Compared with low-fat diets, the traditional Mediterranean diet (MedDiet), supplemented with extra virgin olive oil (EVOO) or nuts, reduces cardiovascular mortality and major cardiovascular events in both primary prevention and secondary prevention following an acute coronary syndrome (ACS).1,2 However, the actual impact of the MedDiet on coronary plaque characteristics is unknown. Consequently, we conducted the Impact of Mediterranean Diet, Inflammation, and Microbiome After an Acute Coronary Syndrome (MEDIMACS) clinical trial, a randomized, controlled, parallel study (ClinicalTrials.gov; NCT03842319). The trial compared the effects of a 12-month intensive MedDiet intervention vs conventional MedDiet (standard of care) on atherosclerotic plaque progression and coronary endothelial and microvascular dysfunction.3
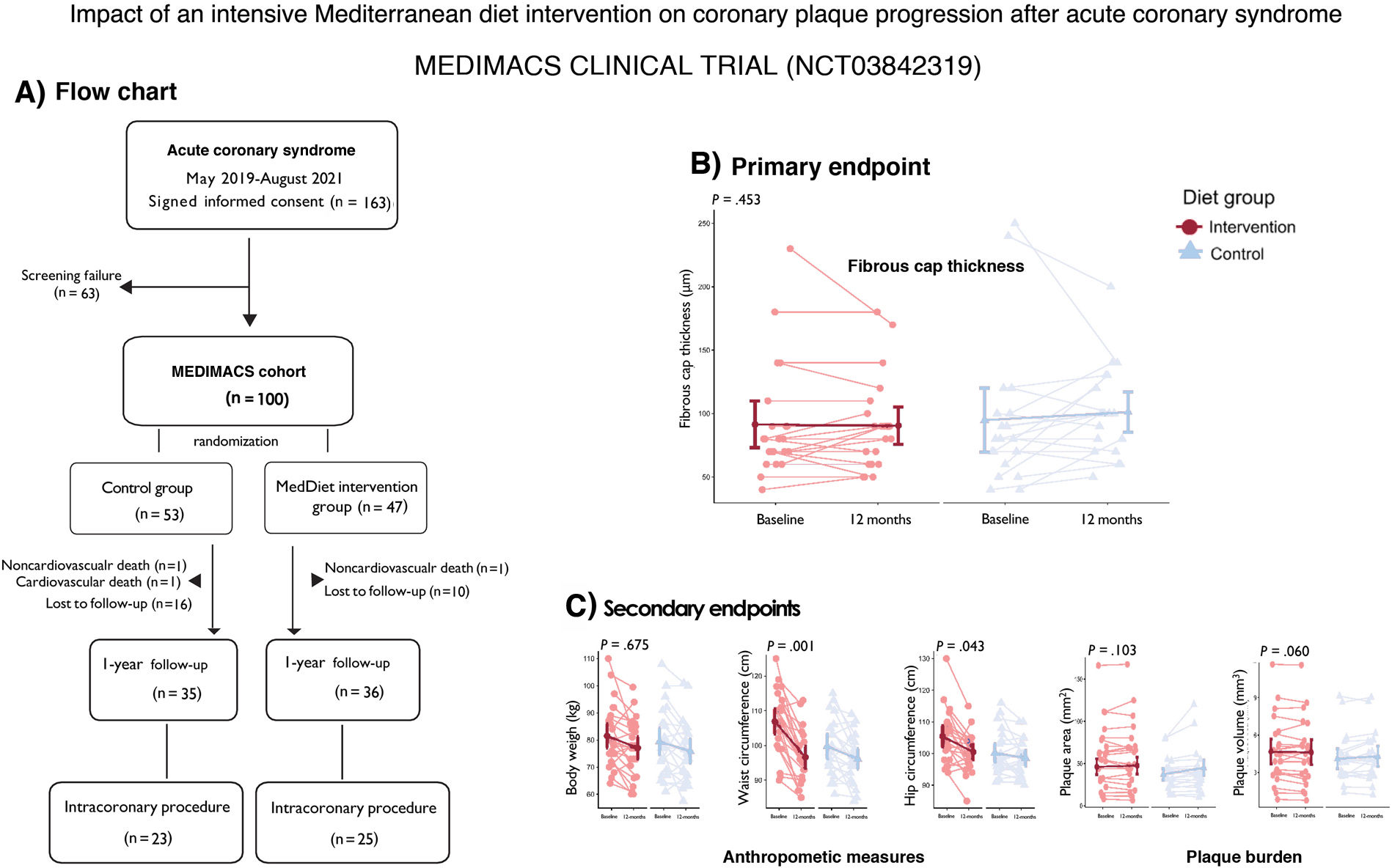
The primary endpoint was the change in fibrous cap thickness (FCT) of a nonculprit coronary plaque, measured by optical coherence tomography (OCT). FCT is a validated marker of plaque vulnerability, with thinner caps being associated with a higher risk of rupture.4 Secondary endpoints included coronary plaque burden (also derived from OCT), endothelial and microvascular function (not reported herein), and anthropometric measurements of cardiovascular risk.
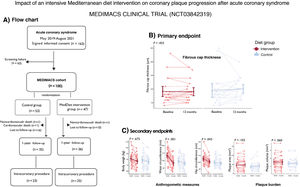
Between May 2019 and August 2021, 163 ACS patients admitted with a diagnosis of ACS provided informed consent to enter the MEDIMACS clinical trial. Eligible patients had non–ST-elevation ACS with an intermediate coronary lesion (20%-60% stenosis) and functionally nonsignificant (fractional flow reserve >0.80) in a nonculprit vessel, designated as the target lesion for the primary endpoint. A total of 100 patients met the inclusion/exclusion criteria3 and were randomly assigned (1:1) to either an intensive MedDiet intervention or a low-intensity MedDiet with lifestyle recommendations (control) for 12 months. Randomization was blinded and performed using a computer-generated system. The intensive MedDiet group received personalized guidance from a nutritionist and 4 liters of EVOO per month, while the control group received standard MedDiet recommendations as part of routine secondary prevention after ACS. All patients underwent clinical evaluations at baseline and at 3, 6, 9, and 12 months. At study completion, a follow-up intracoronary procedure was performed, and anthropometric changes were recorded. Group allocation was blinded to clinicians, nurses, and researchers. The full methodology for the MEDIMACS trial has been published.3
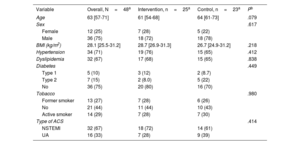
Of the 100 patients, 53 were allocated to the control arm and 47 to the intervention arm. During follow-up, 1 patient died due to noncardiovascular causes, and 1 died due to a second ACS 3 months after inclusion. Of note, the performance of the clinical trial was affected by the COVID pandemic. Although 71 out of the 100 patients completed the 12-month follow-up, only 48 underwent the repeat intracoronary procedure to assess the primary endpoint (figure 1A). Thus, the modified intention-to-treat (mITT) population included 48 patients (25 patients allocated to the intervention group and 23 to the control group). The median age was 63 [57-71] years, 75% were male, and the median body mass index was 28 [26-31] kg/m2 (table 1). The etiology of the ACS was unstable angina in 16 patients and non–ST-segment elevation myocardial infarction in 32. A total of 3 events were recorded during follow-up in this mITT population, and 3 patients required a second revascularization during the lesion re-evaluation (2 in the intervention group, of which 1 resulted in a fatal event, and 1 in the control group).
Baseline demographic and clinical characteristics of modified intention to treat population
| Variable | Overall, N=48a | Intervention, n=25a | Control, n=23a | Pb |
|---|---|---|---|---|
| Age | 63 [57-71] | 61 [54-68] | 64 [61-73] | .079 |
| Sex | .617 | |||
| Female | 12 (25) | 7 (28) | 5 (22) | |
| Male | 36 (75) | 18 (72) | 18 (78) | |
| BMI (kg/m2) | 28.1 [25.5-31.2] | 28.7 [26.9-31.3] | 26.7 [24.9-31.2] | .218 |
| Hypertension | 34 (71) | 19 (76) | 15 (65) | .412 |
| Dyslipidemia | 32 (67) | 17 (68) | 15 (65) | .838 |
| Diabetes | .449 | |||
| Type 1 | 5 (10) | 3 (12) | 2 (8.7) | |
| Type 2 | 7 (15) | 2 (8.0) | 5 (22) | |
| No | 36 (75) | 20 (80) | 16 (70) | |
| Tobacco | .980 | |||
| Former smoker | 13 (27) | 7 (28) | 6 (26) | |
| No | 21 (44) | 11 (44) | 10 (43) | |
| Active smoker | 14 (29) | 7 (28) | 7 (30) | |
| Type of ACS | .414 | |||
| NSTEMI | 32 (67) | 18 (72) | 14 (61) | |
| UA | 16 (33) | 7 (28) | 9 (39) |
ACS, acute coronary syndrome; BMI, body mass index; NSTEMI, non–ST-segment elevation myocardial infarction; UA, unstable angina.
We assessed compliance with the MedDiet in both arms using the 14-item dietary screener (MEDAS).5 Good compliance was defined as a score of 10 or more points on the 14-item MEDAS questionnaire in patients in the intervention group. Diet compliance in the intervention group was very good (mean score during follow-up visits 11.6±0.2), which was significantly higher than in the control group (mean score during follow-up visits 8.9±0.3) during follow-up (P<.00001). The biological markers of compliance (urinary levels of tyrosol, hydroxytyrosol and homovanillic acid), determined in a random subset of patients from each group, suggested higher consumption of olive oil and a more pronounced reduction in alcohol intake, but these differences were nonsignificant.
The effect of the intervention on the primary endpoint, the baseline to-1-year change in FCT, was null. The between-group difference in this change was −7.2μm (95% confidence interval [95%CI], −11.4 to 25.8μm, P=.453, figure 1B). There was a near-significant trend toward a favorable effect of the intervention on between-group differences in the 1-year change in plaque area, with an effect size of −0.25 mm2, (95%CI, −0.50 to 0.01; P=.060; figure 1C). The between-group difference for the baseline to 1-year change in plaque volume also favored in the intervention, with an effect size of −4.21 mm3 (95%CI, −9.27 to 0.85; P=.103; figure 1C). One-year changes in plaque burden were similar between groups, as were changes in endothelial and microvascular function. However, the 1-year changes in waist and hip circumferences were significantly more pronounced in the intervention group: the intervention had positive effects on waist circumference (magnitude of the effect: −6.6 [−10.5 to −2.8] cm, P<.005) and hip circumference (magnitude of the effect: −3.27 [−6.5 to −0.1] cm, P=.045), whereas differences in changes in body weight were not significant (magnitude of the effect: −0.91 [−5.2 to 3.3] kg, P<.675; figure 1C).
Our data, limited by sample size, short-term intervention and the natural crossover between the diet arms, suggest that, compared with current standard recommendations of MedDiet, an intensive intervention including supplemental EVOO had no significant impact on the 1-year FCT of coronary plaque after ACS. Our previous study indicated that systemic inflammation and gut microbiota longitudinal responses influence plaque remodeling after ACS.6 The observed effects could be further clarified by investigating the role of the MedDiet in the inflammation-microbiota-coronary plaque interplay. While the intensive intervention may not affect plaque vulnerability, our anthropometric results and a marginally significant trend toward slower plaque progression support the clinical benefits of a MedDiet with EVOO for secondary cardiovascular prevention.
FUNDINGThis trial was supported financially by the National Institute of Health Carlos III (Spain), the French National Research Agency (France), the Ministry of Science, Technology and Space (Israel), the Swedish Research Council Formas (Sweden) and the European Union's H2020 Research and Innovation Programme under the HDHL-INTIMIC Cofounded Call Joint Transnational Research Proposals on “Interrelation of the Intestinal Microbiome, Diet and Health” (AC17/00086). This trial was sponsored by Centro de Investigación Biomédica en Red (CIBER).
ETHICAL CONSIDERATIONSThe study received approval of the Ethics Committee of the Hospital General Universitario Gregorio Marañón, Madrid, Spain (CEIm 209/18), followed the sex and gender equity in research (SAGER) guidelines and was registered as NCT03842319 in ClinicalTrial.gov. All patients provided informed consent for inclusion in the study.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEThe authors declare that they have not used any type of generative artificial intelligence.
AUTHORS’ CONTRIBUTIONSJ. Bermejo, M.A. Martínez-Gonzalez, A.I. Fernández-Ávila and E. Gutiérrez-Ibañes participated in the conception of the research questions and study design. J. Bermejo, E. Gutiérrez-Ibañes, M.A. Martínez-Gonzalez, and A.I. Fernández-Ávila supervised the clinical trial. A.I. Fernández-Ávila and A. Gabaldón conducted patient data and sample collection. J. Gómez-Lara, A. Gabaldón and E. Gutiérrez-Ibañes conducted the coronary imaging study. J. Bermejo, A.I. Fernández-Ávila and E. Gutiérrez-Ibañes conducted data analysis and image creation. A.I. Fernández-Ávila and J. Bermejo wrote the original draft of the manuscript.
CONFLICTS OF INTERESTThe authors declare that they have no competing interest.
We are in debt to all the personnel of the MEDIMACS project and the Department of Cardiology of the Hospital General Universitario Gregorio Marañón, to Sandra Vazquez for her assistance in sampling and recording patient data and to Ana Fernández Baza for her assistance in the logistics of the study. We thank the staff of the Consorcio Centro de Investigación Biomédica en Red, M.P. (CIBER) for their help in the performance of the MEDIMACS clinical trial. We acknowledge Arteoliva Company for the free supply of extra virgin olive oil for the clinical trial.
Irene Martín de Miguel (Departamento de Cardiología, Hospital General Universitario Gregorio Marañón, Facultad de Medicina, Universidad Complutense de Madrid, Instituto de Investigación Sanitaria Gregorio Marañón, and CIBERCV, Madrid, Spain), Ricardo Sanz-Ruiz Departamento de Cardiología, Hospital General Universitario Gregorio Marañón, Facultad de Medicina, Universidad Complutense de Madrid, Instituto de Investigación Sanitaria Gregorio Marañón, and CIBERCV, Madrid, Spain), Elena Jurado (Departamento de Cardiología, Hospital General Universitario Gregorio Marañón, Facultad de Medicina, Universidad Complutense de Madrid, Instituto de Investigación Sanitaria Gregorio Marañón, and CIBERCV, Madrid, Spain), Francisco Fernández-Avilés (Departamento de Cardiología, Hospital General Universitario Gregorio Marañón, Facultad de Medicina, Universidad Complutense de Madrid, Instituto de Investigación Sanitaria Gregorio Marañón, and CIBERCV, Madrid, Spain), Cristina Razquin Burillo (Departamento de Medicina Preventiva y Salud Pública, Universidad de Navarra, Instituto de Investigación Sanitaria de Navarra, CIBEROBN, Pamplona, Spain), Rafael de la Torre (Hospital del Mar de Investigaciones, Barcelona, Spain), Pablo Martínez-Legazpi (Departamento de Física Matemática y de Fluidos, Facultad de Ciencias, Universidad Nacional de Educación a Distancia, UNED, and CIBERCV, Madrid, Spain), Raquel Yotti (Departamento de Cardiología, Hospital General Universitario Gregorio Marañón, Facultad de Medicina, Universidad Complutense de Madrid, Instituto de Investigación Sanitaria Gregorio Marañón, and CIBERCV, Madrid, Spain), Alex Mira (Área de Genómica y Salud, Centro de Investigación Avanzada en Salud Pública, CSISP-FISABIO, and CIBERESP, Valencia, Spain), Uri Gophna (The Shmunis School of Biomedicine and Cancer Research, George S. Wise Faculty of Life Sciences, Tel Aviv University, Ramat Aviv, Tel Aviv, Israel), Roger Karlsson (Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska Academy of the University of Gothenburg; Sweden Nanoxis Consulting AB, Clinical microbiology, Sahlgrenska University Hospital, Västra Götaland Region, Gothenburg, Sweden), Reem Al-Daccak (Institut National de la Santé et de la Recherche Médicale (INSERM) UMRS-97f, Université Paris-Diderot, HLA et Médecine, Labex Transplantex, Hôpital Saint-Louis, Paris, France) and Dominique Charron (Institut National de la Santé et de la Recherche Médicale (INSERM) UMRS-97f, Université Paris-Diderot, HLA et Médecine, Labex Transplantex, Hôpital Saint-Louis, Paris, France).