We compared the effects of 12 weeks of low-volume high-intensity interval training (LV-HIIT) vs moderate-intensity continuous exercise training (MICET) on cardiopulmonary exercise test parameters and the proportion of non/low responders (NLR) to exercise training in post-acute coronary syndrome (ACS) patients.
MethodsPatients with a recent ACS were randomized to LV-HIIT, MICET, or a usual care group. LV-HIIT consisted of 2 to 3 sets of 6 to 10minutes with repeated bouts of 15 to 30seconds at 100% of peak workload alternating with 15 to 30seconds of passive recovery. Cardiopulmonary exercise test parameters were assessed, and key exercise variables were calculated. Training response was assessed according to the median VO2peak change post vs pretraining in the whole cohort (stratification NLR vs high response).
ResultsFifty patients were included in the analysis (LV-HIIT, n=23; MICET, n=18; usual care, n=9) and 74% were male. The proportion of NLR was higher in the LV-HIIT group than in the MICET group (LV-HIIT 61%, MICET 21%, and usual care 80%; P=.0040). VO2peak-dependent variables (VO2peak, percent-predicted VO2peak) improved in both training groups (P=.002 and P <.0001 for time with LV-HIIT and MICET, respectively), but the improvement was more pronounced with MICET (P=.004 and P=.001 for interaction, respectively). The ΔVO2/Δworkload slope improved only with MICET (P=.021).
ConclusionsIn patients with a recent ACS, several prognostic VO2peak-dependent variables were improved after LV-HIIT, but the improvement was more pronounced or only found after MICET. Low-volume HIIT resulted in a higher proportion of NLR than isocaloric MICET.
Clinical trialsregistered at ClinicalTrials.gov (Identifiers: NCT03414996 and NCT02048696)
Keywords
Exercise-based secondary prevention programs reduce cardiovascular mortality and morbidity in patients with coronary heart disease (CHD), including patients after acute coronary syndrome (ACS).1,2 Maximal cardiorespiratory fitness (ie, VO2peak) is a powerful predictor for all-cause mortality in CHD patients,3,4 and a VO2peak improvement is associated with a reduction in mortality, morbidity, and health care costs.4–7 However, there is a considerable individual heterogeneity in VO2peak improvement to standardized exercise training programs in patients with CHD.8–11 In this population, 14% to 22% can be classified as non/low responders (VO2peak improvement),8,10,11 which has recently been associated with a higher mortality risk.8
Moderate continuous exercise training (MICET) is a guidelines-based aerobic endurance training modality for CHD patients.12–14 High-intensity interval training (HIIT) has been proposed as a modality that is complementary to MICET.14,15 In CHD patients, HIIT protocols have been previously classified as having short (≤ 60seconds), medium (1-3minutes) or long intervals (> 3minutes).16 A recent meta-analysis comparing VO2peak improvements with either HIIT or MICET in stable CHD patients found more pronounced effects with HIIT.17 Of note, most of the studies included (70%) used long interval HIIT protocols, and the superiority of HIIT over MICET disappeared when isocaloric protocols were used. Moreover, the intensity of the HIIT protocol (4minutes at 90%-95% of peak heart rate) in the SAINTEX-CAD study was hard to maintain for most of the CHD patients.18 Similarly, we showed that longer interval HIIT protocols (60 to 90seconds) were less well tolerated and were associated with lower total exercise time in CHD.19
Accordingly, we developed an optimized HIIT protocol with short intervals (15-30seconds) that is safe, very well tolerated by CHD patients, and produces physiological responses very similar to those of MICET.20,21 However, this optimized LV-HIIT protocol has not been compared with isocaloric MICET with regards to the proportion of non/low responders (based on changes in VO2peak) and key cardiopulmonary exercise test (CPET) variables in post-ACS patients. We hypothesized that optimized LV-HIIT would result in a similar proportion of training non/low responders (NLR) and similar VO2peak changes compared with MICET.
The main aims of our study were: a) To assess the proportion of NLR and high responders (based on VO2peak) in post-ACS patients after structured aerobic exercise training (LV-HIIT, MICET) or usual care; b) to compare peak and submaximal CPET parameters between the 2 training modalities; c) to assess the independent predictors of VO2peak NLR in post-ACS patients.
MethodsParticipantsAll patients were referred for a multi-disciplinary secondary prevention program at the Cardiovascular Prevention and Rehabilitation Center (EPIC Center) of the Montreal Heart Institute and included in a randomized training intervention study. Details on the inclusion and exclusion criteria have been previously described elsewhere.22,23 Essentially, all CHD patients were under optimal medical therapy following coronary revascularization for ACS. Patients had to be stable with regard to symptoms and medication doses during the 4 weeks prior to enrolment. For this analysis, data from 2 prospective randomized exercise-intervention studies were pooled. The first study comprised post-ACS patients who were randomized (1:1) to either LV-HIIT or MICET. The primary endpoint was VO2peak. In the second pilot study, post-ACS patients were randomized (1:1) to either LV-HIIT or usual care, with lymphocyte GRK2 mRNA levels as the primary endpoint. This explains the disproportionate number of patients randomized in each group (LV-HIIT, MICET, usual care). The study protocols were approved by the Research Ethics and New Technology Development Committee of the Montreal Heart Institute. Both studies were registered on ClinicalTrials.gov (ClinicalTrials.gov identifier numbers: NCT03414996 and NCT02048696). Written informed consent was obtained by each patient.
Study design and measurementBaseline clinical data, and CPET were assessed at baseline and after completion of the program. Baseline clinical data assessment included data on personal medical history, event details, and cardiovascular risk factor profile.
Maximal cardiopulmonary exercise testingMaximal CPET was performed on a cycle ergometer (Ergoline 800S, Bitz, Germany) according to the recommendations of the American Heart Association, and as previously published.19,21,24,25 Following a 3-minute warm-up phase at an initial workload of 20W, an incremental exercise test was performed with 15 Watt increments per minute until exhaustion at a pedaling speed >60rpm. The recovery phase consisted of 2minutes of active recovery at 20W at pedaling speed between 50rpm and 60rpm, followed by 3minutes of passive recovery. Gas exchange parameters were continuously measured at rest, during exercise, and during recovery using a metabolic system (Oxycon Pro, CareFusion, Jaeger, Germany) as recently published.19,21,25 There was continuous ECG monitoring (Marquette, case 12, St. Louis, Missouri, USA). Blood pressure and rate of perceived exertion were measured every 3minutes throughout the test. The highest VO2 value reached during the exercise phase was considered as the VO2peak and peak workload was defined as the workload reached at the last fully completed stage. Oxygen uptake efficiency slope, ventilatory efficiency (VE/VCO2) slope, and ΔVO2/Δworkload slope were calculated according to recent recommendations.26
Exercise training interventionAll patients performed 2 to 3 exercise training sessions a week on a bicycle ergometer. The aerobic exercise training consisted of 2 different training modalities: low-volume high-intensity interval training (LV-HIIT) or moderate-intensity continuous exercise training (MICET), which were isocaloric according to previously published methods.21 Additional resistance training was performed following each aerobic exercise session. All training was center-based and performed under the supervision of a certified kinesiologist.
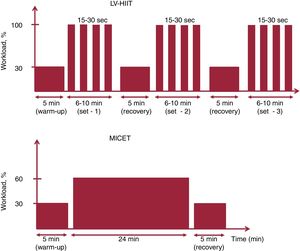
Low-volume high-intensity interval trainingThe HIIT protocol was evaluated in a prospective randomized trial and optimized in that specific population (ie, CHD patients) as recently published.19,21 Following a 5-minute warm-up at 30% of peak workload obtained at the CPET, patients performed 2 to 3 sets of 6 to 10minutes with repeated bouts of 15 to 30seconds at 100% of peak workload alternating with 15 to 30seconds of passive recovery. The target rating of perceived exertion (rate of perceived exertion 6-20) was set at 15 during the HIIT bouts. The sets were separated by a 5-minute active recovery phase at 30% of peak workload. The training session was terminated by a 5-minute cool-down phase at 30% of peak workload (figure 1).27 The term low-volume refers to the fact that the weekly training volume with the protocols used was <150minutes (MICET) or <75minutes (LV-HIIT) for high/vigorous intensity, which are the minimal thresholds recommended by most international guidelines.28,29
Moderate-intensity continuous exercise trainingFollowing a 5-minute warm-up at 30% of peak workload, patients performed continuous exercise at 60% of peak workload for 24minutes. At the end of the session, the patients performed 5minutes of recovery at 30% of peak workload (figure 1). The total time was 34minutes and the training was isocaloric with the LV-HIIT session.21 A recent meta-analysis in CHD patients underlined the importance of matching energy expenditure during the training when comparing exercise modalities (HIIT vs MICET).17 Indeed, the superiority of HIIT vs MICET on VO2peak improvement disappears when both protocols are isocaloric.17 Our isocaloric calculation method was strongly based on a direct measure of metabolic energy expenditure with gas exchange (VO2 uptake) during similar acute HIIT and MICET protocols in CHD patients.21
Resistance training programResistance training consisted of 20minutes of circuit weight training performed with elastic bands and free weights adapted to each patient's capacity. For each muscle group, patients performed 1 set of 15 to 20 repetitions, followed by a 30-second rest at a target rate of perceived exertion of 15.27
Usual care groupThe control group received recommendations on physical activity for a period of 12 weeks by their discharging cardiologist. If there were no recommendations at discharge, physical activity recommendations consistent with recent guidelines were given. Patients were encouraged to take 30 to 60minutes of moderate-intensity exercise at least 5 days per week (target rate of perceived exertion of 12-14).30
Statistical analysesData are presented as mean±standard deviation or median±interquartile range as appropriate for continuous variables, while frequencies and percentages are presented for categorical variables. Baseline characteristics were compared between the 3 groups using 1-way ANOVA and categorical variables were compared using the chi-square or Fisher exact tests. Repeated measures ANOVA models were used to study the CPET parameters across time and between groups. Models with time, group and group × time interaction as independent variables were used. The main focus of the analysis was the group × time interaction as it tested the difference in the change (post-pre) between the 3 groups. In addition, under the repeated measures ANOVA model, the change (post-pre) within each group was formally tested against zero. For the analysis of training response, VO2peak high response vs NLR was defined as the median value for change in peak oxygen uptake (ΔVO2peak in mL/min/kg) post- and pretraining in the whole cohort.8 Training response with a ΔVO2peak <2.1mL/min/kg was defined as NLR, while a ΔVO2peak >2.1mL/min/kg was defined as VO2peak high response. Univariate and multivariate logistic regression was used to generate a predictive model for training NLR. Predictors of training NLR for univariate logistic regression were selected as follows: sex, age, VO2peak percent predicted at baseline, presence of type 2 diabetes mellitus, and training modality. All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and conducted at the .05 significance level.
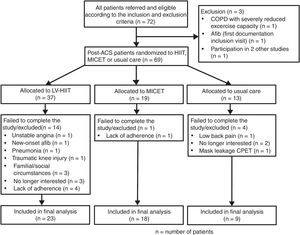
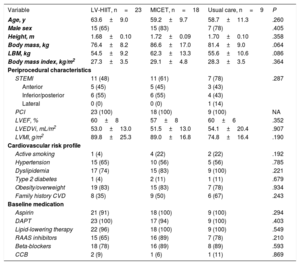
ResultsClinical characteristicsThe study flowchart is presented in figure 2. In the final analysis, we included a total of 50 patients (LV-HIIT, n=23, MICET, n=18, usual care, n=9). Patients in the HIIT group tended to have lower body mass and lean body mass than patients in the MICET and usual care group. Otherwise, there were no differences with regards to baseline clinical characteristics (table 1).
Baseline characteristics of post-ACS patients randomized to LV-HIIT, MICET or usual care
| Variable | LV-HIIT, n=23 | MICET, n=18 | Usual care, n=9 | P |
|---|---|---|---|---|
| Age, y | 63.6±9.0 | 59.2±9.7 | 58.7±11.3 | .260 |
| Male sex | 15 (65) | 15 (83) | 7 (78) | .405 |
| Height, m | 1.68±0.10 | 1.72±0.09 | 1.70±0.10 | .358 |
| Body mass, kg | 76.4±8.2 | 86.6±17.0 | 81.4±9.0 | .064 |
| LBM, kg | 54.5±9.2 | 62.3±13.3 | 55.6±10.6 | .086 |
| Body mass index, kg/m2 | 27.3±3.5 | 29.1±4.8 | 28.3±3.5 | .364 |
| Periprocedural characteristics | ||||
| STEMI | 11 (48) | 11 (61) | 7 (78) | .287 |
| Anterior | 5 (45) | 5 (45) | 3 (43) | |
| Inferior/posterior | 6 (55) | 6 (55) | 4 (43) | |
| Lateral | 0 (0) | 0 (0) | 1 (14) | |
| PCI | 23 (100) | 18 (100) | 9 (100) | NA |
| LVEF, % | 60±8 | 57±8 | 60±6 | .352 |
| LVEDVi, mL/m2 | 53.0±13.0 | 51.5±13.0 | 54.1±20.4 | .907 |
| LVMI, g/m2 | 89.8±25.3 | 89.0±16.8 | 74.8±16.4 | .190 |
| Cardiovascular risk profile | ||||
| Active smoking | 1 (4) | 4 (22) | 2 (22) | .192 |
| Hypertension | 15 (65) | 10 (56) | 5 (56) | .785 |
| Dyslipidemia | 17 (74) | 15 (83) | 9 (100) | .221 |
| Type 2 diabetes | 1 (4) | 2 (11) | 1 (11) | .679 |
| Obesity/overweight | 19 (83) | 15 (83) | 7 (78) | .934 |
| Family history CVD | 8 (35) | 9 (50) | 6 (67) | .243 |
| Baseline medication | ||||
| Aspirin | 21 (91) | 18 (100) | 9 (100) | .294 |
| DAPT | 23 (100) | 17 (94) | 9 (100) | .403 |
| Lipid-lowering therapy | 22 (96) | 18 (100) | 9 (100) | .549 |
| RAAS inhibitors | 15 (65) | 16 (89) | 7 (78) | .210 |
| Beta-blockers | 18 (78) | 16 (89) | 8 (89) | .593 |
| CCB | 2 (9) | 1 (6) | 1 (11) | .869 |
ACS, acute coronary syndrome; CCB, calcium channel blocker; CVD, cardiovascular disease; DAPT, dual antiplatelet therapy; LBM, lean body mass; LV-HIIT, low-volume high-intensity interval training; LVEDVi, left ventricular end diastolic volume index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MICET, moderate-intensity continuous exercise training; PCI, percutaneous coronary intervention; RAAS, renin angiotensin aldosterone system; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as mean±standard deviation or No. (%).
The median value for ΔVO2peak (in mL/min/kg) post and pretraining in the whole cohort was 2.1mL/min/kg. MICET was associated with a significantly lower proportion of training NLR compared with LV-HIIT and usual care (21% in MICET, 61% in LV-HIIT, and 80% in the usual care group; P=.004). Of note, for the LV-HIIT and MICET program, adherence (percentage) was defined as the number of attended sessions divided by the total planned sessions × 100. Patients were only included in the analysis if they attended at least 75% of the training sessions and 1.5 weekly training sessions. Patients completed 2.4±0.5 and 2.4±0.4 weekly training sessions in the LV-HIIT and MICET groups, respectively (P=.946). Weekly training duration was 83±12minutes with LV-HIIT and 80±14minutes with MICET (P=.487). Adherence was 100 (97) in the LV-HIIT and 100 (95) in the MICET group, respectively (P=.456).
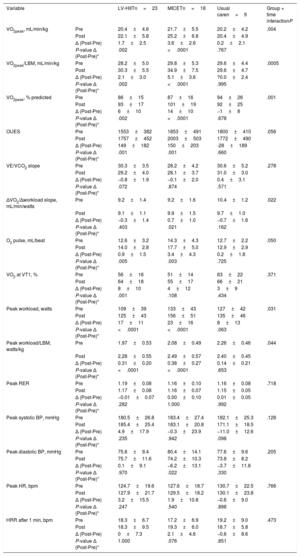
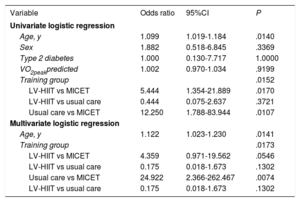
Cardiopulmonary exercise test parameters in the groups (LV-HIIT, MICET, usual care)As shown in table 2, VO2peak (normalized for body mass and lean body mass, respectively), predicted VO2peak, and peak workload (absolute and normalized for lean body mass) improved with training in the LV-HIIT and in the MICET group (P <.05 for time), while there was no effect in the usual care group. Significant group × time interaction was observed for these parameters (P <.05). The oxygen uptake efficiency slope and O2 pulse improved with LV-HIIT and MICET (P <.05 for time), but not in the usual care group. Moreover, the ΔVO2/Δworkload slope increased only in the MICET group (P <.05 for time), while there was no change in the LV-HIIT and the usual care groups. There was a significant group × time interaction for this variable (P <.05). In table 3, initial fitness (expressed as percent-predicted VO2peak) was not related to training NLR either in the univariate or in the multivariate analysis. In the multivariate regression model, age remained a predictor of training NLR (P <.05), while there was a trend for LV-HIIT vs MICET (P=.054).
Cardiopulmonary exercise test parameters in post-ACS patients randomized to LV-HIIT, MICET, or usual care
| Variable | LV-HIITn=23 | MICETn=18 | Usual caren=9 | Group × time interactionP | |
|---|---|---|---|---|---|
| VO2peak, mL/min/kg | Pre | 20.4±4.6 | 21.7±5.5 | 20.2±4.2 | .004 |
| Post | 22.1±5.8 | 25.2±6.8 | 20.4±4.9 | ||
| Δ (Post-Pre) | 1.7±2.5 | 3.6±2.6 | 0.2±2.1 | ||
| P-value Δ (Post-Pre)* | .002 | <.0001 | .767 | ||
| VO2peak/LBM, mL/min/kg | Pre | 28.2±5.0 | 29.8±5.3 | 29.6±4.4 | .0005 |
| Post | 30.3±5.5 | 34.9±7.5 | 29.6±4.7 | ||
| Δ (Post-Pre) | 2.1±3.0 | 5.1±3.6 | ?0.0±2.4 | ||
| P-value Δ (Post-Pre)* | .002 | <.0001 | .995 | ||
| VO2peak, % predicted | Pre | 86±15 | 87±16 | 94±26 | .001 |
| Post | 93±17 | 101±19 | 92±25 | ||
| Δ (Post-Pre) | 6±10 | 14±10 | −1±8 | ||
| P-value Δ (Post-Pre)* | .002 | <.0001 | .678 | ||
| OUES | Pre | 1553±382 | 1853±491 | 1800±410 | .056 |
| Post | 1757±452 | 2003±503 | 1772±490 | ||
| Δ (Post-Pre) | 149±182 | 150±203 | -28±189 | ||
| P-value Δ (Post-Pre)* | .001 | .001 | .660 | ||
| VE/VCO2 slope | Pre | 30.3±3.5 | 28.2±4.2 | 30.6±5.2 | .278 |
| Post | 29.2±4.0 | 28.1±3.7 | 31.0±3.0 | ||
| Δ (Post-Pre) | −0.8±1.9 | −0.1±2.0 | 0.4±3.1 | ||
| P-value Δ (Post-Pre)* | .072 | .874 | .571 | ||
| ΔVO2/Δworkload slope, mL/min/watts | Pre | 9.2±1.4 | 9.2±1.6 | 10.4±1.2 | .022 |
| Post | 9.1±1.1 | 9.9±1.5 | 9.7±1.0 | ||
| Δ (Post-Pre) | −0.3±1.4 | 0.7±1.0 | −0.7±1.6 | ||
| P-value Δ (Post-Pre)* | .403 | .021 | .162 | ||
| O2 pulse, mL/beat | Pre | 12.6±3.2 | 14.3±4.3 | 12.7±2.2 | .050 |
| Post | 14.0±2.8 | 17.7±5.0 | 12.9±2.9 | ||
| Δ (Post-Pre) | 0.9±1.5 | 3.4±4.3 | 0.2±1.8 | ||
| P-value Δ (Post-Pre)* | .005 | .003 | .725 | ||
| VO2 at VT1, % | Pre | 56±16 | 51±14 | 63±22 | .371 |
| Post | 64±18 | 55±17 | 66±21 | ||
| Δ (Post-Pre) | 8±10 | 4±12 | 3±9 | ||
| P-value Δ (Post-Pre)* | .001 | .108 | .434 | ||
| Peak workload, watts | Pre | 109±39 | 133±43 | 127±42 | .031 |
| Post | 125±43 | 156±51 | 135±46 | ||
| Δ (Post-Pre) | 17±11 | 23±16 | 8±13 | ||
| P-value Δ (Post-Pre)* | <.0001 | <.0001 | .063 | ||
| Peak workload/LBM, watts/kg | Pre | 1.97±0.53 | 2.08±0.49 | 2.26±0.46 | .044 |
| Post | 2.28±0.55 | 2.49±0.57 | 2.40±0.45 | ||
| Δ (Post-Pre) | 0.31±0.20 | 0.38±0.27 | 0.14±0.21 | ||
| P-value Δ (Post-Pre)* | <.0001 | <.0001 | .653 | ||
| Peak RER | Pre | 1.19±0.08 | 1.16±0.10 | 1.16±0.08 | .718 |
| Post | 1.17±0.08 | 1.16±0.07 | 1.15±0.05 | ||
| Δ (Post-Pre) | −0.01±0.07 | 0.00±0.10 | 0.01±0.05 | ||
| P-value Δ (Post-Pre)* | .282 | 1.000 | .992 | ||
| Peak systolic BP, mmHg | Pre | 180.5±26.8 | 183.4±27.4 | 182.1±25.3 | .128 |
| Post | 185.4±25.4 | 183.1±20.8 | 171.1±18.5 | ||
| Δ (Post-Pre) | 4.9±17.9 | −0.3±23.9 | −11.0±12.6 | ||
| P-value Δ (Post-Pre)* | .235 | .942 | .098 | ||
| Peak diastolic BP, mmHg | Pre | 75.6±9.4 | 80.4±14.1 | 77.6±9.6 | .205 |
| Post | 75.7±11.6 | 74.2±10.3 | 73.8±8.2 | ||
| Δ (Post-Pre) | 0.1±9.1 | −6.2±13.1 | −3.7±11.8 | ||
| P-value Δ (Post-Pre)* | .970 | .022 | .330 | ||
| Peak HR, bpm | Pre | 124.7±19.6 | 127.6±18.7 | 130.7±22.5 | .766 |
| Post | 127.9±21.7 | 129.5±18.2 | 130.1±23.8 | ||
| Δ (Post-Pre) | 3.2±15.5 | 1.9±10.8 | −0.6±9.0 | ||
| P-value Δ (Post-Pre)* | .247 | .540 | .898 | ||
| HRR after 1 min, bpm | Pre | 18.3±6.7 | 17.2±6.9 | 19.2±9.0 | .473 |
| Post | 18.3±9.5 | 19.3±6.0 | 18.7±5.8 | ||
| Δ (Post-Pre) | 0±7.3 | 2.1±4.6 | −0.6±8.6 | ||
| P-value Δ (Post-Pre)* | 1.000 | .076 | .851 |
ACS, acute coronary syndrome; BP, blood pressure; HR, heart rate; HRR, heart rate recovery; LBM, lean body mass; LV-HIIT, low-volume high-intensity interval training; MICET, moderate-intensity continuous exercise training; OUES, oxygen uptake efficiency slope; RER, respiratory exchange ratio; VE/VCO2 slope, ventilatory efficiency slope; VO2, oxygen uptake; VT1, first ventilatory threshold.
Data are expressed as means±standard deviation.
Predictors for training non/low response
| Variable | Odds ratio | 95%CI | P |
|---|---|---|---|
| Univariate logistic regression | |||
| Age, y | 1.099 | 1.019-1.184 | .0140 |
| Sex | 1.882 | 0.518-6.845 | .3369 |
| Type 2 diabetes | 1.000 | 0.130-7.717 | 1.0000 |
| VO2peakpredicted | 1.002 | 0.970-1.034 | .9199 |
| Training group | .0152 | ||
| LV-HIIT vs MICET | 5.444 | 1.354-21.889 | .0170 |
| LV-HIIT vs usual care | 0.444 | 0.075-2.637 | .3721 |
| Usual care vs MICET | 12.250 | 1.788-83.944 | .0107 |
| Multivariate logistic regression | |||
| Age, y | 1.122 | 1.023-1.230 | .0141 |
| Training group | .0173 | ||
| LV-HIIT vs MICET | 4.359 | 0.971-19.562 | .0546 |
| LV-HIIT vs usual care | 0.175 | 0.018-1.673 | .1302 |
| Usual care vs MICET | 24.922 | 2.366-262.467 | .0074 |
| LV-HIIT vs usual care | 0.175 | 0.018-1.673 | .1302 |
95%CI, 95% confidence interval; LV-HIIT, low-volume high-intensity interval training; MICET, moderate-intensity continuous exercise training; VO2, oxygen consumption.
Univariate and multivariate logistic regression analysis including age, sex, type 2 diabetes (0=no, 1=yes), VO2peak predicted and training group.
The main findings of our study can be summarized as follows: a) For the first time, we show that optimized LV-HIIT exhibited a higher proportion of NLR to training than isocaloric MICET (61% in the HIIT vs 21% in the MICET group). b) VO2peak-dependent variables (ie VO2peak, percent-predicted VO2peak), peak workload, and O2 pulse were improved after LV-HIIT, but the improvement was more pronounced in the MICET group. c) The ΔVO2/Δworkload slope increased only in the MICET group. d) Age and training group were independent predictors of the non/low response in our patients with a recent ACS.
This is the first study to compare the proportion of responders (non/low vs high) to aerobic exercise training with different modalities in patients with a recent ACS. Contrary to our initial hypothesis, our data revealed a disproportionally higher proportion of VO2peak NLR with LV-HIIT than with isocaloric MICET. Our results disagree with those of the SAINTEX-CAD study in that the proportion of nonresponders (14%) was equivalent after HIIT and MICET.10 However, the criteria for VO2peak nonresponse were less conservative in this study (ΔVO2peak <1mL/min/kg), and the training volume was higher (114min/wk to 141min/wk) compared with our study (80minutes to 83min).10 Recently, a multicenter study in adults with different CV status showed that high-volume HIIT led to a lower proportion of nonresponders vs MICET and LV-HIIT.31 Finally, it has been consistently shown that lower exercise intensity is an independent predictor of training nonresponse in cardiac patients (together with age, initial VO2peak, and comorbidities).10,11 Therefore, our patient cohort performed an exercise volume at the lower range of current international recommendations, but this reflects common clinical practice in cardiovascular secondary prevention in our center and more generally in our province.28,29
The recommendations for exercise prescription based on the FITT principle (FIIT: frequency, intensity, type, and time)29,32 can influence the proportion of exercise responders, as recently suggested in young and obese adults.33,34 In obese adults, Ross et al.34 showed that, at fixed exercise intensity and frequency, increasing the exercise volume (session duration) reduced the proportion of nonresponders by 50% after 24 weeks. In the same study, for a fixed exercise volume (frequency/duration), increasing training intensity eliminated nonresponders completely. Similarly, in young adults, Montero et al.33 showed a higher proportion of nonresponders in individuals performing 1 to 3 aerobic exercise training sessions/wk compared with those performing 4 to 5 sessions/wk (6 weeks of training). Training nonresponse was abolished by adding another 120min/wk to the 4 to 5 sessions for another 6 week training period.33
Based on these previous elements, our results with a higher proportion of NLR after LV-HIIT could be explained by several hypotheses: First, our LV-HIIT might not provide a sufficient total exercise duration due to the passive recovery used.35 Patients in our study effectively pedaled only half of the time during LV-HIIT (9 to 15minutes for 1 session), whereas exercise was not stopped during MICET (24minutes for 1 session). Due to the nature of LV-HIIT (very short stage/passive recovery) might have a lower impact on the ventilatory and cardiac function adaptations (ie, ventilation-cardiac output, a major determinant of VO2peak) compared with MICET, as demonstrated acutely.19,21
Regarding our CPET variables, we observed substantial training-induced improvements after LV-HIIT and MICET training compared with usual care in our post-ACS patients. Contrary to our initial hypothesis, MICET led to greater improvements with regards to VO2peak-dependent variables (ie, VO2peak, percent-predicted VO2peak) compared with LV-HIIT. Indeed, the mean ΔVO2peak improvement was 1.7mL/min/kg (or 8.3%) for LV-HIIT and 3.6mL/min/kg (or 16.1%) in the MICET group. This improvement in our LV-HIIT group is lower than the improvements reported in a recent meta-analysis comparing HIIT and MICET training in CHD patients.17 Some factors in our LV-HIIT protocol, such as the use of passive recovery, a lower session frequency (2-3/wk) and therefore a lower exercise volume, may have influenced our results, as recently documented in a meta-analysis in cardiac patients.35 Although previously optimized regarding acute physiological and clinical responses, our LV-HIIT protocol was not equivalent regarding VO2peak improvement compared with isocaloric MICET. However, some clinical benefits have been documented for modest improvements of VO2peak in CHD patients: a VO2peak increase of 1 mL/min/kg confers a 15% mortality reduction and being a lower responder (< 2.5mL/min/kg) is associated with a better prognosis compared with a nonresponder.8,36
Regarding other CPET variables, oxygen uptake efficiency slope improved similarly in the MICET and LV-HIIT group, indicating a similar impact of both modalities on this parameter of ventilatory efficiency. Indeed, the oxygen uptake efficiency slope reflects the efficiency of O2 extracted by the lungs and used by the peripheral muscle and is also an independent predictor of cardiovascular and total mortality in CHD patients.37 An increase in the oxygen uptake efficiency slope of 100 can be associated with a 4.4% reduction in cardiovascular mortality in CHD patients. Our patients improved their oxygen uptake efficiency slope by a mean of 150 (table 2) in both groups.37 We observed an improvement of the ΔVO2/Δworkload slope only in the MICET group, reflecting an improvement in the adequacy of O2 transport to the peripheral muscle.26 Moreover, O2 pulse was improved to a greater level in the MICET group (table 2) compared with the LV-HIIT group. This suggests an improvement in central cardiac function, because O2 pulse is an indirect surrogate for stroke volume.26 Finally, VO2 at the first ventilatory threshold (VO2 at VT1) was only improved in the LV-HIIT group, which reflects an improvement in aerobic endurance.
LimitationsIn our study, data from 2 prospective randomized control trials were pooled for analysis from a single institution and with the participants composed mainly of men. This explains why a disproportionate number of patients were randomized to the 3 groups. This might have affected our results. However, a carefully selected and highly homogenous population of patients who experienced an ACS in the preceding 6 weeks before inclusion were included and randomized to either HIIT vs MICET or HIIT vs usual care. While our optimized LV-HIIT protocol has been well evaluated regarding the acute responses in CHD patients,19,21 our protocol is not the most commonly used in clinical research in patients with a recent ACS.17,31,35 Therefore, the results of our findings cannot be generalized, particularly not to a cohort using a different HIIT protocol.
ConclusionsIn patients with a recent ACS, optimized LV-HIIT resulted in a higher proportion of NLR to training compared with isocaloric MICET. Substantial improvements were observed in both aerobic exercise training groups compared with usual care with a training frequency and duration at the lower range of recommended international guidelines. Key VO2peak-dependent variables and peak workload improved significantly with LV-HIIT, but the improvement was more pronounced with MICET. Other CPET variables related to ventilatory efficiency or aerobic endurance (oxygen uptake efficiency slope, VO2 VT1) were also improved with LV-HIIT. Based on these findings, we believe that MICET remains an important exercise modality to use in patients with recent ACS during the initiation/improvement phase.16 Because it is well tolerated, our LV-HIIT protocol could be used during the initiation phase (1 to 2 weeks) to familiarize patients with the HIIT modality. Future research in this field should take into consideration and compare alternative training models such as training periodization (including HIIT and MICET), as recently proposed.16
FundingThe study was funded by the Montreal Heart Institute Foundation and the EPIC Center Foundation.
Conflicts of interestNone declared.
- -
Long interval HIIT can be equivalent to isocaloric MICET for VO2peak improvement in CHD patients.
- -
Long interval HIIT is less well tolerated and its intensity is hard to maintain for CHD patients.
- -
LV-HIIT is safe, well tolerated by CHD patients, and produces similar acute physiological responses to MICET.
- -
LV-HIIT resulted in a higher proportion of NLR to training vs isocaloric MICET.
- -
In post-ACS patients, key VO2peak variables showed a greater improvement after isocaloric MICET than after LV-HIIT.
- -
In post-ACS patients, LV-HIIT and MICET similarly improve aerobic endurance and ventilatory efficiency.