-term (65.8%). This information is difficult to compare with that of other series, because of differences in definitions and follow-up times. In the series reported by Gurvitch et al,33 the composite endpoint of death, infarction, stroke, or aortic valve replacement was reached in 48.6% at 3 years. Heart failure not associated with prosthetic valve dysfunction was the primary cause of nonfatal adverse events, which, together with the relevant residual cardiovascular mortality data, indicates the need for more investigation in this field.
Prosthetic Function at Follow-upA notable finding was the durability of the prosthetic valve used in this study at follow-up: a 15.3% prosthetic valve dysfunction rate according to VARC-2 (moderate AR and/or mean gradient of 20mmHg to 25mmHg) without need for repeat valve replacement. Long-term prosthetic valve dysfunction has been reported in a heterogeneous manner, focusing on moderate or severe AR, which in the long-term is very low (2.3%, 0.0%, and 0.0% at 4-5 years in the series reported by Toggweiler et al,27 Rodés-Cabau et al,28 and PARTNER B,30 respectively), although there may be a bias, as the patients in these studies had higher mortality. The need for repeat valve replacement was minimal (Table 5).
LimitationsThe number of patients in this registry was smaller than in other multicenter registries, but reflects the normal practice of a Spanish tertiary center, and the inclusion of consecutive patients makes the results more representative of clinical practice. In our center, a mean of 19.8 procedures were performed each year during the study period, whereas in Spain the average was 15.8 per center in 2012.34 The number of patients may have limited the capacity to find differences regarding the experience of the center or between different access routes. The follow-up time was not the same for all patients, but this was the method used in many similar studies.15,28 The study was designed with a minimum follow-up of 2.5 years for living patients, and no patients were lost to clinical follow-up.
CONCLUSIONSIn this unselected population of 79 consecutive patients with severe AS, indication for valve replacement, and high surgical risk who underwent TAVI, the median all-cause death-free survival was 47.6 months (4 years). If we selected the 70 patients who survived to discharge, the median survival would be 50.3 months (4.2 years).
A third of deaths occurred in the first 30 days, most of which were from cardiovascular causes (70%). The other deaths were evenly distributed in time, and were most commonly due to noncardiovascular causes (65.5%), particularly infection associated with respiratory disease. However, there was a residual cardiovascular morbidity and mortality that was not associated with prosthetic valve dysfunction, which was not insignificant.
The improvement in hemodynamic parameters post-TAVI was maintained throughout follow-up, and although 15.3% of patients met VARC-2 criteria of prosthetic valve dysfunction at follow-up, no patients required subsequent valve replacement.
FUNDINGFrom 2010-2012, P. Salinas received training grants from the Sociedad Española de Cardiología (Spanish Society of Cardiology): “Cordis grant for hemodynamics training in national centers”.
CONFLICTS OF INTERESTNone declared.
Many professionals from different specialties have contributed to the TAVI program at Hospital Universitario La Paz and deserve our acknowledgement. As regards this study, Diego Iglesias, Sergio García Blas, Omar Razzo, David Dobarro, and David Filgueiras deserve special recognition.