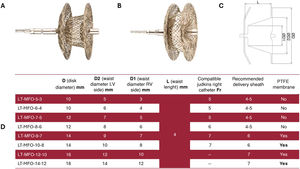
The Lifetech KONAR-MF ventricular septal defect occluder (MFO) is increasingly used for transcatheter perimembranous ventricular septal defect (pmVSD) closure. We aim to collect real-world data on patient outcomes and MFO performance in pmVSD cases.
MethodsThis was a nonrandomized, retrospective, multicenter, postmarketing clinical follow-up study of pmVSD patients implanted with the MFO device between 2018 and 2023. The primary endpoint was 6-month composite clinical success, defined as technical success (successful implantation and device retention at the end of the procedure), closure success (trivial or no residual shunt), absence of serious adverse events at 30 days, and no device removal or reintervention. Secondary endpoints consisted of technical success, procedural success (technical success and less than moderate residual shunt), closure success (clinically insignificant or absent residual shunt), and safety.
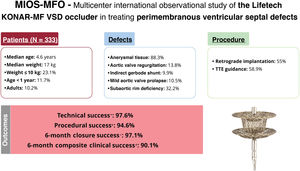
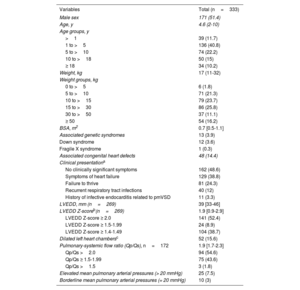
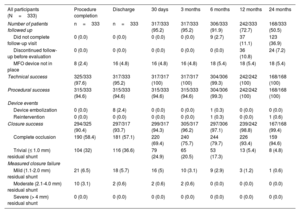
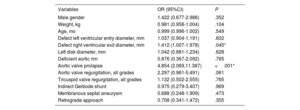
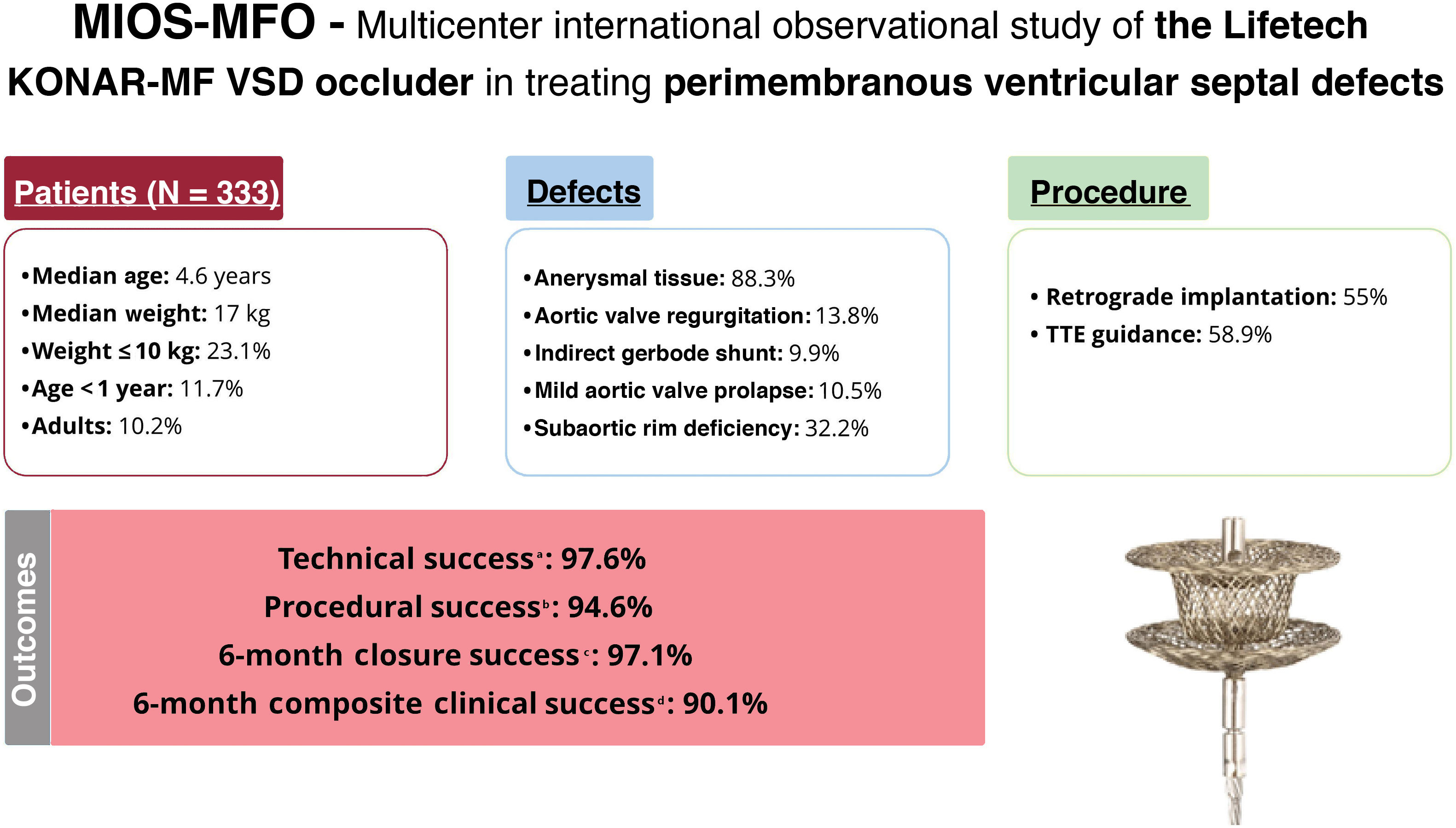
ResultsOur cohort comprised 333 patients (51.4% male, 10.2% adults), with a median age of 4.6 [IQR, 2-10] years and a median weight of 17 [IQR, 11-32] kg. Baseline defect characteristics consisted of a left ventricular defect diameter of 8 [IQR, 7-10] mm, with 13.8% of patients having aortic valve regurgitation, 9.9% having an indirect Gerbode shunt, 10.5% having mild aortic valve prolapse, and 32.2% having subaortic rim deficiency. Closure via a retrograde approach was performed in 183 patients (55%). Technical success at procedure completion was 97.6%, and procedural success was 94.6%. Closure success at 6 months was 97.1%, while composite clinical success was 90.1%. Freedom from serious adverse events was 95.2% at 30 days. Freedom from device-related events was 96.8% at 6 months and 93.8% at 24 months. Risk factors for 6-month composite clinical failure were aortic valve prolapse (OR, 4.85; 95%CI, 2.07-11.39) and right ventricular defect diameter (OR, 1.41; 95%CI, 1.01-1.98).
ConclusionsTranscatheter closure of pmVSD with the MFO device demonstrated feasibility, effectiveness, and safety in real-world settings.
Keywords
Identify yourself
Not yet a subscriber to the journal?
Purchase access to the article
By purchasing the article, the PDF of the same can be downloaded
Price: 19,34 €
Phone for incidents
Monday to Friday from 9am to 6pm (GMT+1) except for the months of July and August, which will be from 9am to 3pm